Taxonomical Identification and Safety Characterization of Lactobacillaceae from Mediterranean Natural Fermented Sausages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sausage Samples and Lactobacillaceae Isolation
2.2. DNA Extraction and REP-PCR Analysis
2.3. Genotyping Identification of Isolates
2.4. Antibiotic Susceptibility Testing
2.5. PCR-Based Screening of Resistance Genes
2.6. Biogenic Amines (BAs) Production
3. Results and Discussion
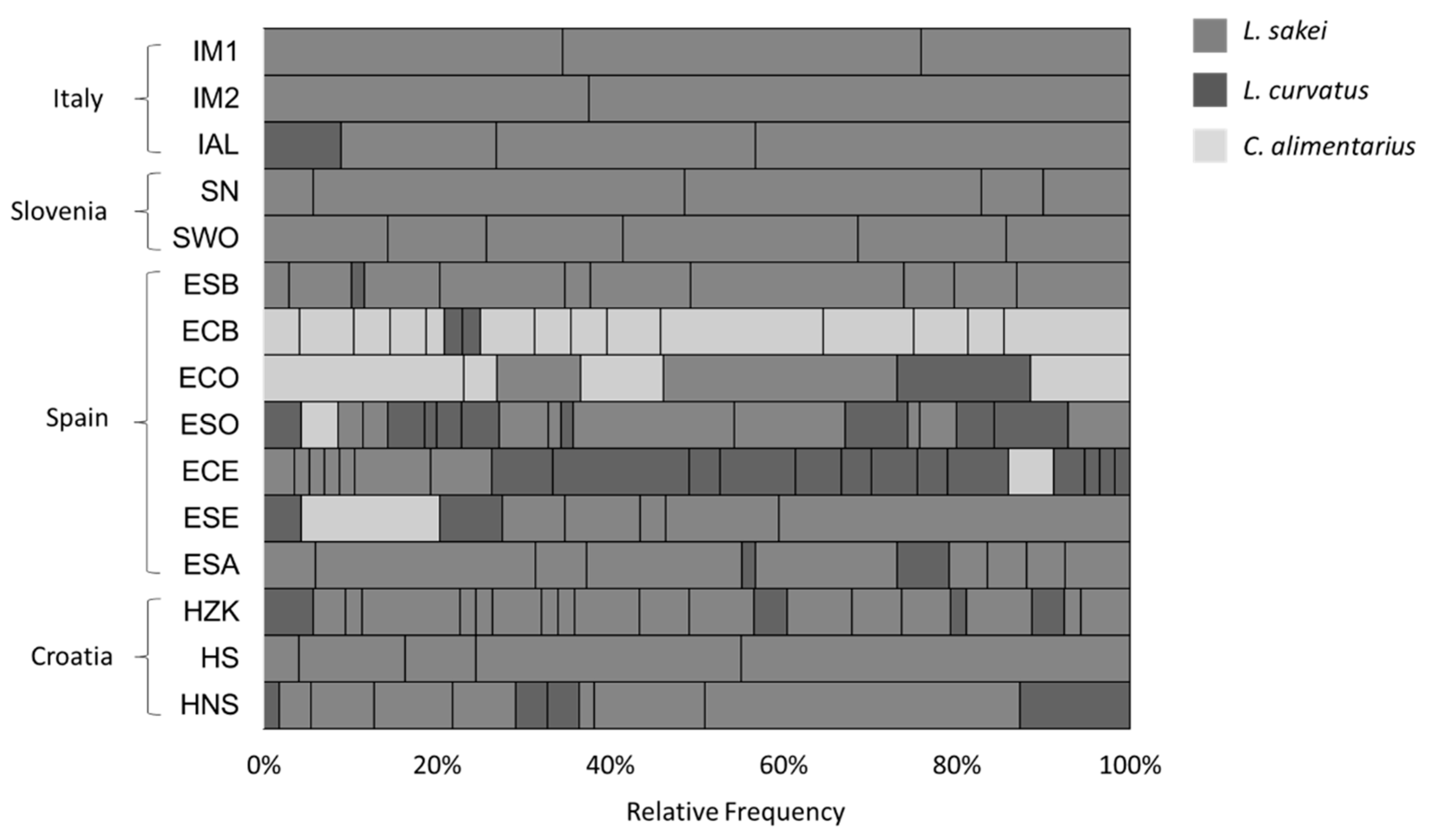
3.1. Strain Genotyping and Identification of Biotypes
3.2. Safety Assessment of Isolated Strains
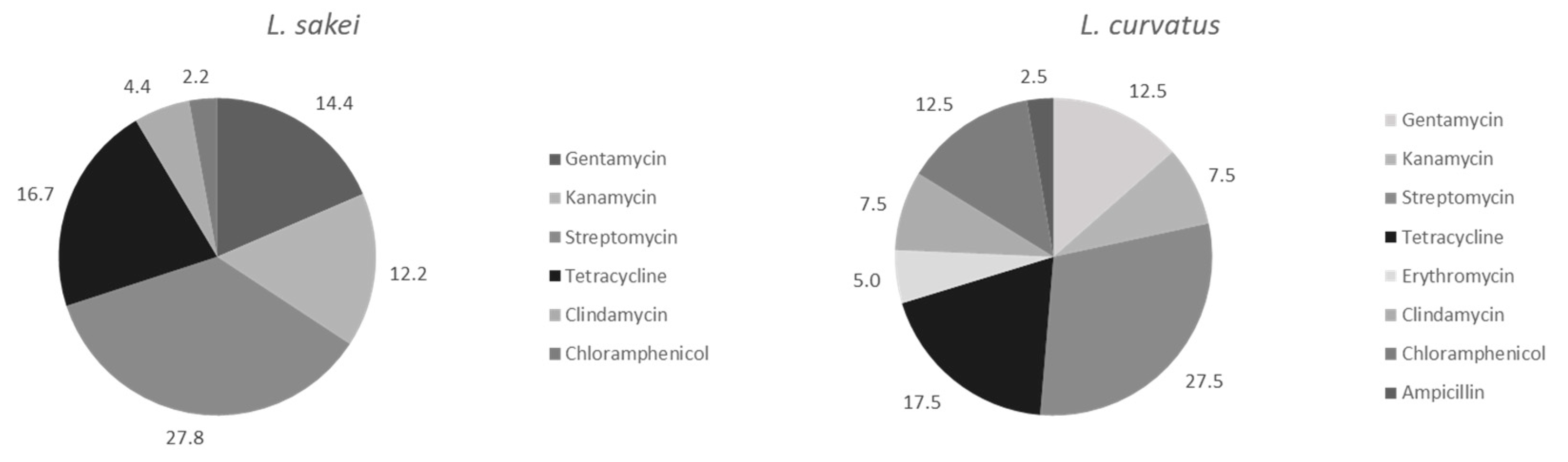
Antibiotic Resistance Assessment
3.3. Biogenic Amines (BAs) Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aquilanti, L.; Garofalo, C.; Osimani, A.; Clementi, F. Ecology of Lactic Acid Bacteria and Coagulase Negative Cocci in Fermented Dry Sausages Manufactured in Italy and Other Mediterranean Countries: An Overview. Int. Food Res. J. 2016, 23. Available online: http://www.ifrj.upm.edu.my/23%20(02)%202016/(1).pdf (accessed on 28 August 2022).
- Franciosa, I.; Alessandria, V.; Dolci, P.; Rantsiou, K.; Cocolin, L. Sausage fermentation and starter cultures in the era of molecular biology methods. Int. J. Food Microbiol. 2018, 279, 26–32. [Google Scholar] [CrossRef]
- Cocolin, L.; Dolci, P.; Rantsiou, K.; Urso, R.; Cantoni, C.; Comi, G. Lactic acid bacteria ecology of three traditional fermented sausages produced in the North of Italy as determined by molecular methods. Meat Sci. 2009, 82, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chatli, M.K.; Verma, A.K.; Mehta, N.; Malav, O.P.; Kumar, D.; Sharma, N. Quality, functionality, and shelf life of fermented meat and meat products: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 2844–2856. [Google Scholar] [CrossRef] [PubMed]
- Niinivaara, F.P. Starter Cultures in the Processing of Meat by Fermentation and Dehydration. 1991, pp. 59–63. Available online: https://meatscience.org/docs/default-source/publications-resources/rmc/1991/starter-cultures-in-the-processing-of-meat-by-fermentation-and-dehydration.pdf?sfvrsn=2 (accessed on 28 August 2022).
- Comi, G.; Muzzin, A.; Corazzin, M.; Iacumin, L. Lactic Acid Bacteria: Variability Due to Different Pork Breeds, Breeding Systems and Fermented Sausage Production Technology. Foods 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Luecke, F.K. Microbiological Processes in the Manufacture of Dry Sausage and Raw Ham. Fleischwirtschaft 1986, 66, 1505–1509. [Google Scholar]
- Lücke, F.-K. Utilization of microbes to process and preserve meat. Meat Sci. 2000, 56, 105–115. [Google Scholar] [CrossRef]
- Cocconcelli, P.S.; Fontana, C. Bacteria. In Handbook of Fermented Meat and Poultry; Wiley: New York, NY, USA, 2014; pp. 117–128. [Google Scholar]
- Carballo, J. Sausages: Nutrition, Safety, Processing and Quality Improvement. Foods 2021, 10, 890. [Google Scholar] [CrossRef]
- Capozzi, V.; Spano, G. Food Microbial Biodiversity and “Microbes of Protected Origin”. Front. Microbiol. 2011, 2, 237. [Google Scholar] [CrossRef]
- Montanari, C.; Barbieri, F.; Magnani, M.; Grazia, L.; Gardini, F.; Tabanelli, G. Phenotypic Diversity of Lactobacillus sakei Strains. Front. Microbiol. 2018, 9, 2003. [Google Scholar] [CrossRef]
- Van Reckem, E.; Geeraerts, W.; Charmpi, C.; Van Der Veken, D.; De Vuyst, L.; Leroy, F. Exploring the Link between the Geographical Origin of European Fermented Foods and the Diversity of Their Bacterial Communities: The Case of Fermented Meats. Front. Microbiol. 2019, 10, 2302. [Google Scholar] [CrossRef] [PubMed]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ameer, A.; Seleshe, S.; Kim, B.-J.; Kang, A.S.N. Inoculation of Lactobacillus sakei on Quality Traits of Dry Fermented Sausages. Prev. Nutr. Food Sci. 2021, 26, 476–484. [Google Scholar] [CrossRef]
- Barat, J.M.; Toldrá, F. Reducing Salt in Processed Meat Products. Processed Meats: Improving Safety, Nutrition and Quality; Woodhead Publishing Limited: Sawston, UK. [CrossRef]
- Bover-Cid, S.; Holzapfel, W.H. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef]
- Suzzi, G. Biogenic amines in dry fermented sausages: A review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef]
- Pasini, F.; Soglia, F.; Petracci, M.; Caboni, M.F.; Marziali, S.; Montanari, C.; Gardini, F.; Grazia, L.; Tabanelli, G. Effect of Fermentation with Different Lactic Acid Bacteria Starter Cultures on Biogenic Amine Content and Ripening Patterns in Dry Fermented Sausages. Nutrients 2018, 10, 1497. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Landete, J.M.; Rivas, B.D.L.; Marcobal, A.; Muñoz, R. Updated Molecular Knowledge about Histamine Biosynthesis by Bacteria. Crit. Rev. Food Sci. Nutr. 2008, 48, 697–714. [Google Scholar] [CrossRef]
- Marcobal, A.; Sonnenburg, J.L. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 2012, 18, 12. [Google Scholar] [CrossRef]
- Dos Santos Cruxen, C.E.; Funck, G.D.; Haubert, L.; da Silva Dannenberg, G.; de Lima Marques, J.; Chaves, F.C.; da Silva, W.P.; Fiorentini, Â.M. Selection of native bacterial starter culture in the production of fermented meat sausages: Application potential, safety aspects, and emerging technologies. Food Res. Int. 2019, 122, 371–382. [Google Scholar] [CrossRef]
- Ammor, M.S.; Flórez, A.B.; Mayo, B. Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol. 2007, 24, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Daza, M.V.B.; Milani, G.; Cortimiglia, C.; Pietta, E.; Bassi, D.; Cocconcelli, P.S. Genomic Insight of Enterococcus faecium UC7251, a Multi-Drug Resistance Strain from Ready-to-Eat Foods, Highlights the Risk of Antimicrobial Resistance in the Food Chain. Front. Microbiol. 2022, 13, 894241. [Google Scholar] [CrossRef] [PubMed]
- Zonenschain, D.; Rebecchi, A.; Morelli, L. Erythromycin- and tetracycline-resistant lactobacilli in Italian fermented dry sausages. J. Appl. Microbiol. 2009, 107, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Patrone, V.; Lopez, C.M.; Morelli, L.; Rebecchi, A. Incidence of Tetracycline and Erythromycin Resistance in Meat-Associated Bacteria: Impact of Different Livestock Management Strategies. Microorganisms 2021, 9, 2111. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Tabanelli, G.; Montanari, C.; Dall’Osso, N.; Šimat, V.; Možina, S.S.; Baños, A.; Özogul, F.; Bassi, D.; Fontana, C.; et al. Mediterranean Spontaneously Fermented Sausages: Spotlight on Microbiological and Quality Features to Exploit Their Bacterial Biodiversity. Foods 2021, 10, 2691. [Google Scholar] [CrossRef]
- Gevers, D.; Huys, G.; Swings, J. Applicability of rep-PCR fingerprinting for identification of Lactobacillus species. FEMS Microbiol. Lett. 2001, 205, 31–36. [Google Scholar] [CrossRef]
- Klijn, N.; Weerkamp, A.H.; de Vos, W.M. Identification of mesophilic lactic acid bacteria by using polymerase chain reaction-amplified variable regions of 16S rRNA and specific DNA probes. Appl. Environ. Microbiol. 1991, 57, 3390–3393. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; Da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.-W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar] [CrossRef]
- International Organization for Standardization. Milk and Milk Products: Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria; International Organization for Standardization: Geneva, Switzerland; International Dairy Federation: Brussels, Belgium.
- Villedieu, A.; Diaz-Torres, M.L.; Hunt, N.; McNab, R.; Spratt, D.A.; Wilson, M.; Mullany, P. Prevalence of Tetracycline Resistance Genes in Oral Bacteria. Antimicrob. Agents Chemother. 2003, 47, 1028–1036. [Google Scholar] [CrossRef]
- Olsvik, B.; Olsen, I.; Tenover, F.C. Detection of tet(M) and tet(Q) using the polymerase chain reaction in bacteria isolated from patients with periodontal disease. Oral Microbiol. Immunol. 1995, 10, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, E.; Korhonen, J.M.; Heikkinen, J.; Morelli, L.; Von Wright, A. Transfer of plasmid-mediated resistance to tetracycline in pathogenic bacteria from fish and aquaculture environments. FEMS Microbiol Lett 2009, 293, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Danielsen, M.; Huys, G.; Swings, J. Molecular Characterization of tet (M) Genes in Lactobacillus Isolates from Different Types of Fermented Dry Sausage. Appl. Environ. Microbiol. 2003, 69, 1270–1275. [Google Scholar] [CrossRef]
- Sutcliffe, J.; Grebe, T.; Tait-Kamradt, A.; Wondrack, L. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 1996, 40, 2562–2566. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.B.; Frimodt-Møller, N.; Aarestrup, F.M. Presence of erm gene classes in Gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol. Lett. 1999, 170, 151–158. [Google Scholar] [CrossRef]
- Casaburi, A.; Di Martino, V.; Ferranti, P.; Picariello, L.; Villani, F. Technological properties and bacteriocins production by Lactobacillus curvatus 54M16 and its use as starter culture for fermented sausage manufacture. Food Control 2016, 59, 31–45. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Nevado, F.P.; de Guía Córdoba, M. Screening of lactic acid bacteria and bifidobacteria for potential probiotic use in Iberian dry fermented sausages. Meat Sci 2008, 80, 715–721. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.; Mosleth, E.F.; Rud, I.; Dos Santos, F.B.; Snipen, L.; Liland, K.H.; Axelsson, L. Effects of glucose availability in Lactobacillus sakei; metabolic change and regulation of the proteome and transcriptome. PLoS ONE 2017, 12, e0187542. [Google Scholar] [CrossRef]
- Widenmann, A.; Schiffer, C.; Ehrmann, M.; Vogel, R. Impact of different sugars and glycosyltransferases on the assertiveness of Latilactobacillus sakei in raw sausage fermentations. Int. J. Food Microbiol. 2022, 366, 109575. [Google Scholar] [CrossRef]
- Janßen, D.; Eisenbach, L.; Ehrmann, M.A.; Vogel, R.F. Assertiveness of Lactobacillus sakei and Lactobacillus curvatus in a fermented sausage model. Int. J. Food Microbiol. 2018, 285, 188–197. [Google Scholar] [CrossRef]
- Garciafontan, M.; Lorenzo, J.; Parada, A.; Franco, I.; Carballo, J. Microbiological characteristics of “androlla”, a Spanish traditional pork sausage. Food Microbiol. 2007, 24, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Fontán, M.C.G.; Lorenzo, J.M.; Martínez, S.; Franco, I.; Carballo, J. Microbiological characteristics of Botillo, a Spanish traditional pork sausage. LWT 2007, 40, 1610–1622. [Google Scholar] [CrossRef]
- Coppola, S.; Mauriello, G.; Aponte, M.; Moschetti, G.; Villani, F. Microbial succession during ripening of Naples-type salami, a southern Italian fermented sausage. Meat Sci. 2000, 56, 321–329. [Google Scholar] [CrossRef]
- Ammor, M.S.; Mayo, B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: An update. Meat Sci. 2007, 76, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Coton, M.; Lebreton, M.; Salas, M.L.; Garnier, L.; Navarri, M.; Pawtowski, A.; Le Blay, G.; Valence, F.; Coton, E.; Mounier, J. Biogenic amine and antibiotic resistance profiles determined for lactic acid bacteria and a propionibacterium prior to use as antifungal bioprotective cultures. Int. Dairy J. 2018, 85, 21–26. [Google Scholar] [CrossRef]
- Holck, A.; Axelsson, L.; McLeod, A.; Rode, T.M.; Heir, E. Health and Safety Considerations of Fermented Sausages. J. Food Qual. 2017, 2017, 9753894. [Google Scholar] [CrossRef]
- Freiding, S.; Gutsche, K.A.; Ehrmann, M.A.; Vogel, R.F. Genetic screening of Lactobacillus sakei and Lactobacillus curvatus strains for their peptidolytic system and amino acid metabolism, and comparison of their volatilomes in a model system. Syst. Appl. Microbiol. 2011, 34, 311–320. [Google Scholar] [CrossRef]
- Romano, A.; Trip, H.; Lonvaud-Funel, A.; Lolkema, J.S.; Lucas, P.M. Evidence of Two Functionally Distinct Ornithine Decarboxylation Systems in Lactic Acid Bacteria. Appl. Environ. Microbiol. 2012, 78, 1953–1961. [Google Scholar] [CrossRef]
- Parente, E.; Grieco, S.; Crudele, M. Phenotypic diversity of lactic acid bacteria isolated from fermented sausages produced in Basilicata (Southern Italy). J. Appl. Microbiol. 2001, 90, 943–952. [Google Scholar] [CrossRef]
- Samelis, J.; Maurogenakis, F.; Metaxopoulos, J. Characterisation of lactic acid bacteria isolated from naturally fermented Greek dry salami. Int. J. Food Microbiol. 1994, 23, 179–196. [Google Scholar] [CrossRef]
- Hugas, M.; Garriga, M.; Aymerich, T.; Monfort, J. Biochemical characterization of lactobacilli from dry fermented sausages. Int. J. Food Microbiol. 1993, 18, 107–113. [Google Scholar] [CrossRef]
- Santos, E.M.; González-Fernández, C.; Jaime, I.; Rovira, J. Comparative study of lactic acid bacteria house flora isolated in different varieties of ‘chorizo’. Int. J. Food Microbiol. 1998, 39, 123–128. [Google Scholar] [CrossRef]
- Monger, X.C.; Gilbert, A.-A.; Saucier, L.; Vincent, A.T. Antibiotic Resistance: From Pig to Meat. Antibiotics 2021, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
| Primers Name | Oligonucleotide Sequence (5′-3′) | Expected Band (bp) | Positive Control Strain | Reference |
|---|---|---|---|---|
| ermA1 | TCTAAAAAGCATGTAAAAGAA | 645 | E. faecium PE1 | [38] |
| ermA2 | CTTCGATAGTTTATTAATATTAGT | |||
| ermB1 | GAAAAGGTACTCAACCAAATA | 639/694 | E. faecium PE1 | [38] |
| ermB2 | AGTAACGGTACTTAAATTGTTTAC | |||
| ermC1 | ATCTTTGAAATCGGCTCAGG | 275/294 | L. reuteri 70 | [39] |
| ermC2 | CAAACCCGTATTCCACGATT | |||
| tetL1 | GTMGTTGCGCGCTATATTCC | 696 | E. faecium LMG 20927 | [29] |
| tetL2 | GTGAAMGRWAGCCCACCTAA | |||
| tetM1 | GAACTCGAACAAGAGGAAAGC | 740 | L. plantarum 146 | [35] |
| tetM2 | ATGGAAGCCCAGAAAGGAT | |||
| tetS1 | GGAGTACAGTCACAAACTCG | 335 | L. reuteri 541 | [36] |
| tetS2 | GGATATAAGGAGCAACTTTG | |||
| tetW1 | GAGAGCCTGCTATATGCCAGC | 168 | L. reuteri 534 | [34] |
| tetW2 | GGGCGTATCCACAATGTTAAC |
| Production Country | Type of Sausage | Sample Name | Number of Isolates | Biotypes |
|---|---|---|---|---|
| Italy | Salame Fabriano-producer 1 (Marche) | IM1 | 58 | 3 |
| Salame Fabriano-producer 2 (Marche) | IM2 | 48 | 2 | |
| Salame Alfianello (Brescia), Lombardy | IAL | 67 | 4 | |
| Slovenia | Traditional smoked salami with nitrates | SN | 70 | 5 |
| Traditional smoked salami without nitrates | SWO | 70 | 6 | |
| Spain | Salchichòn Bérchules | ESB | 69 | 11 |
| Chorizo Bérchules | ECB | 48 | 16 | |
| Chorizo Olvera | ECO | 52 | 7 | |
| Salchichon Olvera | ESO | 70 | 19 | |
| Chorizo Ecija | ECE | 69 | 22 | |
| Salchichon Ecija | ESE | 69 | 8 | |
| Salchichon Alhendin | ESA | 67 | 11 | |
| Croatia | Salami ZminjskaKlobasica | HZK | 53 | 21 |
| Traditional smoked salami | HS | 49 | 5 | |
| Traditional unsmoked salami | HNS | 55 | 11 | |
| Total | 15 | 914 | 151 |
| Antibiotic a | Species | MIC Value (µg mL−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.016 | 0.032 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ||
| Gen | L. sakei | 1 | 1 | 4 | 11 | 33 | 27 | 10 | 3 | |||||||
| L. curvatus | 1 | 3 | 2 | 9 | 10 | 10 | 5 | |||||||||
| Kan | L. sakei | 1 | 5 | 12 | 20 | 20 | 21 | 5 | 6 | |||||||
| L. curvatus | 1 | 3 | 8 | 11 | 7 | 7 | 3 | |||||||||
| Str | L. sakei | 1 | 5 | 10 | 10 | 17 | 22 | 22 | 3 | |||||||
| L. curvatus | 1 | 2 | 3 | 7 | 7 | 9 | 4 | 7 | ||||||||
| Tet | L. sakei | 7 | 5 | 9 | 26 | 17 | 5 | 6 | 1 | 14 | ||||||
| L. curvatus | 2 | 4 | 3 | 9 | 9 | 6 | 6 | 1 | ||||||||
| Ery | L. sakei | 4 | 11 | 37 | 17 | 13 | 3 | 5 | ||||||||
| L. curvatus | 3 | 7 | 15 | 7 | 6 | 2 | ||||||||||
| Clin | L. sakei | 66 | 6 | 1 | 9 | 2 | 2 | 2 | 1 | 1 | ||||||
| L. curvatus | 20 | 3 | 11 | 1 | 2 | 2 | 1 | |||||||||
| Chlor | L. sakei | 7 | 2 | 10 | 33 | 29 | 7 | 2 | ||||||||
| L. curvatus | 1 | 4 | 4 | 27 | 3 | 1 | ||||||||||
| Amp | L. sakei | 7 | 2 | 9 | 20 | 12 | 40 | |||||||||
| L. curvatus | 1 | 15 | 11 | 7 | 4 | 2 | ||||||||||
| Countries of Origin | L. Curvatus Aminobiogenic Strains | Tyramine | Histamine | Putrescine | Cadaverine |
|---|---|---|---|---|---|
| Italy | L. curvatus IAL6 (S) * | + | − | − | − |
| Spain | L. curvatus ESB8 (S) | − | − | + | − |
| L. curvatus ECB11 (S) | + | − | − | − | |
| L. curvatus ECB12 (S) | + | − | − | − | |
| L. curvatus ECO46 (S) | + | − | − | − | |
| L. curvatus ESO6 (R) | − | − | − | − | |
| L. curvatus ESO13 (R) | + | − | − | − | |
| L. curvatus ESO14 (R) | + | − | + | − | |
| L. curvatus ESO16 (R) | + | − | − | − | |
| L. curvatus ESO19 (R) | − | − | − | − | |
| L. curvatus ESO25 (R) | − | − | − | − | |
| L. curvatus ESO52 (R) | − | − | − | − | |
| L. curvatus ESO59 (S) | + | − | + | − | |
| L. curvatus ESO61 (R) | − | − | + | − | |
| L. curvatus ECE16 (R) | + | − | + | − | |
| L. curvatus ECE25 (R) | − | − | − | − | |
| L. curvatus ECE27 (R) | − | + | − | − | |
| L. curvatus ECE32 (S) | + | − | − | − | |
| L. curvatus ECE35 (S) | + | − | − | − | |
| L. curvatus ECE37 (S) | + | − | − | − | |
| L. curvatus ECE40 (R) | − | − | − | − | |
| L. curvatus ECE42 (S) | + | − | − | − | |
| L. curvatus ECE46 (S) | + | − | + | − | |
| L. curvatus ECE51 (S) | − | − | + | − | |
| L. curvatus ECE52 (S) | + | − | − | − | |
| L. curvatus ECE53 (R) | − | − | − | − | |
| L. curvatus ECE54 (S) | + | − | − | − | |
| L. curvatus ECE57 (S) | − | − | + | − | |
| L. curvatus ESE3 (S) | − | − | + | − | |
| L. curvatus ESE19 (S) | − | − | + | − | |
| L. curvatus ESA38 (S) | + | − | − | − | |
| L. curvatus ESA53 (S) | + | − | − | − | |
| Croatia | L. curvatus HZK3 (R) | + | − | − | − |
| L. curvatus HZK32 (R) | − | − | − | − | |
| L. curvatus HZK43 (R) | − | − | − | − | |
| L. curvatus HZK49 (R) | − | − | − | − | |
| L. curvatus HNS1 (R) | − | − | − | − | |
| L. curvatus HNS18 (R) | − | − | − | − | |
| L. curvatus HNS20 (R) | − | − | − | − | |
| L. curvatus HNS55 (S) | − | − | − | − | |
| Total strains | 40 | 19 | 1 | 10 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassi, D.; Milani, G.; Belloso Daza, M.V.; Barbieri, F.; Montanari, C.; Lorenzini, S.; Šimat, V.; Gardini, F.; Tabanelli, G. Taxonomical Identification and Safety Characterization of Lactobacillaceae from Mediterranean Natural Fermented Sausages. Foods 2022, 11, 2776. https://doi.org/10.3390/foods11182776
Bassi D, Milani G, Belloso Daza MV, Barbieri F, Montanari C, Lorenzini S, Šimat V, Gardini F, Tabanelli G. Taxonomical Identification and Safety Characterization of Lactobacillaceae from Mediterranean Natural Fermented Sausages. Foods. 2022; 11(18):2776. https://doi.org/10.3390/foods11182776
Chicago/Turabian StyleBassi, Daniela, Giovanni Milani, Mireya Viviana Belloso Daza, Federica Barbieri, Chiara Montanari, Silvia Lorenzini, Vida Šimat, Fausto Gardini, and Giulia Tabanelli. 2022. "Taxonomical Identification and Safety Characterization of Lactobacillaceae from Mediterranean Natural Fermented Sausages" Foods 11, no. 18: 2776. https://doi.org/10.3390/foods11182776
APA StyleBassi, D., Milani, G., Belloso Daza, M. V., Barbieri, F., Montanari, C., Lorenzini, S., Šimat, V., Gardini, F., & Tabanelli, G. (2022). Taxonomical Identification and Safety Characterization of Lactobacillaceae from Mediterranean Natural Fermented Sausages. Foods, 11(18), 2776. https://doi.org/10.3390/foods11182776









