Preparation and Characterization of Plant Protein Adhesives with Strong Bonding Strength and Water Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HPM, SBM and CSM Protein Adhesives
2.3. Characterization of Adhesive Samples
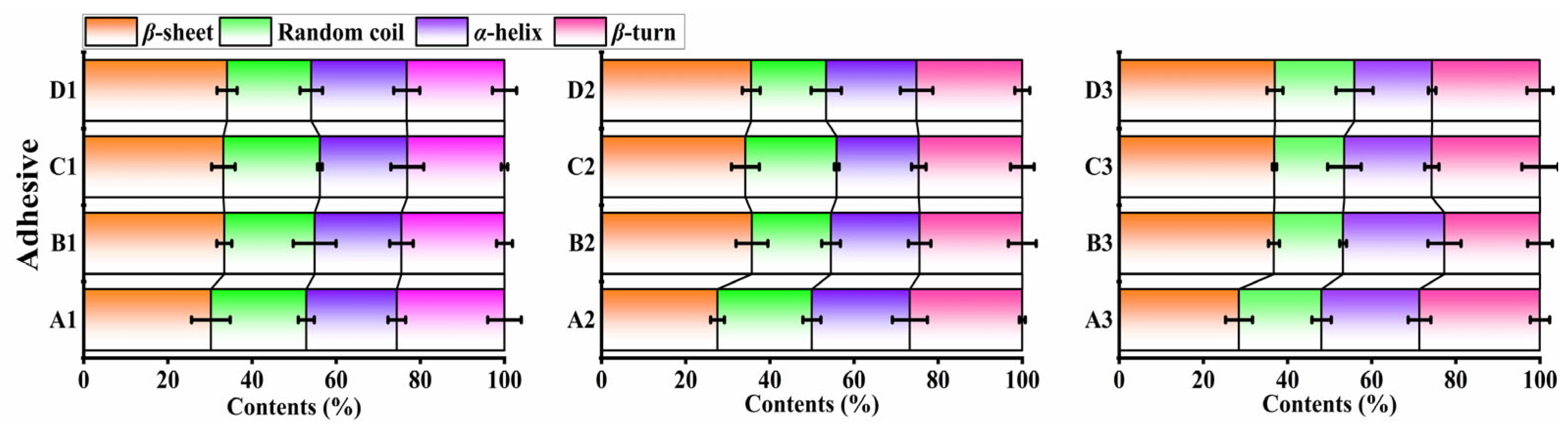
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
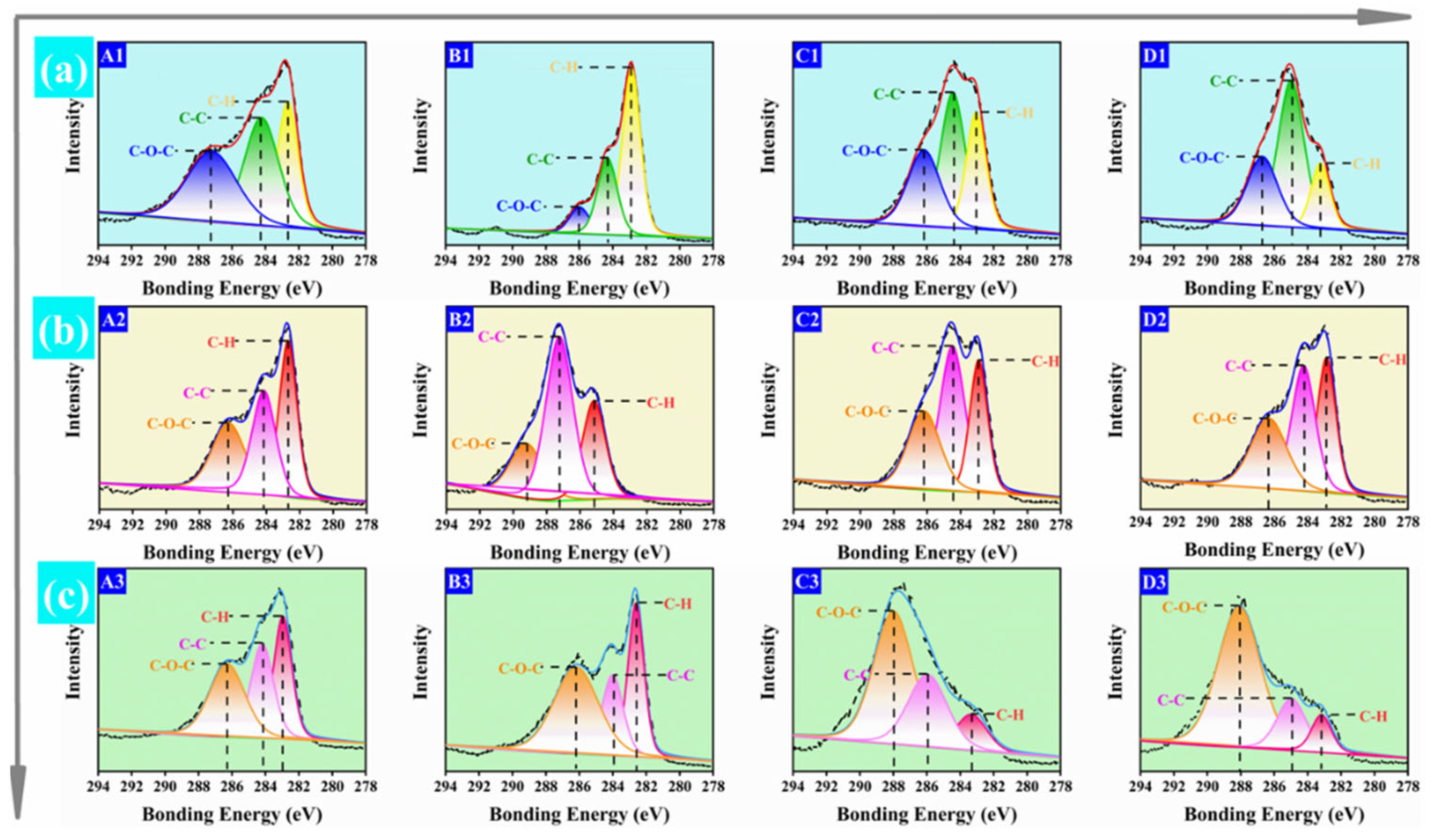
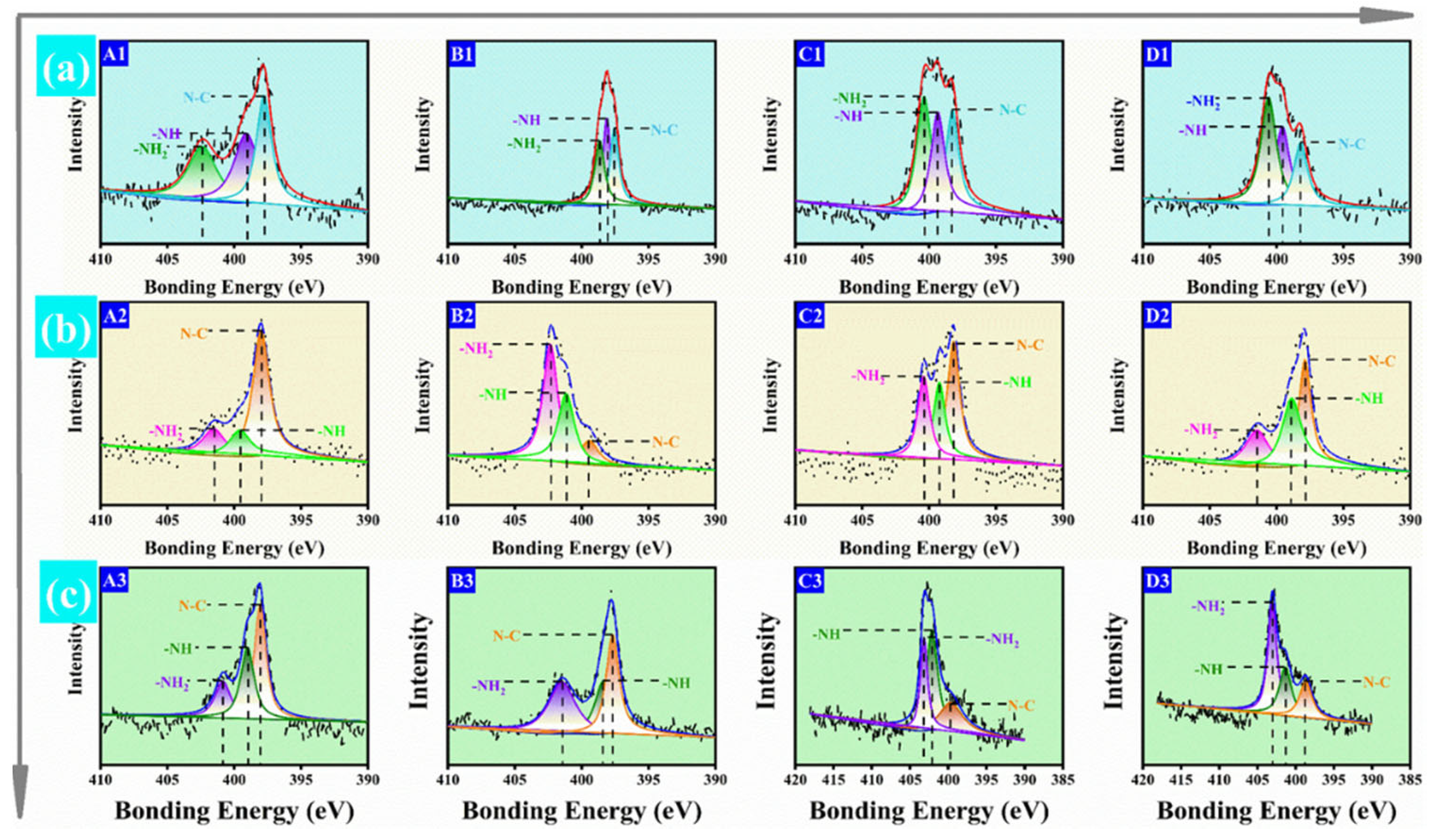
2.3.2. X-ray Photoelectron Spectroscopy (XPS)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Thermogravimetric (TGA)
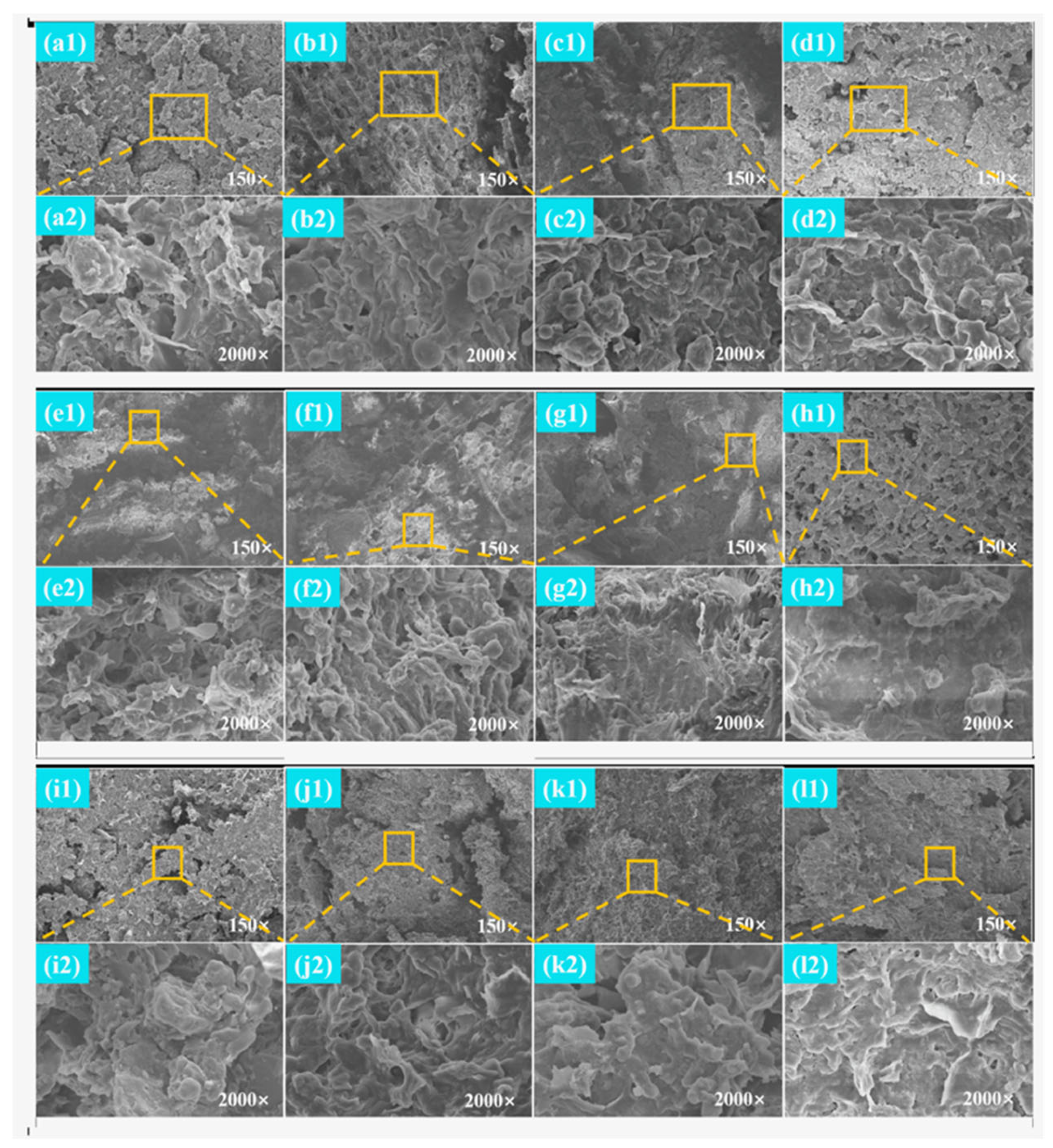
2.3.5. Scanning Electron Microscope (SEM)
2.4. Sol–Gel Test
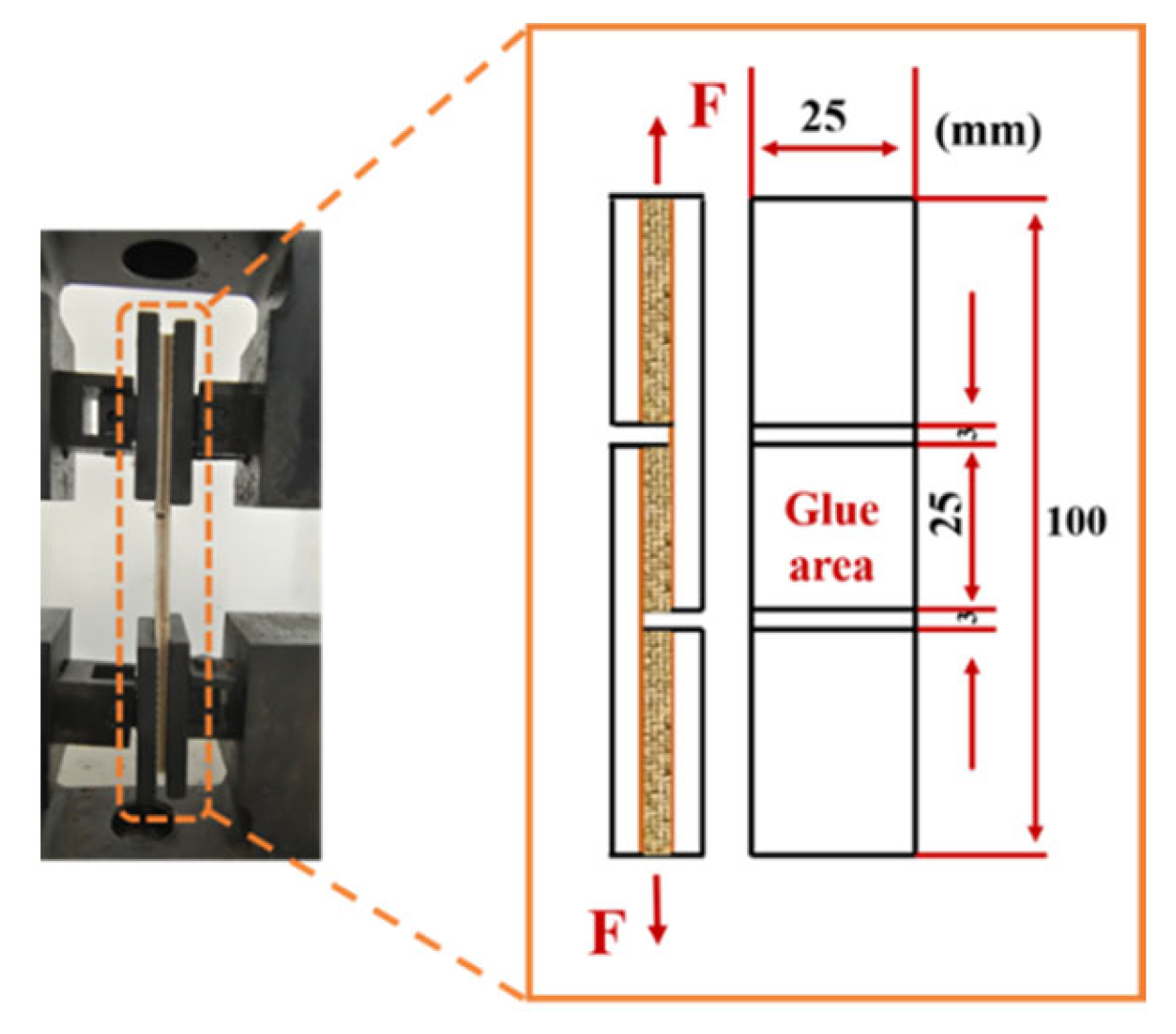
2.5. Preparation of Three-Ply Plywood Specimens and Bonding Strength Testing
2.6. Physical Property Measurements of Adhesive Sample
2.7. Crack Observations
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of HPMP, SBMP, and CSMP-Based Adhesives
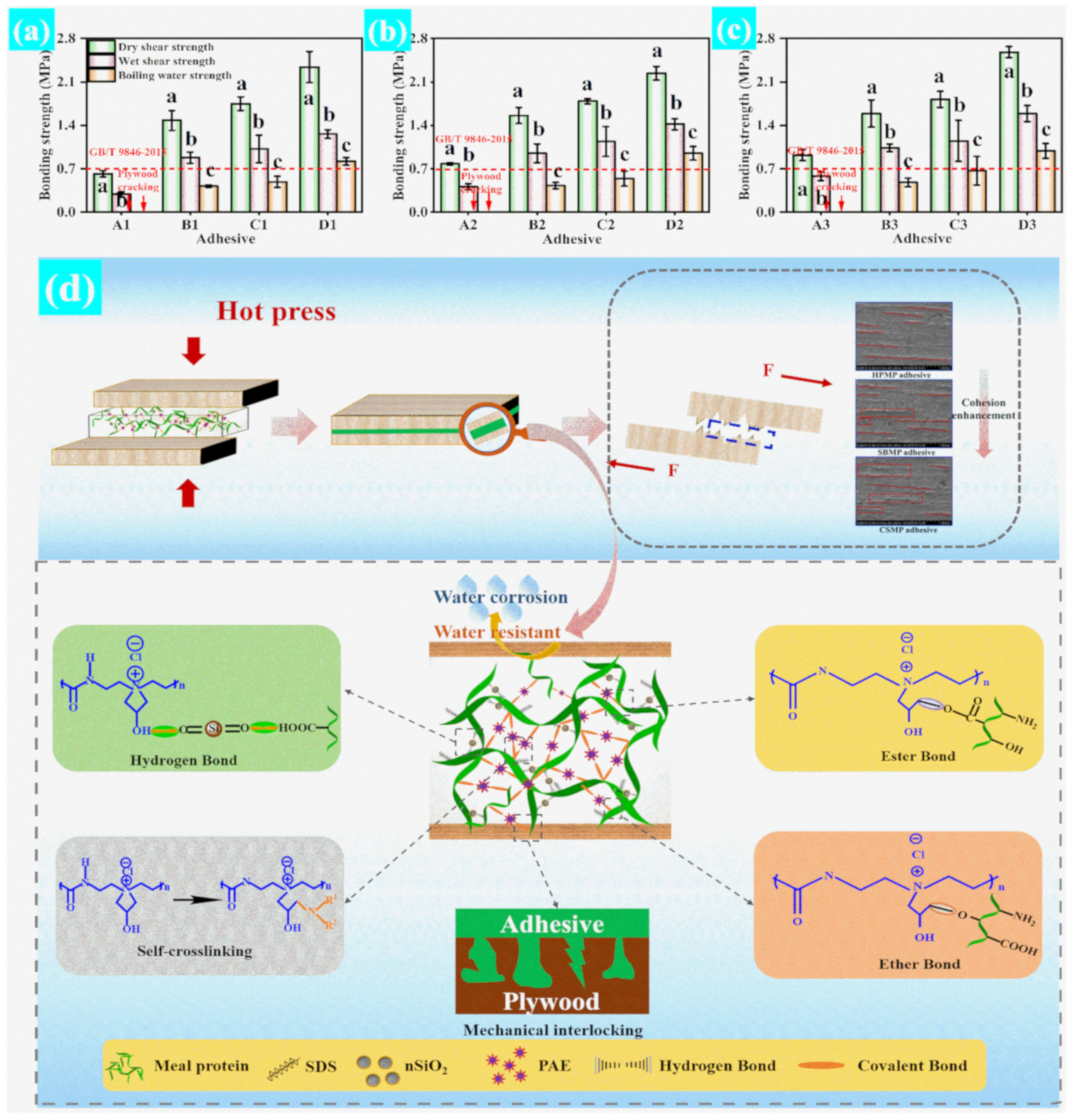
3.2. Water Resistance and Bonding Strength Test
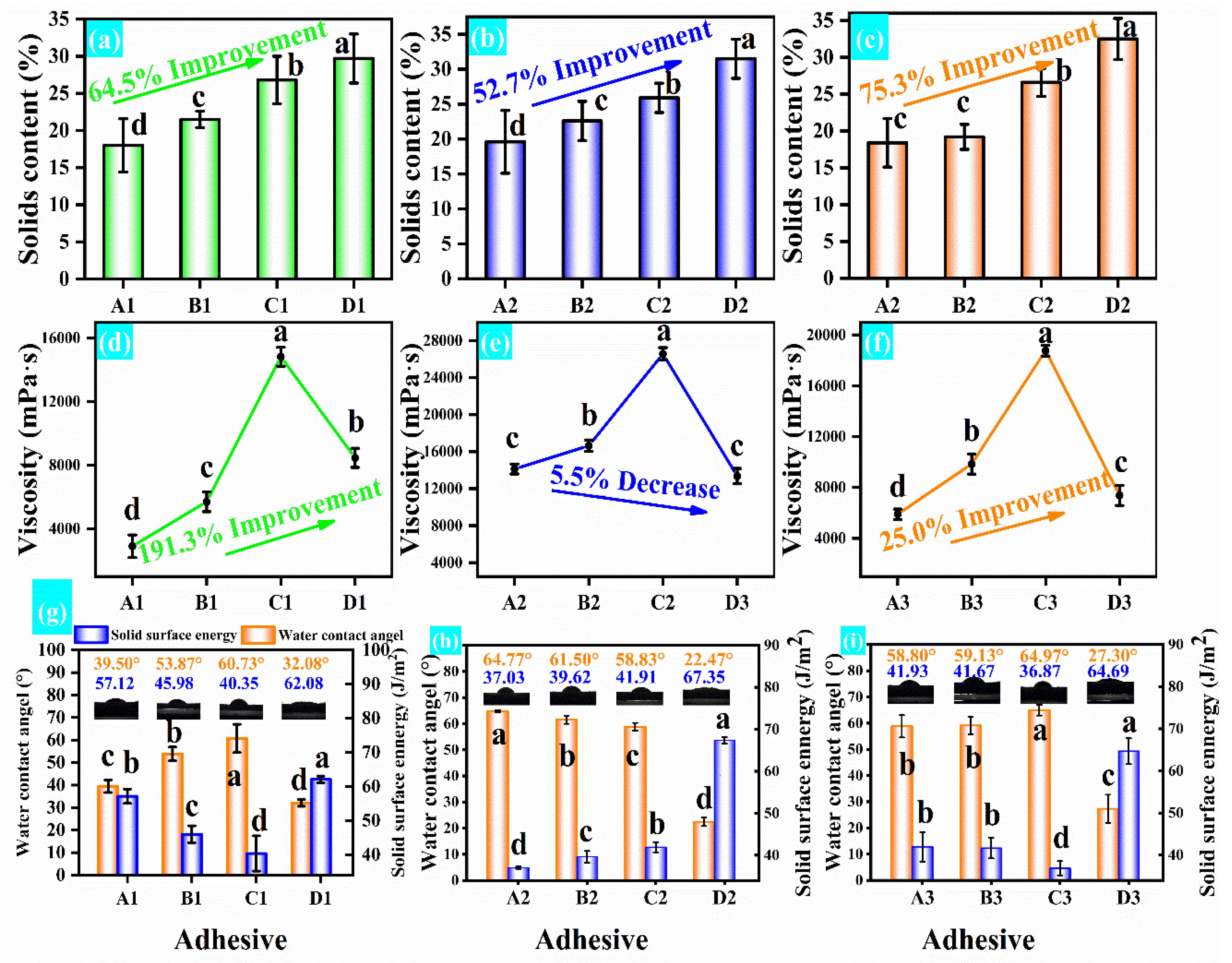
3.3. Physical Property
3.4. Microscopic Morphology Observation
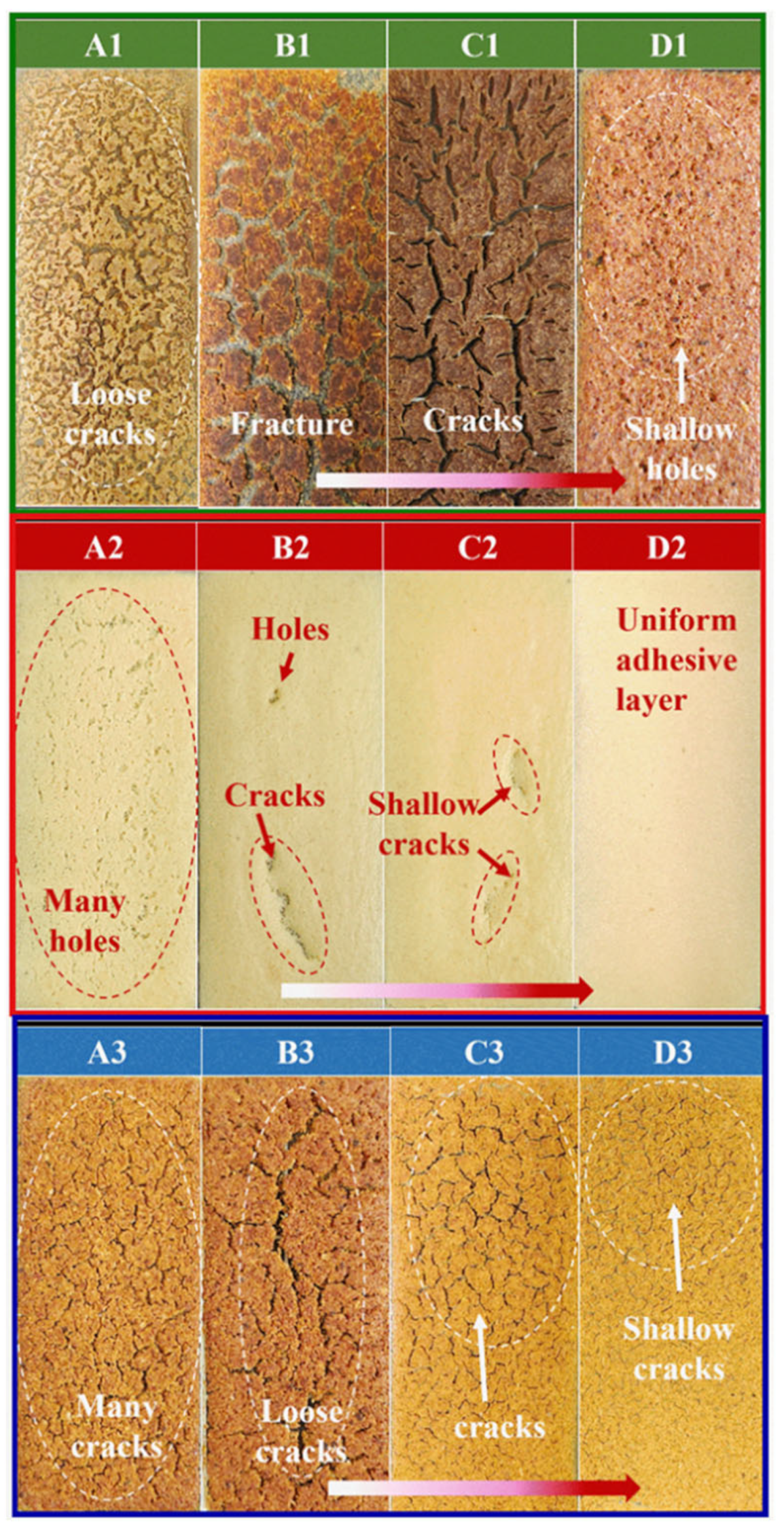
3.5. Crack Observation
3.6. Current Problems and Future Prospects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Chen, S.; Fan, D.; Wang, Y. Preparation and Characterization of a Robust, High Strength, and Mildew Resistant Fully Biobased Adhesive from Agro-Industrial Wastes. ACS Appl. Polym. Mater. 2021, 3, 5197–5206. [Google Scholar] [CrossRef]
- Zajforoushan Moghaddam, S.; Qie, R.; Thormann, E. Making Protein-Based Adhesives Water Resistant: Role of Protein Water Solubility, Galloyl Modification, and Complexation. ACS Appl. Polym. Mater. 2022, 4, 18–23. [Google Scholar] [CrossRef]
- Chen, X.; Ji, N.; Li, F.; Qin, Y.; Wang, Y.; Xiong, L.; Sun, Q. Dual Cross-Linked Starch–Borax Double Network Hydrogels with Tough and Self-Healing Properties. Foods 2022, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, E.; Zhu, Z.; Han, L.; Zheng, P.; Zeng, H.; Chen, N. Soy-Based Adhesives Functionalized with Pressure-Responsive Crosslinker Microcapsules for Enhanced Wet Adhesion. ACS Appl. Polym. Mater. 2021, 3, 1032–1041. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Wang, Z.; Zhang, S.; Li, J. Biomimetic water-in-oil water/pMDI emulsion as an excellent ecofriendly adhesive for bonding wood-based composites. J. Hazard. Mater. 2020, 396, 122722. [Google Scholar] [CrossRef]
- Zheng, P.; Chen, N.; Mahfuzul Islam, S.M.; Ju, L.-K.; Liu, J.; Zhou, J.; Chen, L.; Zeng, H.; Lin, Q. Development of self-cross-linked soy adhesive by enzyme complex from aspergillus niger for production of all-biomass composite materials. ACS Sustain. Chem. Eng. 2019, 7, 3909–3916. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Luo, J.; Li, K.; Gao, Q.; Li, J. A novel eco-friendly blood meal-based bio-adhesive: Preparation and performance. J. Polym. Environ. 2018, 26, 607–615. [Google Scholar] [CrossRef]
- Chen, C.; Chen, F.; Liu, B.; Du, Y.; Liu, K. Peanut meal-based wood adhesives enhanced by urea and epichlorohydrin. R. Soc. Open Sci. 2019, 6, 191154. [Google Scholar] [CrossRef] [Green Version]
- Khoo, S.C.; Peng, W.X.; Yang, Y.; Ge, S.B.; Soon, C.F.; Ma, N.L.; Sonne, C. Development of formaldehyde-free bio-board produced from mushroom mycelium and substrate waste. J. Hazard. Mater. 2020, 400, 123296. [Google Scholar] [CrossRef]
- Krüger, J.M.; Brner, H.G. Accessing the next generation of synthetic mussel-glue polymers via mussel-inspired polymerization. Angew. Chem. Int. Ed. 2021, 60, 6408–6413. [Google Scholar] [CrossRef]
- Jin, S.; Li, K.; Zhang, X.; Gao, Q.; Zeng, L.; Shi, S.Q.; Li, J. Phytic acid-assisted fabrication for soybean meal/nanofiber composite adhesive via bioinspired chelation reinforcement strategy. J. Hazard. Mater. 2020, 399, 123064. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Use of additives to enhance the properties of cottonseed protein as wood adhesives. Int. J. Adhes. Adhes. 2016, 68, 156–160. [Google Scholar] [CrossRef]
- Gu, W.; Liu, X.; Ye, Q.; Gao, Q.; Gong, S.; Li, J.; Shi, S.Q. Bio-inspired co-deposition strategy of aramid fibers to improve performance of soy protein isolate-based adhesive. Ind. Crops Prod. 2020, 150, 112424. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Ren, B.; Zhang, Y.; Ma, J.; Xu, L.; Chen, Q.; Zheng, J. Super bulk and interfacial toughness of physically crosslinked double-network hydrogels. Adv. Funct. Mater. 2017, 27, 1703086. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Liu, D.; Yuan, Z.; Hung, H.-C.; Luozhong, S.; Gu, W.; Wu, K.; Jiang, S. High-strength and nonfouling zwitterionic triple-network hydrogel in saline environments. Adv. Mater. 2021, 33, 2102479. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Liu, X.; Guan, X.; Zhang, C.; Niu, Y. Polyglycerol-based organic-inorganic hybrid adhesive with high early strength. Mater. Des. 2017, 117, 1–6. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Chen, M.; Luo, J.; Shi, S.Q.; Li, J.; Gao, Q. Constructing a triple network structure to prepare strong, tough, and mildew resistant soy protein adhesive. Compos. B Eng. 2021, 211, 108677. [Google Scholar] [CrossRef]
- Li, X.; Deng, Y.; Lai, J.; Zhao, G.; Dong, S. Tough, long-eerm, water-resistant, and underwater adhesion of low-molecular-weight supramolecular adhesives. J. Am. Chem. Soc. 2020, 142, 5371–5379. [Google Scholar] [CrossRef]
- Qu, Y.; Guo, Q.; Li, T.; Zhang, Y.; Gao, Q.; Liu, H.; Wang, Q. A novel environmentally friendly hot-pressed peanut meal protein adhesive. J. Clean. Prod. 2021, 327, 129473. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, X.; Zhang, Y.; Liu, Z.; Luo, J.; Li, J.; Li, J.; Gao, Q. A high bonding performance and antibacterial soybean meal adhesive with maillard reaction based cross-linked structure. Compos. B Eng. 2021, 227, 109403. [Google Scholar] [CrossRef]
- GB/T 9846; China National Standard Plywood for General Use. Standardization Administration of the Peoples Republic of China: Beijing, China, 2015.
- Costa, S.M.; Ferreira, D.P.; Ferreira, A.; Vaz, F.; Fangueiro, R. Multifunctional flax fibres based on the combined effect of silver and zinc oxide (Ag/ZnO) nanostructures. Nanomaterials 2018, 8, 1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bean, S.; Sun, X.S. Camelina protein adhesives enhanced by polyelectrolyte interaction for plywood applications. Ind. Crops Prod. 2018, 124, 343–352. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, G.; Zhang, F.; Luo, J.; Li, K.; Li, X.; Li, J.; Fang, Z. High strength and flame retardant soybean polysaccharide-based wood adhesive produced by borate chemistry and crosslinking strategy. Eur. Polym. J. 2022, 164, 110973. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Li, Z.; Li, L.; Zhang, S. Core-shell nanohybrid elastomer based on co-deposition strategy to improve performance of soy protein adhesive. ACS Appl. Mater. Interfaces 2019, 11, 32414–32422. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Kang, H.; Li, J.; Zhang, S.; Han, C.; Huang, A. Fully bio-based soybean adhesive in situ cross-linked by interactive network skeleton from plant oil-anchored fiber. Ind. Crops Prod. 2018, 122, 366–374. [Google Scholar] [CrossRef]
- Li, J.; Xiao, F.; Amirkhanian, S.N. Storage, fatigue and low temperature characteristics of plasma treated rubberized binders. Constr. Build. Mater. 2019, 209, 454–462. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; He, Z.; Wan, H. “Greener” adhesives composed of urea-formaldehyde resin and cottonseed meal for wood-based composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- Xu, H.; Yang, M.; Hou, X.; Li, W.; Su, X.; Yang, Y. Industrial trial of high-quality all green sizes composed of soy-derived protein and glycerol. J. Clean. Prod. 2016, 135, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mazzotta, M.G.; Putnam, A.A.; North, M.A.; Wilker, J.J. Weak bonds in a biomimetic adhesive enhance toughness and performance. J. Am. Chem. Soc. 2020, 142, 4762–4768. [Google Scholar] [CrossRef]
- Zhao, S.; Pang, H.; Li, Z.; Wang, Z.; Kang, H.; Zhang, W.; Zhang, S.; Li, J.; Li, L. Polyurethane as high-functionality crosslinker for constructing thermally driven dual-crosslinking plant protein adhesion system with integrated strength and ductility. Chem. Eng. J. 2021, 422, 130152. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, A.; Wang, Q. Peanut meal as plywood adhesives: Preparation and characterization. J. Adhes. Sci. Technol. 2018, 32, 2450–2463. [Google Scholar] [CrossRef]
- Li, H.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Physicochemical properties of modified soybean-flour adhesives enhanced by carboxylated styrene-butadiene rubber latex. Int. J. Adhes. Adhes. 2016, 66, 59–64. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Chen, S.; Chu, F.; Zhang, R.; Wang, Y.; Fan, D. Preparation and characterization of a soy protein based bio-adhesive crosslinked by waterborne epoxy resin and polyacrylamide. RSC Adv. 2019, 9, 35273–35279. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Soy and cottonseed protein blends as wood adhesives. Ind. Crops Prod. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, J.; Shao, Z.; Chen, X. Water-resistant zein-based adhesives. ACS Sustain. Chem. Eng. 2020, 8, 7668–7679. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Gao, Q.; Zhang, S.; Li, J. Properties of soybean-flour-based adhesives enhanced by attapulgite and glycerol polyglycidyl ether. Ind. Crops Prod. 2014, 59, 35–40. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysaccharide and soy protein. Carbohydr. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Zhang, W.; Qi, C.; Zhang, S.; Li, J. Bio-inspired cellulose nanofiber-reinforced soy protein resin adhesives with dopamine-induced codeposition of “water-resistant” interphases. Appl. Surf. Sci. 2019, 478, 441–450. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, Y.; Shi, S.Q.; Gao, Q. Improving bond performance and reducing cross-linker dosage for soy flour adhesives inspired by spider silk. ACS Sustain. Chem. Eng. 2020, 9, 168–179. [Google Scholar] [CrossRef]
- Li, X.; Xia, C.; Li, J.; Zhou, X. Design and build an elastic crosslinked network to strengthen and toughen soybean-meal based bioadhesive using organo-sepiolite and greener crosslinker triglycidylamine. Polym. Test. 2020, 89, 106648. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, R.; Xu, Y.; Chen, M.; Zhang, J.; Gao, Q.; Li, J. Developing a stable high-performance soybean meal-based adhesive using a simple high-pressure homogenization technology. J. Clean. Prod. 2020, 256, 120336. [Google Scholar] [CrossRef]
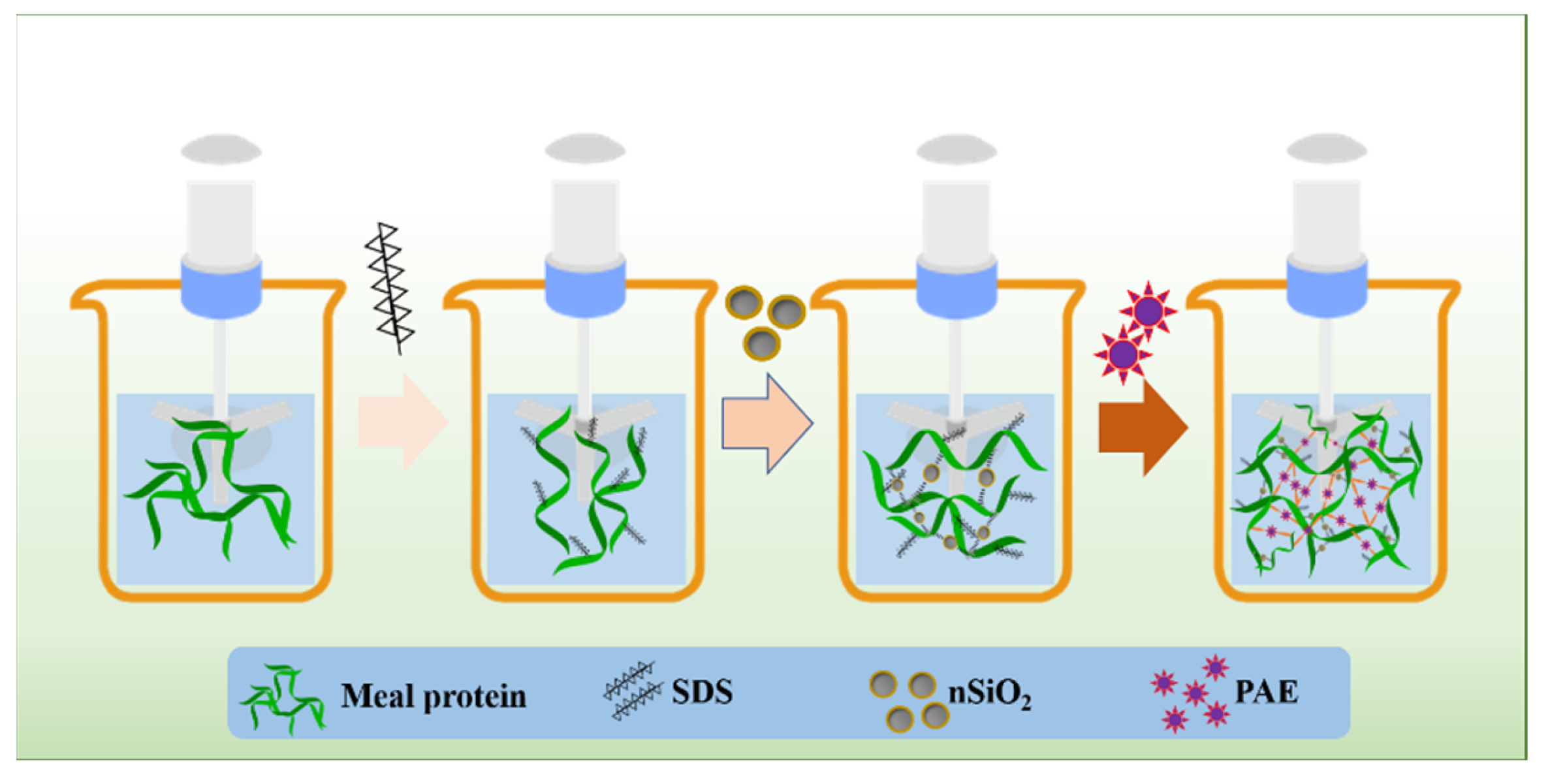



| Adhesive | HPM (g) | SBM (g) | CSM (g) | Water (g) | SDS (g) | nSiO2 | PAE (g) |
|---|---|---|---|---|---|---|---|
| A1 | 40 | 120 | |||||
| B1 | 40 | 120 | 1.28 | ||||
| C1 | 40 | 120 | 1.28 | 0.8 | |||
| D1 | 40 | 120 | 1.28 | 0.8 | 34 | ||
| A2 | 40 | 120 | |||||
| B2 | 40 | 120 | 1.28 | ||||
| C2 | 40 | 120 | 1.28 | 0.8 | |||
| D2 | 40 | 120 | 1.28 | 0.8 | 34 | ||
| A3 | 40 | 120 | |||||
| B3 | 40 | 120 | 1.28 | ||||
| C3 | 40 | 120 | 1.28 | 0.8 | |||
| D3 | 40 | 120 | 1.28 | 0.8 | 34 |
| Matrix | Adhesive | Dry Shear Strength/MPa | Wet Shear Strength/MPa | Boiling Water Strength/MPa | Testing Standards | Refs |
|---|---|---|---|---|---|---|
| Peanut meal | HPMP | 2.34 | 1.26 | 0.82 | GB/T 9846–2015 | This work |
| Peanut meal/U/ECH | 1.79 | 0.92 | - | GB/T 9846–2015 | [10] | |
| HPM/SDS | - | 1.05 | - | GB/T 9846–2015 | [34] | |
| Soybean meal | SBMP | 2.24 | 1.42 | 0.95 | GB/T 9846–2015 | This work |
| AC@CNF-PA/SM-2 | 1.71 | 1.19 | - | GB/T 9846–2015 | [13] | |
| 6-SM/BD/5%HPFC | 2.04 | 1.05 | - | GB/T 9846–2015 | [22] | |
| SF/XSBRL | 1.44 | 0.97 | GB/T 9846–2015 | [35] | ||
| SP/WE-PAM | - | 1.16 | - | GB/T 9846–2015 | [36] | |
| Cottonseed meal | CSMP | 2.58 | 1.59 | 0.99 | GB/T 9846–2015 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Guo, Q.; Huang, X.; Li, T.; Liang, M.; Qin, J.; Gao, Q.; Liu, H.; Wang, Q. Preparation and Characterization of Plant Protein Adhesives with Strong Bonding Strength and Water Resistance. Foods 2022, 11, 2839. https://doi.org/10.3390/foods11182839
Qu Y, Guo Q, Huang X, Li T, Liang M, Qin J, Gao Q, Liu H, Wang Q. Preparation and Characterization of Plant Protein Adhesives with Strong Bonding Strength and Water Resistance. Foods. 2022; 11(18):2839. https://doi.org/10.3390/foods11182839
Chicago/Turabian StyleQu, Yang, Qin Guo, Xuegang Huang, Tian Li, Manzhu Liang, Jingjing Qin, Qiang Gao, Hongzhi Liu, and Qiang Wang. 2022. "Preparation and Characterization of Plant Protein Adhesives with Strong Bonding Strength and Water Resistance" Foods 11, no. 18: 2839. https://doi.org/10.3390/foods11182839
APA StyleQu, Y., Guo, Q., Huang, X., Li, T., Liang, M., Qin, J., Gao, Q., Liu, H., & Wang, Q. (2022). Preparation and Characterization of Plant Protein Adhesives with Strong Bonding Strength and Water Resistance. Foods, 11(18), 2839. https://doi.org/10.3390/foods11182839







