Cardioprotective Effects of Grapefruit IntegroPectin Extracted via Hydrodynamic Cavitation from By-Products of Citrus Fruits Industry: Role of Mitochondrial Potassium Channels
Abstract
:1. Introduction
2. Materials and Methods
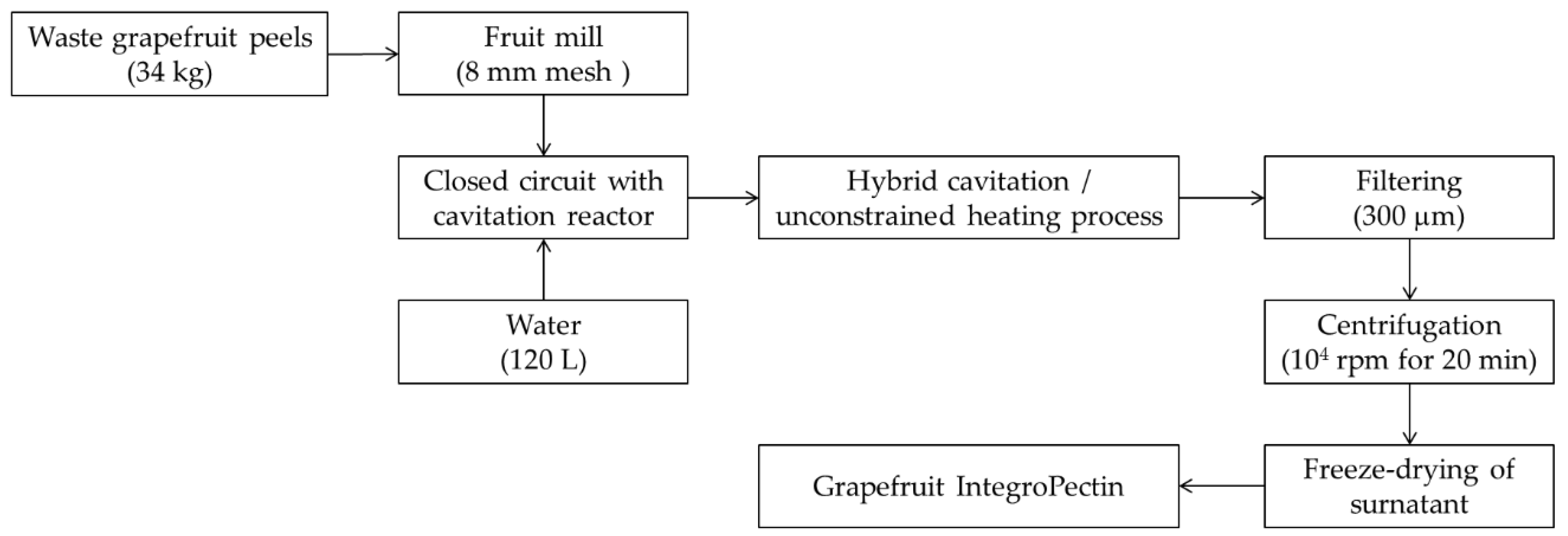
2.1. Production of Grapefruit IntegroPectin
2.2. Animal Experimentation and Data Analysis
2.2.1. Ischemia/Reperfusion on Langendorff Isolated and Perfused Heart
2.2.2. Mitochondrial Isolation Protocol
2.2.3. Cardiac Mitochondrial Membrane Potential
2.2.4. Mitochondrial Changes in Calcium-Uptake
3. Results
3.1. Cardioprotective Effects of Grapefruit IntegroPectin in Ex Vivo Model of Cardiac Ischemia/Reperfusion Injury
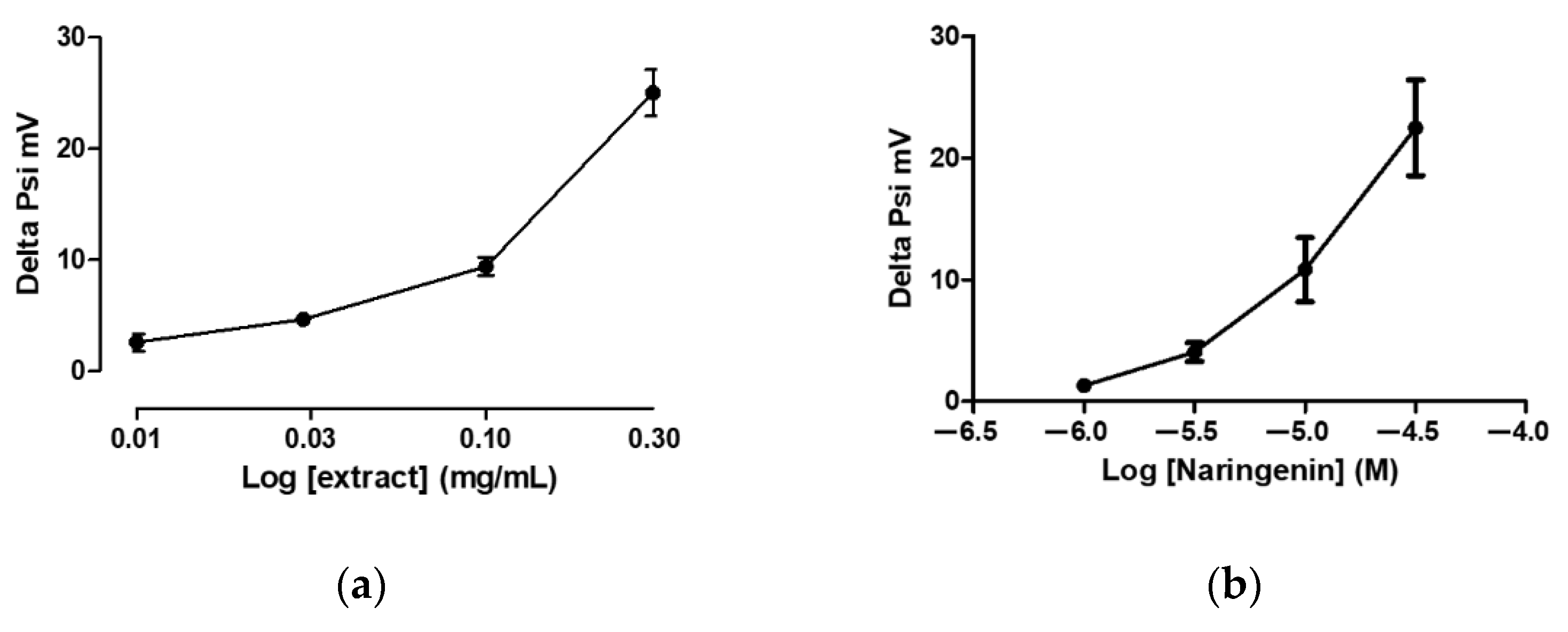
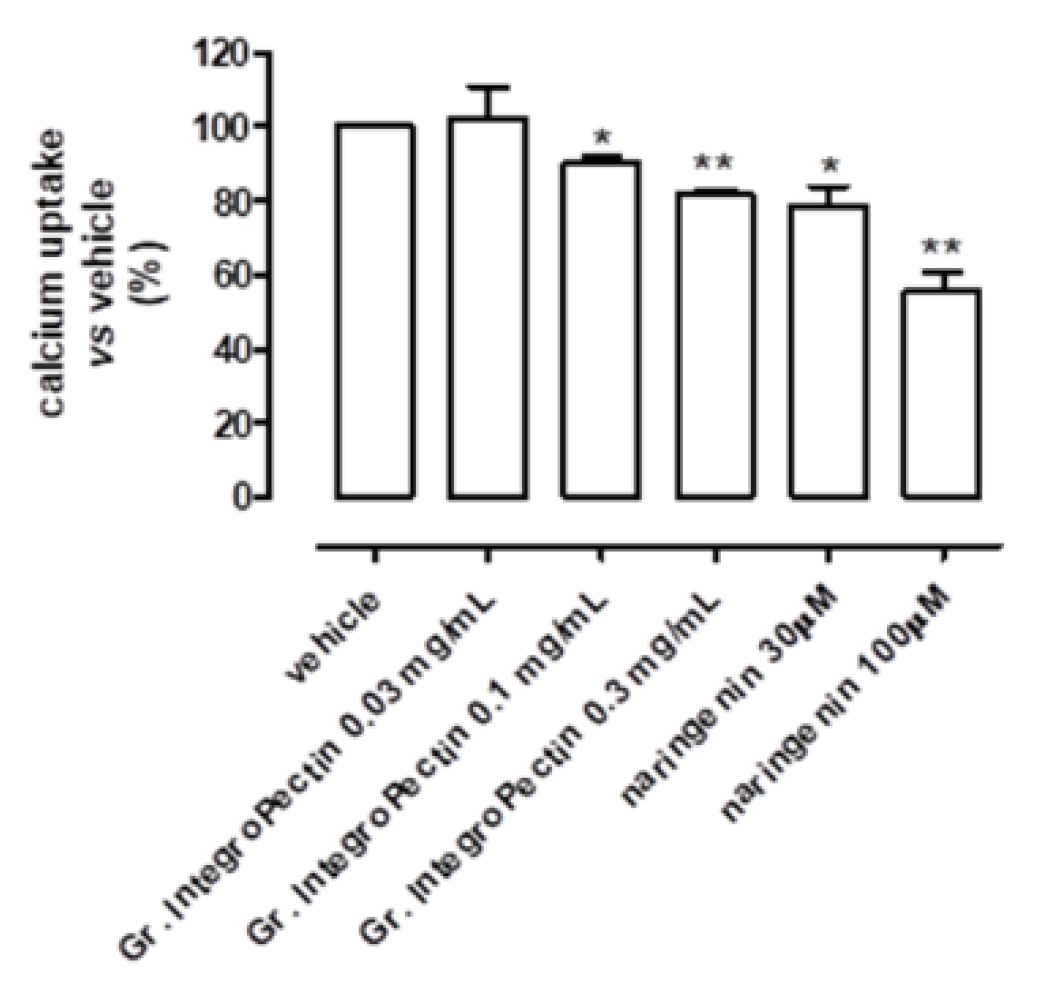
3.2. Mitochondriotropic Effects of Grapefruit IntegroPectin on Cardiac-Isolated Mitochondria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef]
- Pla-Pagà, L.; Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Martín-Luján, F.; Moragas, A.; Canela, N.; Puiggròs, F.; et al. Effects of Hesperidin Consumption on the Cardiovascular System in Pre- and Stage 1 Hypertensive Subjects: Targeted and Non-Targeted Metabolomic Approaches (CITRUS Study). Mol. Nutr. Food Res. 2021, 65, e2001175. [Google Scholar] [CrossRef]
- Naeini, F.; Namkhah, Z.; Tutunchi, H.; Rezayat, S.M.; Mansouri, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. Effects of naringenin supplementation on cardiovascular risk factors in overweight/obese patients with nonalcoholic fatty liver disease: A pilot double-blind, placebo-controlled, randomized clinical trial. Eur. J. Gastroenterol. Hepatol. 2022, 34, 345–353. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Cao, Z.; Li, W.; Liu, R.; Chen, Y.; Li, C.; Song, Y.; Liu, G.; Hu, J.; et al. Naringenin ameliorates homocysteine induced endothelial damage via the AMPKα/Sirt1 pathway. J. Adv. Res. 2021, 34, 137–147. [Google Scholar] [CrossRef]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Di Cesare Mannelli, L.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxidative Med. Cell. Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Marino, A.; D’Antongiovanni, V.; Ciregia, F.; Giusti, L.; Lucacchini, A.; Chericoni, S.; Breschi, M.C.; Calderone, V. The activation of mitochondrial BK potassium channels contributes to the protective effects of naringenin against myocardial ischemia/reperfusion injury. Biochem. Pharmacol. 2013, 85, 1634–1643. [Google Scholar] [CrossRef]
- Saponara, S.; Testai, L.; Iozzi, D.; Martinotti, E.; Martelli, A.; Chericoni, S.; Sgaragli, G.; Fusi, F.; Calderone, V. (+/−)-Naringenin as large conductance Ca2+-activated K+ (BKCa) channel opener in vascular smooth muscle cells. Br. J. Pharmacol. 2006, 149, 1013–1021. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, L.; Cui, L.; Hou, X.; Li, J.; Zhang, X.; Zhang, M. Hesperetin inhibits rat coronary constriction by inhibiting Ca2+ influx and enhancing voltage-gated K+ channel currents of the myocytes. Eur. J. Pharmacol. 2014, 735, 193–201. [Google Scholar] [CrossRef]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Kushwaha, S.; Tripathi, D.N.; Jena, G.B. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovasc. Toxicol. 2011, 11, 215–225. [Google Scholar] [CrossRef]
- Testai, L.; Da Pozzo, E.; Piano, I.; Pistelli, L.; Gargini, C.; Breschi, M.C.; Braca, A.; Martini, C.; Martelli, A.; Calderone, V. The Citrus Flavanone Naringenin Produces Cardioprotective Effects in Hearts from 1 Year Old Rat, through Activation of mitoBK Channels. Front. Pharmacol. 2017, 8, 71. [Google Scholar] [CrossRef]
- Shi, R.; Xu, J.W.; Xiao, Z.T.; Chen, R.F.; Zhang, Y.L.; Lin, J.B.; Cheng, K.L.; Wei, G.Y.; Li, P.B.; Zhou, W.L.; et al. Naringin and Naringenin Relax Rat Tracheal Smooth by Regulating BKCa Activation. J. Med. Food 2019, 22, 963–970. [Google Scholar] [CrossRef]
- Fusi, F.; Trezza, A.; Tramaglino, M.; Sgaragli, G.; Saponara, S.; Spiga, O. The beneficial health effects of flavonoids on the cardiovascular system: Focus on K+ channels. Pharmacol. Res. 2020, 152, 104625. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; dos Santos Nascimento, L.B.; De Carlo, A.; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Albanese, L.; Nuzzo, D.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Presentato, A.; Pagliaro, M.; Avellone, G.; et al. Flavonoids in Lemon and Grapefruit IntegroPectin. ChemistryOpen 2021, 10, 1055–1058. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Garcia, A.R.; Pagliaro, M.; Avellone, G.; Fidalgo, A.; Albanese, L.; Meneguzzo, F.; Ciriminna, R.; Ilharco, L.M. Red Orange and Bitter Orange IntegroPectin: Structure and Main Functional Compounds. Molecules 2022, 27, 3243. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Zabini, F. Hydrodynamic Cavitation in Beer and Other Beverage Processing. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 369–394. [Google Scholar] [CrossRef]
- Presentato, A.; Piacenza, E.; Scurria, A.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Martino, D.C.; Alduina, R. A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains. Antibiotics 2020, 9, 586. [Google Scholar] [CrossRef]
- Nuzzo, D.; Scordino, M.; Scurria, A.; Giardina, C.; Giordano, F.; Meneguzzo, F.; Mudò, G.; Pagliaro, M.; Picone, P.; Attanzio, A.; et al. Protective, Antioxidant and Antiproliferative Activity of Grapefruit IntegroPectin on SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 9368. [Google Scholar] [CrossRef]
- Di Prima, G.; Scurria, A.; Angellotti, G.; Belfiore, E.; Pagliaro, M.; Meneguzzo, F.; De Caro, V.; Ciriminna, R. Grapefruit IntegroPectin isolation via spray drying and via freeze drying: A comparison. Sustain. Chem. Pharm. 2022, 29, 100816. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Presentato, A.; Lino, C.; Piacenza, E.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; et al. Volatile Compounds of Lemon and Grapefruit IntegroPectin. Molecules 2021, 26, 51. [Google Scholar] [CrossRef]
- Calderone, V.; Testai, L.; Martelli, A.; Rapposelli, S.; Digiacomo, M.; Balsamo, A.; Breschi, M.C. Anti-ischemic properties of a new spiro-cyclic benzopyran activator of the cardiac mito-KATP channel. Biochem. Pharmacol. 2010, 79, 39–47. [Google Scholar] [CrossRef]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 17353. [Google Scholar] [CrossRef]
- Rajadurai, M.; Prince, P.S. Preventive effect of naringin on isoproterenol- induced cardiotoxicity in Wistar rats: An in vivo and in vitro study. Toxicology 2007, 232, 216–225. [Google Scholar] [CrossRef]
- You, Q.; Wu, Z.; Wu, B.; Liu, C.; Huang, R.; Yang, L.; Guo, R.; Wu, K.; Chen, J. Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J. Endocrinol. 2016, 230, 197–214. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Manchanda, M.; Nag, T.C.; Ray, R.; Chauhan, S.S.; Kumari, S.; Arya, D.S. Regulation of heat shock proteins 27 and 70, p-Akt/p-eNOS and MAPKs by Naringin Dampens myocardial injury and dysfunction in vivo after ischemia/reperfusion. PLoS ONE 2013, 8, e82577. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, L.; Li, X.; Zhao, L.; Hu, X.; Ma, Z. Short-term pretreatment of naringin isolated from Citrus wilsonii Tanaka attenuates rat myocardial ischemia/reperfusion injury. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1047–1059. [Google Scholar] [CrossRef]
- Li, F.; Zhan, Z.; Qian, J.; Cao, C.; Yao, W.; Wang, N. Naringin attenuates rat myocardial ischemia/reperfusion injury via PI3K/Akt pathway-mediated inhibition of apoptosis, oxidative stress and autophagy. Exp. Ther. Med. 2021, 22, 811. [Google Scholar] [CrossRef]
- Clementi, N.; Scagnolari, C.; D’Amore, A.; Palombi, F.; Criscuolo, E.; Frasca, F.; Pierangeli, A.; Mancini, N.; Antonelli, G.; Clementi, M.; et al. Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol. Res. 2021, 163, 105255. [Google Scholar] [CrossRef]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: An update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Kumar Gaur, V.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of citrus peel waste for the sustainable production of value-added products. Bioresour. Technol. 2022, 351, 127064. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Scurria, A.; Nuzzo, D.; Alduina, R.; Ilharco, L.M.; Pagliaro, M. Pectin: A Long-Neglected Broad-Spectrum Antibacterial. ChemMedChem 2020, 15, 2228–2235. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flori, L.; Albanese, L.; Calderone, V.; Meneguzzo, F.; Pagliaro, M.; Ciriminna, R.; Zabini, F.; Testai, L. Cardioprotective Effects of Grapefruit IntegroPectin Extracted via Hydrodynamic Cavitation from By-Products of Citrus Fruits Industry: Role of Mitochondrial Potassium Channels. Foods 2022, 11, 2799. https://doi.org/10.3390/foods11182799
Flori L, Albanese L, Calderone V, Meneguzzo F, Pagliaro M, Ciriminna R, Zabini F, Testai L. Cardioprotective Effects of Grapefruit IntegroPectin Extracted via Hydrodynamic Cavitation from By-Products of Citrus Fruits Industry: Role of Mitochondrial Potassium Channels. Foods. 2022; 11(18):2799. https://doi.org/10.3390/foods11182799
Chicago/Turabian StyleFlori, Lorenzo, Lorenzo Albanese, Vincenzo Calderone, Francesco Meneguzzo, Mario Pagliaro, Rosaria Ciriminna, Federica Zabini, and Lara Testai. 2022. "Cardioprotective Effects of Grapefruit IntegroPectin Extracted via Hydrodynamic Cavitation from By-Products of Citrus Fruits Industry: Role of Mitochondrial Potassium Channels" Foods 11, no. 18: 2799. https://doi.org/10.3390/foods11182799
APA StyleFlori, L., Albanese, L., Calderone, V., Meneguzzo, F., Pagliaro, M., Ciriminna, R., Zabini, F., & Testai, L. (2022). Cardioprotective Effects of Grapefruit IntegroPectin Extracted via Hydrodynamic Cavitation from By-Products of Citrus Fruits Industry: Role of Mitochondrial Potassium Channels. Foods, 11(18), 2799. https://doi.org/10.3390/foods11182799