Analysis of Cobalamin (Vit B12) in Ripened Cheese by Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sampling
2.3. Sample Preparation
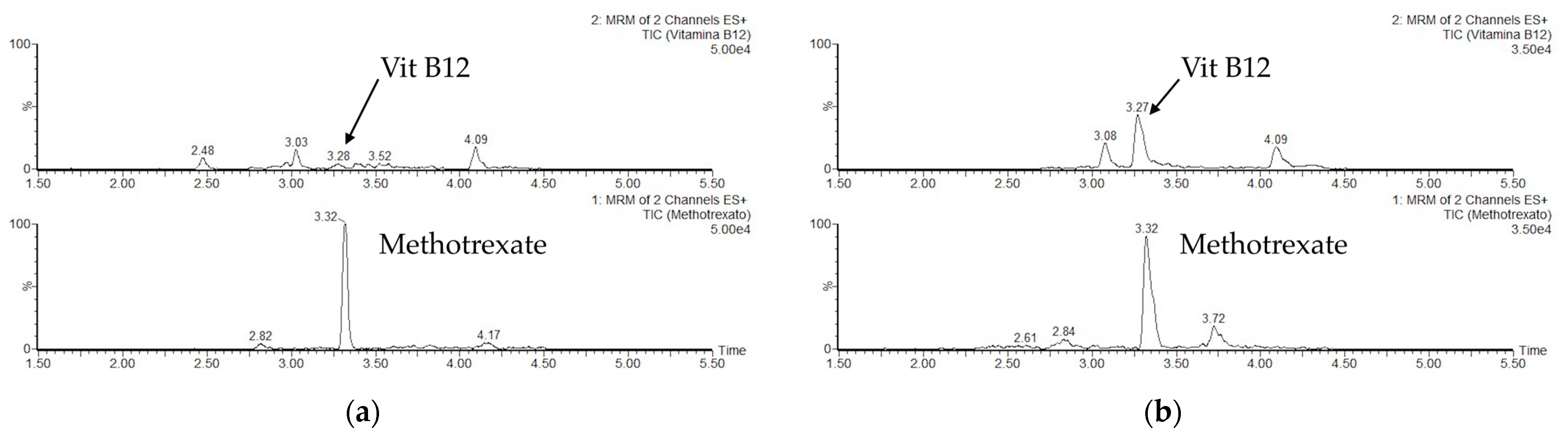
2.4. UHPLC-MS/MS Analysis
2.5. Method Validation
3. Results and Discussion
3.1. Optimization of the Extraction Procedures
3.2. Optimization of the Chromatographic (LC) Conditions
3.3. Method Validation
3.4. Application of the Method to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farquharson, J.; Adams, J.F. The forms of vitamin B12 in foods. Br. J. Nutr. 1976, 36, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Miller, J.W.; Zempleni, J.; Rucker, R.B.; McCormick, D.B.; Suttie, J.W. Encyclopaedia of Human Nutrition, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 413–457. [Google Scholar]
- Rathod, R.; Kale, A.; Joshi, S. Novel insights into the effect of vitamin B12 and omega-3 fatty acids on brain function. J. Biomed. Sci. 2016, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- EFSA. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef]
- Obeid, R.S.; Heil, G.; Verhoeven, M.M.; Van den Heuvel, E.G.; De Groot, L.C.; Eussen, S.J. Vitamin B12 intake from animal foods, biomarkers, and health aspects. Front. Nutr. 2019, 6, 93. [Google Scholar] [CrossRef]
- Bueno Dalto, D.; Audet, I.; Girard, C.L.; Matte, J.J. Bioavailability of vitamin B12 from dairy products using a pig model. Nutrients 2018, 10, 1134. [Google Scholar] [CrossRef]
- EFSA. Food Composition Data. Available online: https://www.efsa.europa.eu/it/microstrategy/food-composition-data (accessed on 26 July 2022).
- Federal Office of Public Health. The Swiss Food Composition Database. Available online: https://naehrwertdaten.ch/en/ (accessed on 26 July 2022).
- U.S. Department of Agriculture. Food Data Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171278/nutrients (accessed on 26 July 2022).
- Karlin, R. Vitamin B12 and folinic acid contents of varieties of cheese. Ann. Nutr. Aliment. 1957, 11, 91–97. [Google Scholar]
- Zironi, E.; Gazzotti, T.; Barbarossa, A.; Farabegoli, F.; Serraino, A.; Pagliuca, G. Determination of vitamin B12 in dairy products by ultra performance liquid chromatography-tandem mass Spectrometry. Ital. J. Food Saf. 2014, 18, 4513. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A. Vitamin B12 in meat and dairy products. Nutr. Rev. 2015, 73, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sela, I.; Yaskolka Meir, A.; Brandis, A.; Krajmalnik-Brown, R.; Zeibich, L.; Chang, D.; Dirks, B.; Tsaban, G.; Kaplan, A.; Rinott, E.; et al. Wolffia globosa-Mankai Plant-Based Protein Contains Bioactive Vitamin B12 and Is Well Absorbed in Humans. Nutrients 2020, 12, 3067. [Google Scholar] [CrossRef]
- Karmi, O.; Zayed, A.; Baraghethi, S.; Qadi, M.; Ghanem, R. Measurement of vitamin B12 concentration: A review on available methods. IIOAB J. 2011, 2, 23–32. [Google Scholar]
- Arkbåge, K.; Witthöft, C.; Fondén, R.; Jägerstad, M. Retention of vitamin B12 during manufacture of six fermented dairy products using a validated radio protein-binding assay. Int. Dairy J. 2003, 13, 101–109. [Google Scholar] [CrossRef]
- Campos-Giménez, E.; Fontannaz, P.; Trisconi, M.J.; Kilinc, T.; Gimenez, C.; Andrieux, P. Determination of vitamin B12 in food products by liquid chromatography/UV detection with immunoaffinity extraction: Single-laboratory validation. J. AOAC Int. 2008, 91, 786–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guggisberg, D.; Risse, M.C.; Hadorn, R. Determination of Vitamin B12 in meat products by RP-HPLC after enrichment and purification on an immunoaffinity column. Meat Sci. 2012, 90, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Heudi, O.; Kilinç, T.; Fontannaz, P.; Marley, E. Determination of Vitamin B12 in food products and in premixes by reversed-phase high performance liquid chromatography and immunoaffinity extraction. J. Chromatogr. A 2006, 1101, 63–68. [Google Scholar] [CrossRef]
- Marley, E.C.; Mackay, E.; Young, G. Characterisation of vitamin B12 immunoaffinity columns and method development for determination of vitamin B12 in a range of foods, juices and pharmaceutical products using immunoaffinity clean-up and high performance liquid chromatography with UV detection. Food Addit. Contam. 2009, 26, 282–288. [Google Scholar] [CrossRef]
- Pakin, C.; Bergaentzlé, M.; Aoudé-Werner, D.; Hasselmann, C. α-Ribazole, a fluorescent marker for the liquid chromatographic determination of vitamin B12 in foodstuffs. J. Chromatogr. A 2005, 108, 181–187. [Google Scholar] [CrossRef]
- Viñas, P.; López-Erroz, C.; Balslobre, N.; Hernández-Córdoba, M. Speciation of cobalamins in biological samples using liquid chromatography with diode-array detection. Chromatographia 2003, 58, 5–10. [Google Scholar] [CrossRef]
- Shetty, S.A.; Young, M.F.; Taneja, S.; Rangiah, K. Quantification of B-vitamins from different fresh milk samples using ultra-high performance liquid chromatography mass spectrometry/selected reaction monitoring methods. J. Chromatogr. A 2020, 1609, 460452. [Google Scholar] [CrossRef]
- Bartosiak, M.; Jankowski, K.; Giersz, J. Determination of cobalt species in nutritional supplements using ICP-OES after microwave-assisted extraction and solid-phase extraction. J. Pharm. Biomed. Anal. 2018, 155, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, B.; Ding, L.; Tang, F.; Yao, S. HPLC-ESI-MS analysis of Vitamin B12 in food products and in multivitamins-multimineral tablets. Anal. Chim. Acta 2006, 562, 185–189. [Google Scholar] [CrossRef]
- Szterk, A.; Roszko, M.; Malek, K.; Czerwonka, M.; Waszkiewicz-Robak, B. Application of the SPE reversed phase HPLC/MS technique to determine vitamin B12 bio-active forms in beef. Meat Sci. 2012, 91, 408–413. [Google Scholar] [CrossRef]
- Van Wyk, J.; Britz, T.J. A rapid HPLC method for the extraction and quantification of vitamin B12 in dairy products and cultures of Propionibacterium freudenreichii. Dairy Sci. Technol. 2010, 90, 509–552. [Google Scholar] [CrossRef] [Green Version]
- Fumio, W.; Tomohiro, B. Determination of Cobalamin and Related Compounds in Foods. J. AOAC Int. 2019, 101, 1308–1313. [Google Scholar] [CrossRef]
- Zironi, E.; Gazzotti, T.; Barbarossa, A.; Devicienti, C.; Scardilli, M.; Pagliuca, G. Development and validation of a method using ultra performance liquid chromatography coupled with tandem mass spectrometry for determination of vitamin B12 concentrations in milk and dairy products. Int. J. Dairy Sci. 2013, 96, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Abe, K.; Fujita, T.; Goto, M.; Hiemori, M.; Nakano, Y. Effects of microwave heating on the loss of vitamin B-12 in foods. J. Agric. Food Chem. 1998, 46, 206–221. [Google Scholar] [CrossRef]
- Renner, E. Milch und Milchprodukte in der Ernahrung des Menschen, 4th ed.; Volkswirtschaftlicher Verlag: Munich, Germany, 1982. [Google Scholar]
- The European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing Decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Union 2021, 64, 84–109. [Google Scholar]
| Compound | RT (min) | Precursor Ion (m/z) | Product Ion 1 (m/z) | CE (eV) | Product Ion 2 (m/z) | CE (eV) |
|---|---|---|---|---|---|---|
| Cyanocobalamin | 3.33 | 678.43 | 147.15 | 18 | 359.15 | 30 |
| Methotrexate (IS) | 3.37 | 455.25 | 308.17 | 48 | 134.17 | 28 |
| Fortification Level (ng/g) | Parameter | Day 1 (n = 3) | Day 2 (n = 3) | Day 3 (n = 3) | Reproducibility (Inter-Day n = 9) |
|---|---|---|---|---|---|
| 0 | Precision (RSD%) | 19.3 | 15.8 | 18.2 | 16.2 |
| Trueness (bias%) | +1.4 | +14.5 | +10.2 | +8.7 | |
| 25 | Precision (RSD%) | 19.2 | 13.6 | 3.6 | 15.4 |
| Trueness (bias%) | −11.8 | +11.9 | +3.1 | −1.1 | |
| 50 | Precision (RSD%) | 2.8 | 11.2 | 11.3 | 9.9 |
| Trueness (bias%) | −9.4 | −4.6 | +2.6 | −3.8 |
| Cheese | Vitamin B12 (ng/g) | ||
|---|---|---|---|
| Type | Original Name | Single Mean ± SD | Total Mean ± SD |
| Cow cheese | Caciocavallo | 5.9 ± 0.6 | 6.5 ± 4.2 |
| Provolone | 14.2 ± 1.2 | ||
| Piave | 5.5 ± 0.8 | ||
| Grana | 4.8 ± 0.5 | ||
| Tête de moine | 2.3 ± 0.4 | ||
| Sheep cheese | Pecorino sardo 1 | 21.0 ± 4.3 | 29.0 ± 8.5 |
| Pecorino sardo 2 | 22.5 ± 5.3 | ||
| Pecorino toscano 1 | 38.9 ± 2.4 | ||
| Pecorino toscano 2 | 31.1 ± 6.1 | ||
| Goat cheese | Caprino 1 | <LOQ * | 12.5 ± 15.8 |
| Caprino 2 | <LOQ * | ||
| Caprino 3 (extra ripened) | 33.5 ± 2.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampazzo, G.; Zironi, E.; Pagliuca, G.; Gazzotti, T. Analysis of Cobalamin (Vit B12) in Ripened Cheese by Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry. Foods 2022, 11, 2745. https://doi.org/10.3390/foods11182745
Rampazzo G, Zironi E, Pagliuca G, Gazzotti T. Analysis of Cobalamin (Vit B12) in Ripened Cheese by Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry. Foods. 2022; 11(18):2745. https://doi.org/10.3390/foods11182745
Chicago/Turabian StyleRampazzo, Giulia, Elisa Zironi, Giampiero Pagliuca, and Teresa Gazzotti. 2022. "Analysis of Cobalamin (Vit B12) in Ripened Cheese by Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry" Foods 11, no. 18: 2745. https://doi.org/10.3390/foods11182745
APA StyleRampazzo, G., Zironi, E., Pagliuca, G., & Gazzotti, T. (2022). Analysis of Cobalamin (Vit B12) in Ripened Cheese by Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry. Foods, 11(18), 2745. https://doi.org/10.3390/foods11182745