Chemical Composition and Antioxidant Capacity of the Fruits of European Plum Cultivar “Čačanska Lepotica” Influenced by Different Rootstocks
Abstract
1. Introduction
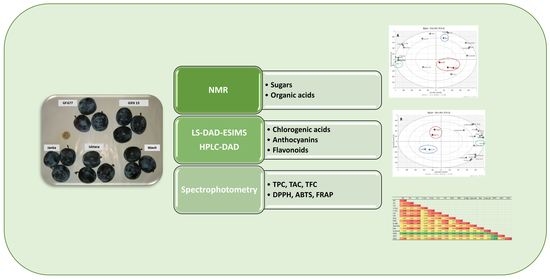
2. Materials and Methods
2.1. Plum Samples
2.2. Extraction
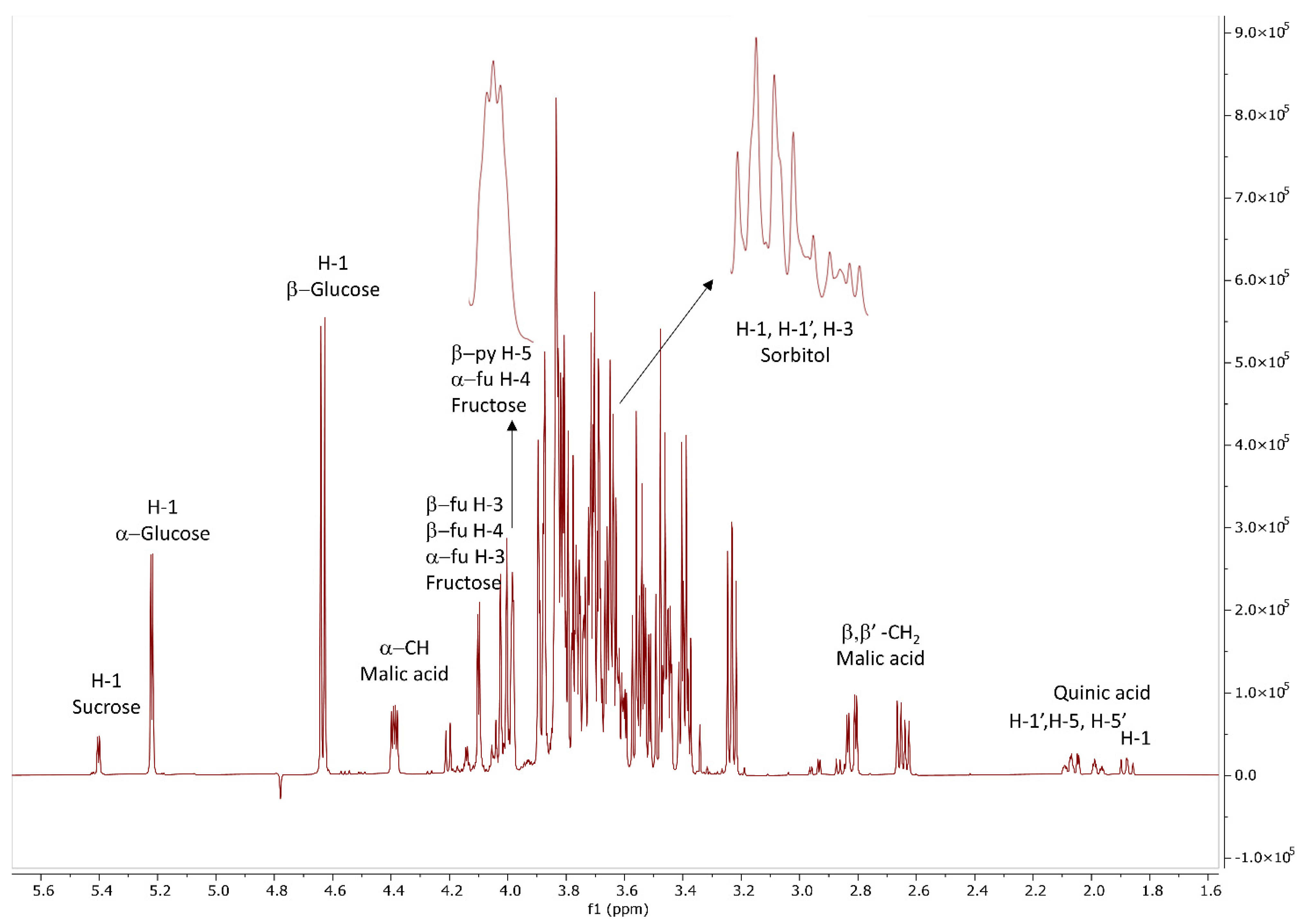
2.3. Qualitative and Quantitative NMR Analysis of Sugars and Organic Acids
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoid Content
2.6. Determination of Total Anthocyanin Content
2.7. LC–DAD–ESIMS Analysis
2.8. Quantitative Determination of Individual Compounds by HPLC–DAD Analysis
2.9. Determination of Antioxidant Capacity
2.9.1. DPPH Radical Scavenging Activity
2.9.2. ABTS Radical-Ion Scavenging Activity
2.9.3. FRAP Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Qualitative and Quantitative Determination of Carbohydrates and Organic Acids by 1H NMR
3.2. Determination of Total Phenolic, Total Flavonoid, and Total Anthocyanin Contents
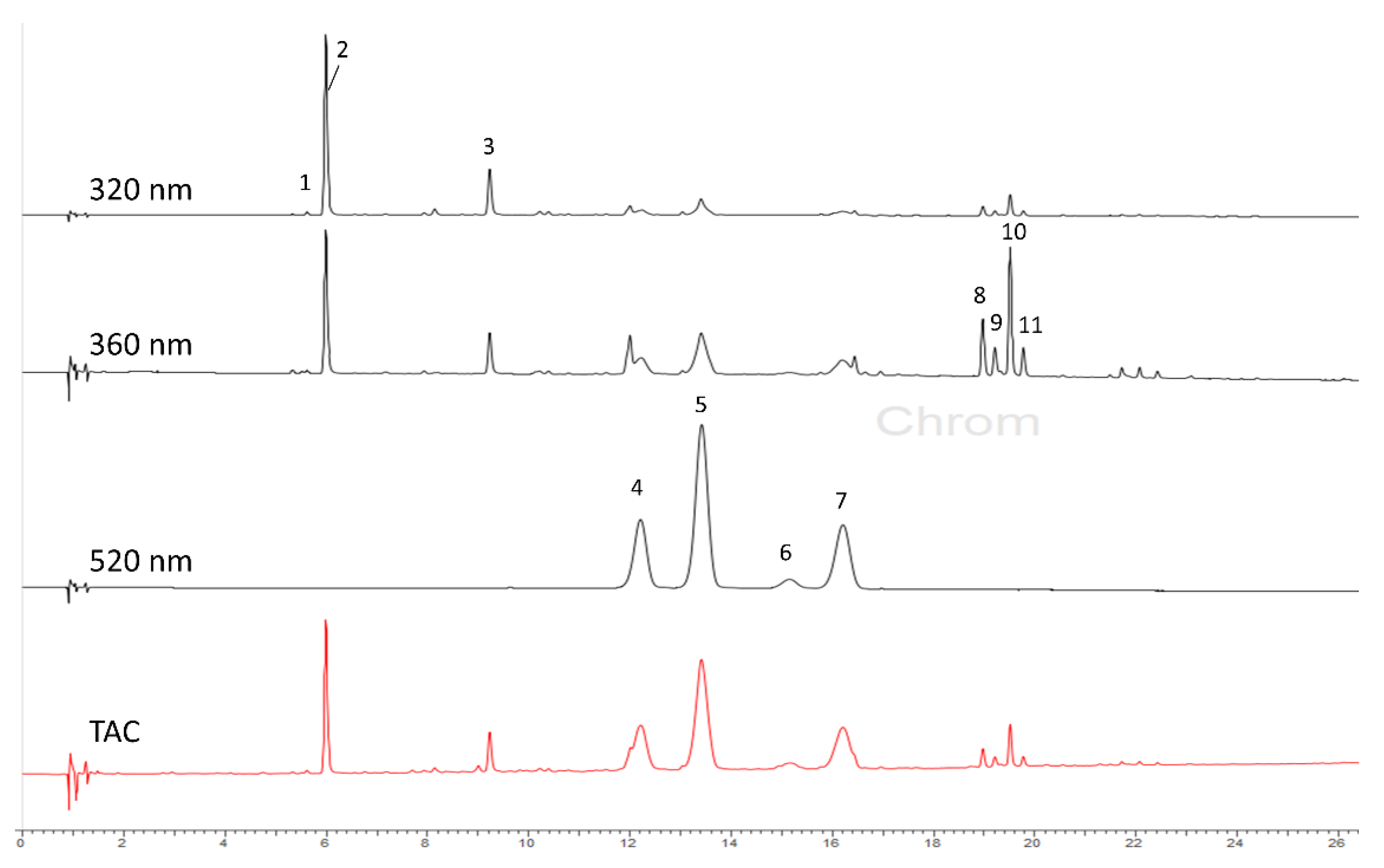
3.3. Identification of Individual Compounds by LC–DAD–ESIMS
3.4. Quantitative Determination of Individual Compounds by HPLC–DAD
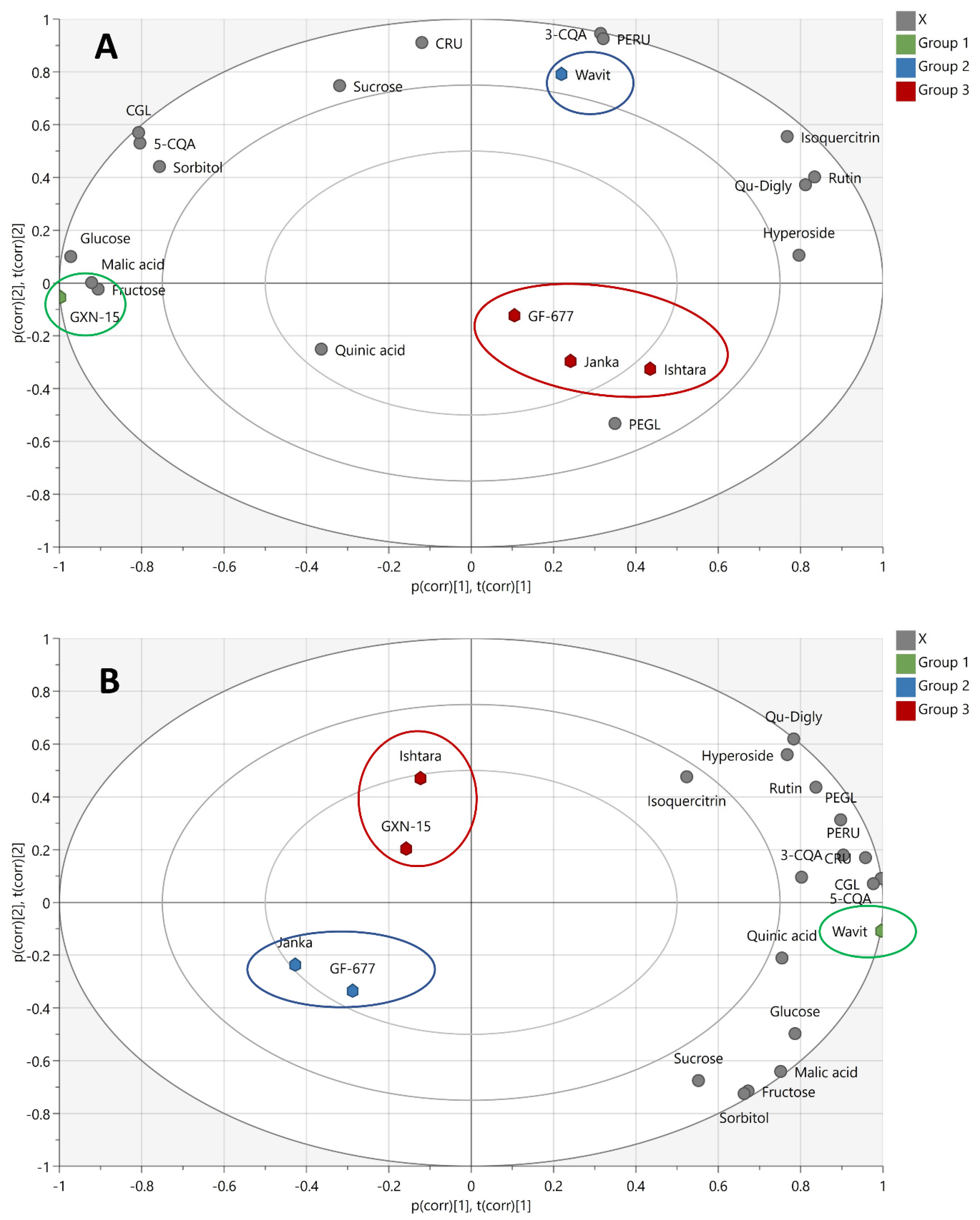
3.5. Comparison of the Plum Fruit and Fruit Skins Samples
3.6. Antioxidant Potential
3.7. Correlation between Phenolic Compounds and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sultana, N.; Tul Muntaha, S.; Haroon, Z.; Fatima, D.; Fakhra, H.; Pakistan Karachi, K. Prunus domestica: A Review. Asian J. Pharmacogn. 2020, 4, 21–29. [Google Scholar]
- Stacewicz-Sapuntzakis, M.; Bowen, P.E.; Hussain, E.A.; Damayanti-Wood, B.I.; Farnsworth, N.R. Chemical Composition and Potential Health Effects of Prunes: A Functional Food? Crit. Rev. Food Sci. Nutr. 2001, 41, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Z.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Miletić, N.; Mitrović, O.; Popović, B.; Nedović, V.; Zlatković, B.; Kandić, M. Polyphenolic Content and Antioxidant Capacity in Fruits of Plum (Prunus domestica L.) Cultivars “Valjevka” and “Mildora” as Influenced by Air Drying. J. Food Qual. 2013, 36, 229–237. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Cvejić Hogervorst, J.; Torović, L. Phenolic Compounds, Chromatic Characteristics and Antiradical Activity of Plum Wines. Int. J. Food Prop. 2017, 20, 2022–2033. [Google Scholar] [CrossRef][Green Version]
- Usenik, V.; Stampar, F.; Kastelec, D. Phytochemicals in Fruits of Two Prunus domestica L. Plum Cultivars during Ripening. J. Sci. Food Agric. 2013, 93, 681–692. [Google Scholar] [CrossRef]
- Treutter, D.; Wang, D.; Farag, M.A.; Baires, G.D.A.; Rühmann, S.; Neumüller, M. Diversity of Phenolic Profiles in the Fruit Skin of Prunus Domestica Plums and Related Species. J. Agric. Food Chem. 2012, 60, 12011–12019. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic Characterization and Antioxidant Activity of Malus domestica and Prunus domestica Cultivars from Costa Rica. Foods 2018, 7, 15. [Google Scholar] [CrossRef]
- Radović, M.; Milatović, D.; Tešić, Ž.; Tosti, T.; Gašić, U.; Dojčinović, B.; Dabić Zagorac, D. Influence of Rootstocks on the Chemical Composition of the Fruits of Plum Cultivars. J. Food Compos. Anal. 2020, 92, 103480. [Google Scholar] [CrossRef]
- Yagmur, C.; Taskin, M. Study on Changes in Mineral Content of Plum (Prunus domestica) and Strawberry (Fragaria×ananassa) during Canning. Indian J. Agric. Sci. 2011, 81, 723–728. [Google Scholar]
- Tabakov, S.G.; Yordanov, A.I. Influence of Different Rootstocks on Growth Characteristics of Cultivars of the Species Prunus domestica in Nursery. Acta Hortic. 2019, 1260, 209–214. [Google Scholar] [CrossRef]
- Angelova, V.R.; Tabakov, S.G.; Petrov, M.N. Influence of Rootstock Cultivar Combination on the Content of Heavy Metals, Micro and Microelements in the Fruits of Plums. Agric. Univ. Agric. Sci. 2020, 12, 128–133. [Google Scholar] [CrossRef]
- Tabakov, S.G.; Yordanov, A.I.; Denev, P.N.; Teneva, D.G. Study of the Influence of Rootstocks on Fruit Flesh Quality of Three Plum Cultivars. Acta Hortic. 2021, 1322, 155–162. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Content of Biologically Active Compounds in Bulgarian Propolis: A Basis for Its Standardization. Bulg. Chem. Commun. 2017, 49, 115–120. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benze, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Nonenzymatic Antioxidant Capacity: Concepts, Procedures, Limita-Tions and Applications. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 76–106. ISBN 9781119135388. [Google Scholar]
- Sobolev, A.P.; Mannina, L.; Proietti, N.; Carradori, S.; Daglia, M.; Giusti, A.M.; Antiochia, R.; Capitani, D. Untargeted NMR-Based Methodology in the Study of Fruit Metabolites. Molecules 2015, 20, 4088. [Google Scholar] [CrossRef]
- Ullah, H.; Sommella, E.; Santarcangelo, C.; D’avino, D.; Rossi, A.; Dacrema, M.; Di Minno, A.; Di Matteo, G.; Mannina, L.; Campiglia, P.; et al. Hydroethanolic Extract of Prunus Domestica L.: Metabolite Profiling and In Vitro Modulation of Molecular Mechanisms Associated to Cardiometabolic Diseases. Nutrients 2022, 14, 340. [Google Scholar] [CrossRef]
- Capitani, D.; Mannina, L.; Proietti, N.; Sobolev, A.P.; Tomassini, A.; Miccheli, A.; Di Cocco, M.E.; Capuani, G.; De Salvador, R.; Delfini, M. Monitoring of Metabolic Profiling and Water Status of Hayward Kiwifruits by Nuclear Magnetic Resonance. Talanta 2010, 82, 1826–1838. [Google Scholar] [CrossRef]
- Ayub, R.A.; Salome, K.S.; Steinkirch, E.M.P.V.; Fonseca, F.A.; Barisson, A. Plum Ripening Evaluation by 1H NMR Spectroscopy. Acta Hortic. 2015, 1099, 277–282. [Google Scholar] [CrossRef]
- Goulas, V.; Minas, I.S.; Kourdoulas, P.M.; Lazaridou, A.; Molassiotis, A.N.; Gerothanassis, I.P.; Manganaris, G.A. 1H NMR Metabolic Fingerprinting to Probe Temporal Postharvest Changes on Qualitative Attributes and Phytochemical Profile of Sweet Cherry Fruit. Front. Plant Sci. 2015, 6, 959. [Google Scholar] [CrossRef] [PubMed]
- Nor, S.M.; Ding, P.; Abas, F.; Mediani, A. 1H NMR Reveals Dynamic Changes of Primary Metabolites in Purple Passion Fruit (Passiflora edulis Sims) Juice during Maturation and Ripening. Agriculture 2022, 12, 156. [Google Scholar] [CrossRef]
- Human Metabolome Database: 1H NMR Spectrum (1D, 500 MHz, H2O, Experimental) (HMDB0000247). Available online: https://hmdb.ca/spectra/nmr_one_d/1273 (accessed on 22 July 2022).
- Babenko, M.; Peron, J.M.R.; Kaialy, W.; Calabrese, G.; Alany, R.G.; ElShaer, A. 1H NMR Quantification of Spray Dried and Spray Freeze-Dried Saccharide Carriers in Dry Powder Inhaler Formulations. Int. J. Pharm. 2019, 564, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.; Petrovsky, N. Observation of the Keto Tautomer of D-Fructose in D2O Using 1H NMR Spectroscopy. Carbohydr. Res. 2012, 347, 136–141. [Google Scholar] [CrossRef]
- Miloševíć, T.; Miloševíć, N. The Physical and Chemical Attributes of Plum Influenced by Rootstock. Acta Aliment. 2012, 41, 293–303. [Google Scholar] [CrossRef]
- Usenik, V. The Influence of the Production System on the Composition of Phytochemicals in Prunus domestica L. Fruit. J. Food Compos. Anal. 2021, 95, 103701. [Google Scholar] [CrossRef]
- Sahamishirazi, S.; Moehring, J.; Claupein, W.; Graeff-Hoenninger, S. Quality Assessment of 178 Cultivars of Plum Regarding Phenolic, Anthocyanin and Sugar Content. Food Chem. 2017, 214, 694–701. [Google Scholar] [CrossRef]
- Vangdal, E.; Døving, A.; Måge, F. The Fruit Quality of Plums (Prunus domestica L.) as Related to Yield and Climatic Conditions. Acta Hortic. 2007, 734, 425–429. [Google Scholar] [CrossRef]
- Forni, E.; Erba, M.L.; Maestrelli, A.; Polesello, A. Sorbitol and Free Sugar Contents in Plums. Food Chem. 1992, 44, 269–275. [Google Scholar] [CrossRef]
- Dimkova, S.; Ivanova, D.; Stefanova, B.; Marinova, N.; Todorova, S. Chemical and Technological Characteristic of Plum Cultivars of Prunus domestica L. Bulg. J. Agric. Sci. 2018, 24, 43–47. [Google Scholar]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS Analysis of Phenolic Compounds in Nectarines, Peaches, and Plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant Capacities, Phenolic Compounds, Carotenoids, and Vitamin C Contents of Nectarine, Peach, and Plum Cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Mitic, V.; Ilic, M.; Dimitrijevic, M.; Cvetkovic, J.; Ciric, S.; Jovanovic, V.S. Chemometric Characterization of Peach, Nectarine and Plum Cultivars According to Fruit Phenolic Content and Antioxidant Activity. Fruits 2016, 71, 57–66. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Botu, M. Total Phenolic, Flavonoid Distribution and Antioxidant Capacity in Skin, Pulp and Fruit Extracts of Plum Cultivars. J. Food Biochem. 2015, 39, 64–69. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Jaiswal, R.; Karaköse, H.; Rühmann, S.; Goldner, K.; Neumüller, M.; Treutter, D.; Kuhnert, N. Identification of Phenolic Compounds in Plum Fruits (Prunus Salicina L. and Prunus domestica L.) by High-Performance Liquid Chromatography/Tandem Mass Spectrometry and Characterization of Varieties by Quantitative Phenolic Fingerprints. J. Agric. Food Chem. 2013, 61, 12020–12031. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purification of Anthocyanins. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.1.1–F1.1.11. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Fossen, T. Characterization of Anthocyanins by NMR. Curr. Protoc. Food Anal. Chem. 2003, 9, F1.4.1–F1.4.23. [Google Scholar] [CrossRef]
- Poonam, V.; Raunak; Kumar, G.; Reddy, L.S.; Jain, R.C.; Sharma, S.K.; Prasad, A.K.; Parmar, V.S. Chemical Constituents of the Genus Prunus and Their Medicinal Properties. Curr. Med. Chem. 2012, 18, 3758–3824. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and Fruit Colour in Plums (Prunus domestica L.) during Ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Okulevičiūtė, R.; Lanauskas, J.; Kviklys, D.; Zymonė, K.; Rendyuk, T.; Žvikas, V.; Uselis, N.; Janulis, V. Variability in the Content of Phenolic Compounds in Plum Fruit. Plants 2020, 9, 1611. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Diuzheva, A.; Carradori, S.; Andruch, V.; Massafra, C.; Moldovan, C.; Sisea, C.; Petzer, J.P.; Petzer, A.; Zara, S.; et al. Development of Novel Techniques to Extract Phenolic Compounds from Romanian Cultivars of Prunus domestica L. and Their Biological Properties. Food Chem. Toxicol. 2018, 119, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. High-Throughput Screening and Characterization of Phenolic Compounds in Stone Fruits Waste by LC-ESI-QTOF-MS/MS and Their Potential Antioxidant Activities. Antioxidants 2021, 10, 234. [Google Scholar] [CrossRef]
| Rootstock | Sucrose | Glucose | Fructose | Sorbitol | TSC | Malic Acid | Quinic Acid | TOAC |
|---|---|---|---|---|---|---|---|---|
| Fruit | ||||||||
| Wavit | 1.60 ± 0.02 a | 3.62 ± 0.1 a–c | 2.48 ± 0.01 a | 3.39 ± 0 a | 11.09 ± 0.05 a | 0.77 ± 0.01 a | 0.26 ± 0 a–c | 1.02 ± 0 a |
| Janka | 1.35 ± 0.02 b | 3.47 ± 0.03 a,d | 2.38 ± 0.01 b | 2.92 ± 0.01 b | 10.12 ± 0.02 b | 0.75 ± 0.02 a,b | 0.3 ± 0.02 a,d–f | 1.05 ± 0.02 a,b |
| CF-677 | 1.19 ± 0 c | 3.67 ± 0.02 b | 2.69 ± 0 c | 3.44 ± 0.01 c | 11.00 ± 0.01 a | 0.83 ± 0 c | 0.26 ± 0.01 b,d,g,h | 1.09 ± 0.01 b |
| Ishtara | 1.34 ± 0.02 b | 3.51 ± 0.02 c,d | 2.44 ± 0.01 a | 2.98 ± 0 d | 10.27 ± 0.01 c | 0.73 ± 0.01 b | 0.25 ± 0.01 c,e | 0.98 ± 0.01 c |
| GXN-15 | 1.5 ± 0 d | 4.09 ± 0.04 e | 2.92 ± 0.02 d | 3.65 ± 0.07 c | 12.16 ± 0.04 d | 0.89 ± 0 d | 0.28 ± 0 f,h | 1.17 ± 0 d |
| Skin | ||||||||
| Wavit | 1.09 ± 0.01 a | 4.79 ± 0.21 a | 2.98 ± 0.08 a | 3.59 ± 0.06 a | 12.45 ± 0.12 a | 0.94 ± 0.03 a | 0.39 ± 0.02 a,b | 1.33 ± 0.02 a |
| Janka | 0.99 ± 0.01 b,c | 4.06 ± 0.05 b | 2.55 ± 0 b | 2.8 ± 0.02 b | 10.40 ± 0.03 b | 0.78 ± 0.01 b | 0.35 ± 0.01 a,c | 1.13 ± 0.01 b,c |
| CF-677 | 1.09 ± 0.01 a | 3.79 ± 0 c | 2.49 ± 0.02 c | 3.17 ± 0 c | 10.53 ± 0.01 b | 0.78 ± 0.01 b,c | 0.32 ± 0 d | 1.10 ± 0.01 b,d |
| Ishtara | 0.97 ± 0.01 b,d | 3.55 ± 0.04 d | 2.01 ± 0.01 d | 2.39 ± 0 d | 8.92 ± 0.02 c | 0.65 ± 0 d | 0.32 ± 0 e | 0.97 ± 0 e |
| GXN-15 | 0.97 ± 0.01 c,d | 3.44 ± 0.02 d | 2.15 ± 0.02 e | 2.54 ± 0.07 d | 9.10 ± 0.04 c | 0.72 ± 0.05 c,d | 0.36 ± 0.04 b–e | 1.08 ± 0.04 c,d,e |
| Rootstock | TPC (mg GAE/100 g FW) | TFC (mg QE/100 g FW) | TAC (mg CGE/100 g FW) | |||
|---|---|---|---|---|---|---|
| Fruit | Skin | Fruit | Skin | Fruit | Skin | |
| Wavit | 156.1 ± 4.7 a | 337.3 ± 0.1 a | 16.63 ± 0.4 a | 94.4 ± 1.9 a | 19.2 ± 0.1 a | 161.9 ± 0.2 a |
| Janka | 114.1 ± 4.0 b | 203.3 ± 4.4 b | 13.3 ± 0.4 b,c | 57.9 ± 2.0 b | 11.9 ± 0.5 b | 79.1 ± 3.0 b |
| GF-677 | 120.6 ± 6.3 b | 233.5 ± 2.0 c | 13.4 ± 0.4 b,d | 56.3 ± 2.6 b | 13.3 ± 0.4 c | 98.1 ± 2.7 c |
| Ishtara | 93.7 ± 1.5 c | 245.8 ± 4.5 d | 11.8 ± 0.2 e | 74.9 ± 3.1 c | 9.6 ± 0.3 d | 116.7 ± 1.7 d |
| GXN-15 | 143.4 ± 1.7 d | 229.5 ± 4.6 c | 12.9 ± 0.5 c,d | 64.5 ± 2.4 d | 12.0 ± 0.5 b | 101.1 ± 1.9 b |
| Peak No. | Compound | Rt (min) | UV (nm) | Molecular Formula | [M + H]+/[M − H]− m/z | Fragmentation Ions m/z | Method of Identification * |
|---|---|---|---|---|---|---|---|
| 1 | Chlorogenic acid–isomer | 5.19 | 325 | C16H18O9 | 355.10/353.05 | 191 [C7H11O6]− | MS |
| 2 | Neochlorogenic acid | 5.82 | 325 | C16H18O9 | 355.10/353.05 | 191 [C7H11O6]− | MS, RS |
| 3 | Chlorogenic acid | 9.05 | 325 | C16H18O9 | 355.10/353.05 | 191 [C7H11O6]− | MS, RS |
| 4 | Cyanidin-3-O-glucoside | 12.05 | 519, 280 | C21H21O11 | 449.15/447.05 | 287 [C15H11O6]+ | MS, NMR, RS |
| 5 | Cyanidin-3-O-rutinoside | 13.25 | 519, 280 | C27H31O15 | 595.25/593.15 | 449 [M-146]+, 287 [C15H11O6]+ | MS, NMR, RS |
| 6 | Peonidin-3-O-glucoside | 15.00 | 519, 280 | C22H23O11 | 463.15/461.15 | 301 [C16H13O6]+ | MS, NMR |
| 7 | Peonidin-3-O-rutinoside | 15.94 | 519, 280 | C28H33O15 | 609.30/607.15 | 463 [M-146]+, 301 [C16H13O6]+ | MS, NMR |
| 8 | Quercetin-3-O-diglycoside | 18.78 | 357, 256 | C27H30O16 | 611.30/609.10 | 303 [C15H11O7]+ | MS |
| 9 | Hyperoside | 19.03 | 355, 255 | C21H20O12 | 465.13/463.15 | 303 [C15H11O7]+ | MS, RS |
| 10 | Rutin | 19.34 | 354, 258 | C27H30O16 | 611.25/609.15 | 465 [M-146]+, 303 [C15H11O7]+ | MS, RS |
| 11 | Isoquercitrin | 19.62 | 355, 255 | C21H20O12 | 465.13/463.15 | 303 [C15H11O7]+ | MS, RS |
| Rootstock | 3-CQA | 5-CQA | CGL | CRU | PEGL | PERU | Rutin | Qu-Digly | Hyperoside | Isoquercitrin |
|---|---|---|---|---|---|---|---|---|---|---|
| Fruit | ||||||||||
| Wavit | 57.83 ± 0.35 a | 4.17 ± 0.02 a | 2.36 ± 0.04 a | 11.87 ± 0.02 a | 0.02 ± 0 a | 3.60 ± 0.07 a | 2.62 ± 0 a | 1.14 ± 0.02 a | 0.05 ± 0 a | 0.08 ± 0.01 a |
| Janka | 34.82 ± 0.12 b | 3.34 ± 0.09 b | 1.07 ± 0.02 b | 7.79 ± 0.03 b | 0.33 ± 0.01 b | 0.82 ± 0.03 b | 2.15 ± 0.02 b | 0.99 ± 0.01 b | 0.03 ± 0 b | 0.04 ± 0 b |
| GF-677 | 38.83 ± 0.04 c | 3.05 ± 0.07 c | 1.65 ± 0.03 c | 8.60 ± 0.10 c | 0.35 ± 0.01 c | 0.68 ± 0.07 c | 2.38 ± 0.05 c | 0.93 ± 0.01 c | 0.05 ± 0 a | 0.06 ± 0.01 c |
| Ishtara | 36.86 ± 0.10 d | 2.56 ± 0.07 d | 0.53 ± 0.06 d | 5.28 ± 0.01 d | 0.10 ± 0.01 d | 0.09 ± 0.01 d | 1.91 ± 0.01 d | 0.60 ± 0.04 d | 0.06 ± 0 c | 0.06 ± 0.01 c |
| GXN-15 | 34.08 ± 0.02 e | 4.80 ± 0.08 e | 3.00 ± 0.04 e | 8.33 ± 0.07 e | 0.04 ± 0 e | 0.02 ± 0 e | 0.92 ± 0.01 e | 0.01 ± 0 e | 0.02 ± 0 c | 0.01 ± 0 d |
| Skin | ||||||||||
| Wavit | 69.13 ± 0.12 a | 19.95 ± 0.02 a | 34.02 ± 0.26 a | 87.39 ± 0.75 a | 2.21 ± 0.11 a | 35.46 ± 0.07 a | 34.07 ± 0.04 a | 23.10 ± 0.05 a | 4.20 ± 0.04 a | 1.69 ± 0.03 a |
| Janka | 48.11 ± 0.04 b | 9.91 ± 0.03 b | 8.74 ± 0.09 b | 44.82 ± 0.06 b | 0.28 ± 0.03 b | 20.42 ± 0.05 b | 21.03 ± 0.02 b | 13.41 ± 0.06 b | 0.66 ± 0.02 b | 1.23 ± 0.04 b |
| GF-677 | 50.16 ± 0.19 c | 10.70 ± 0.02 c | 14.01 ± 0.06 c | 56.94 ± 0.08 c | 0.89 ± 0.06 c | 22.57 ± 0.17 c | 20.85 ± 0.04 c | 13.45 ± 0 c | 1.36 ± 0 c | 0.30 ± 0.01 c |
| Ishtara | 49.96 ± 0.07 c | 12.84 ± 0.01 d | 15.77 ± 0.06 d | 65.40 ± 0.02 d | 1.29 ± 0.05 d | 28.61 ± 0.05 d | 30.63 ± 0.04 d | 21.13 ± 0.01 d | 3.11 ± 0.03 d | 1.73 ± 0.01 a |
| GXN-15 | 64.10 ± 0.16 d | 12.01 ± 0 e | 18.67 ± 0.21 e | 56.96 ± 0.13 c | 1.39 ± 0.12 d | 20.50 ± 0.19 b | 22.77 ± 0.02 e | 18.56 ± 0.01 e | 3.43 ± 0.01 e | 0.72 ± 0.02 d |
| Rootstock | DPPH IC50 (mg/mL) | ABTS (µM Trolox/100 g FW) | FRAP (µM Fe2+/100 g FW) | |||
|---|---|---|---|---|---|---|
| Fruit | Skin | Fruit | Skin | Fruit | Skin | |
| Wavit | 6.49 ± 0.18 a | 4.18 ± 0.02 a | 350.65 ± 0.51 a | 728.69 ± 4.04 a | 2.89 ± 0.10 a,b | 6.16 ± 0.63 a |
| Janka | 9.40 ± 0.15 b | 5.60 ± 0.04 b | 366.56 ± 2.84 b | 732.69 ± 2.27 a | 2.26 ± 0.05 c | 3.49 ± 0.11 b |
| GF-677 | 8.86 ± 0.15 c | 4.42 ± 0.01 c | 354.41 ± 1.17 c | 689.94 ± 0.53 b | 2.92 ± 0.12 a,d | 3.91 ± 0.24 b,c |
| Ishtara | 6.73 ± 0.06 a | 4.21 ± 0.02 a | 345.94 ± 0.11 d | 717.68 ± 1.95 c | 1.89 ± 0.03 e | 4.58 ± 0.12 d |
| GXN-15 | 8.66 ± 0.32 d | 5.39 ± 0.01 d | 345.97 ± 4.23 a,d | 676.98 ± 2.51 d | 2.76 ± 0.1 b,d | 4.02 ± 0.24 c |
| TPC | TFC | TAC | 3-CQA | 5-CQA | CGL | CRU | PEGL | PERU | Qu-Digly | Hyperoside | Rutin | Isoquercetin | DPPH | ABTS | FRAP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | -- | |||||||||||||||
| TFC | 0.962 | -- | ||||||||||||||
| TAC | 0.975 | 0.994 | -- | |||||||||||||
| 3-CQA | 0.826 | 0.771 | 0.785 | -- | ||||||||||||
| 5-CQA | 0.987 | 0.979 | 0.986 | 0.794 | -- | |||||||||||
| CGL | 0.966 | 0.938 | 0.959 | 0.820 | 0.982 | -- | ||||||||||
| CRU | 0.974 | 0.993 | 0.999 | 0.780 | 0.982 | 0.952 | -- | |||||||||
| PEGL | 0.897 | 0.894 | 0.918 | 0.754 | 0.924 | 0.961 | 0.910 | -- | ||||||||
| PERU | 0.962 | 0.993 | 0.992 | 0.757 | 0.969 | 0.921 | 0.993 | 0.871 | -- | |||||||
| Qu-Digly | 0.931 | 0.990 | 0.981 | 0.757 | 0.954 | 0.912 | 0.983 | 0.891 | 0.981 | -- | ||||||
| Hyperoside | 0.898 | 0.919 | 0.928 | 0.796 | 0.928 | 0.945 | 0.922 | 0.966 | 0.890 | 0.936 | -- | |||||
| Rutin | 0.941 | 0.994 | 0.987 | 0.732 | 0.959 | 0.909 | 0.988 | 0.876 | 0.994 | 0.993 | 0.907 | -- | ||||
| Isoquercitrin | 0.831 | 0.907 | 0.872 | 0.609 | 0.868 | 0.793 | 0.865 | 0.756 | 0.897 | 0.898 | 0.809 | 0.917 | -- | |||
| DPPH | −0.828 | −0.866 | −0.870 | −0.756 | −0.817 | −0.768 | −0.873 | −0.692 | −0.891 | −0.864 | −0.759 | −0.876 | −0.750 | -- | ||
| ABTS | 0.872 | 0.950 | 0.932 | 0.661 | 0.887 | 0.810 | 0.940 | 0.748 | 0.956 | 0.953 | 0.793 | 0.960 | 0.845 | −0.856 | -- | |
| FRAP | 0.968 | 0.924 | 0.940 | 0.789 | 0.958 | 0.953 | 0.934 | 0.918 | 0.918 | 0.892 | 0.893 | 0.902 | 0.829 | −0.750 | 0.798 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trendafilova, A.; Ivanova, V.; Trusheva, B.; Kamenova-Nacheva, M.; Tabakov, S.; Simova, S. Chemical Composition and Antioxidant Capacity of the Fruits of European Plum Cultivar “Čačanska Lepotica” Influenced by Different Rootstocks. Foods 2022, 11, 2844. https://doi.org/10.3390/foods11182844
Trendafilova A, Ivanova V, Trusheva B, Kamenova-Nacheva M, Tabakov S, Simova S. Chemical Composition and Antioxidant Capacity of the Fruits of European Plum Cultivar “Čačanska Lepotica” Influenced by Different Rootstocks. Foods. 2022; 11(18):2844. https://doi.org/10.3390/foods11182844
Chicago/Turabian StyleTrendafilova, Antoaneta, Viktoria Ivanova, Boryana Trusheva, Mariana Kamenova-Nacheva, Sava Tabakov, and Svetlana Simova. 2022. "Chemical Composition and Antioxidant Capacity of the Fruits of European Plum Cultivar “Čačanska Lepotica” Influenced by Different Rootstocks" Foods 11, no. 18: 2844. https://doi.org/10.3390/foods11182844
APA StyleTrendafilova, A., Ivanova, V., Trusheva, B., Kamenova-Nacheva, M., Tabakov, S., & Simova, S. (2022). Chemical Composition and Antioxidant Capacity of the Fruits of European Plum Cultivar “Čačanska Lepotica” Influenced by Different Rootstocks. Foods, 11(18), 2844. https://doi.org/10.3390/foods11182844