Analysis of 18 Free Amino Acids in Honeybee and Bumblebee Honey from Eastern and Northern Europe and Central Asia Using HPLC-ESI-TQ-MS/MS Approach Bypassing Derivatization Step
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Chemicals and Reagents
- buffer A 10 mM hydrochloric acid solution (pH 2.04);
- buffer B 6 M ammonium hydroxide solution (pH 11.14).
2.3. Preparation of Amino Acids for HPLC-ESI-TQ-MS/MS Analysis
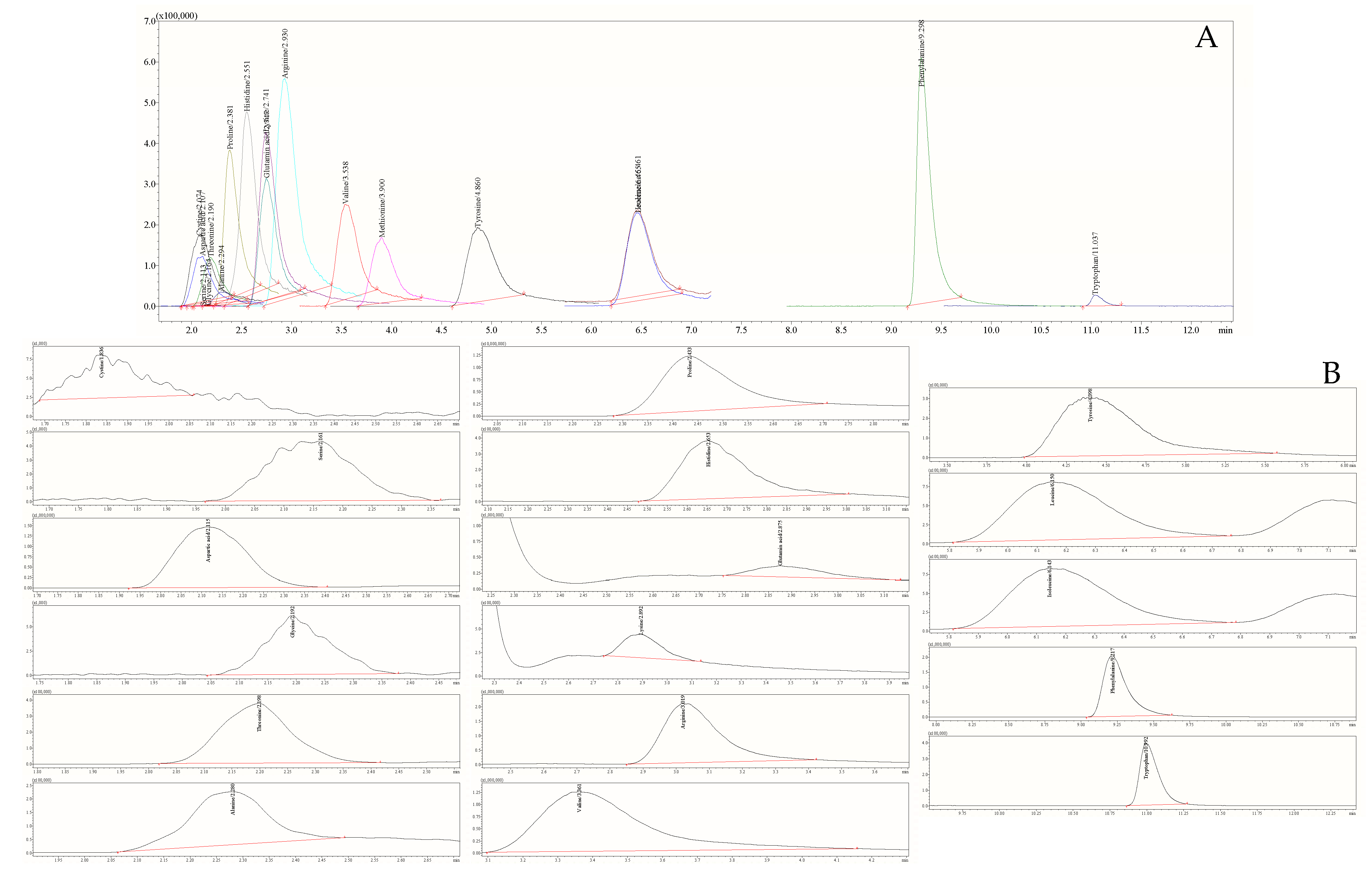
2.4. The HPLC-ESI-TQ-MS/MS Analytical Conditions for Amino Acids
2.5. Preparation of Standard Stock Solution
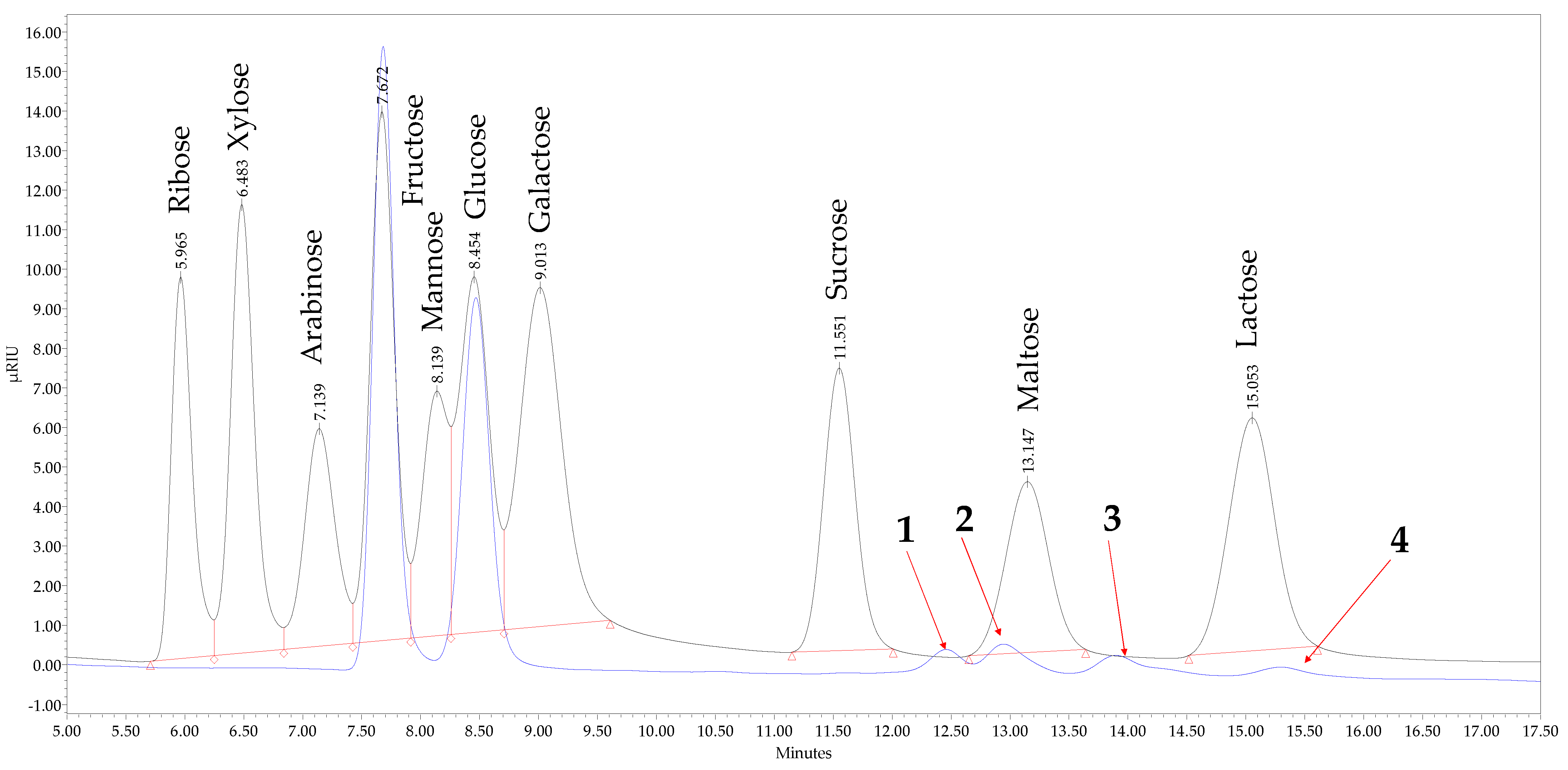
2.6. The HPLC-RID Conditions for Carbohydrates Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Profile of 18 Free Amino Acids in Honey Samples
3.2. Profile of Mono- and Disaccharides in Honey Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Haq, A. Effect of honey on antibody production against thymus-dependent and thymus-independent antigens in primary and secondary immune responses. J. Med. Food 2004, 7, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Emsen, I.M. A different and safe method of split thickness skin graft fixation: Medical honey application. Burns 2007, 33, 782–787, Erratum in Burns 2009, 35, 909. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N. The same, but different: Pollen foraging in honeybee and bumblebee colonies. Apidologie 2012, 43, 449–464. [Google Scholar] [CrossRef]
- Weidenmüller, A.; Kleineidam, C.; Tautz, J. Collective control of nest climate parameters in bumblebee colonies. Anim. Behav. 2002, 63, 1065–1071. [Google Scholar] [CrossRef]
- Svanberg, I.; Berggren, Å. Bumblebee honey in the Nordic countries. Ethnobiol. Lett. 2018, 9, 312–318. [Google Scholar] [CrossRef]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef]
- Madebeikin, I.I. Fauna and Bioecology of Bumblebees in Agrocenoses of the North-Eastern Part of the Volga Upland. Ph.D. Thesis, Russian State Agrarian Correspondence University, Moscow, Russia, 2004. [Google Scholar]
- Sato, H.; Tsukamoto-Yasui, M.; Takado, Y.; Kawasaki, N.; Matsunaga, K.; Ueno, S.; Kanda, M.; Nishimura, M.; Karakawa, S.; Isokawa, M.; et al. Protein deficiency-induced behavioral abnormalities and neurotransmitter loss in aged mice are ameliorated by essential amino acids. Front. Nutr. 2020, 7, 23. [Google Scholar] [CrossRef]
- Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. 2002, Volume L 10/47. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32001L0110 (accessed on 30 August 2022).
- CXS 12-19811; Codex Alimentarius International Food Standards, Standard for Honey. Codex Aliment, 2019; Volume 1, pp. 1–13, Adopted in 1981. Revised in 1987, 2001. Amended in 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 30 August 2022).
- Kowalski, S.; Kopuncová, M.; Ciesarová, Z.; Kukurová, K. Free amino acids profile of Polish and Slovak honeys based on LC–MS/MS method without the prior derivatisation. J. Food Sci. Technol. 2017, 54, 3716–3723. [Google Scholar] [CrossRef]
- Thiele, B.; Füllner, K.; Stein, N.; Oldiges, M.; Kuhn, A.J.; Hofmann, D. Analysis of amino acids without derivatization in barley extracts by LC-MS-MS. Anal. Bioanal. Chem. 2008, 391, 2663–2672. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.L.; Villegas-Portero, M.J.; Bernal-Daza, V. Characterization and analysis of amino acids in orange juice by HPLC-MS/MS for authenticity assessment. Analytica Chimica Acta 2005, 540, 221–230. [Google Scholar] [CrossRef]
- Roucher, V.F.; Desnots, E.; Naël, C.; Agnoux, A.M.; Alexandre-Gouabau, M.C.; Darmaun, D.; Boquien, C.Y. Use of UPLC-ESI-MS/MS to quantitate free amino acid concentrations in micro-samples of mammalian milk. Springerplus 2013, 2, 622. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, B.; Li, J.; Li, X. Development of a simple, underivatized method for rapid determination of free amino acids in honey using dilute-and-shoot strategy and liquid chromatography-tandem mass spectrometry. Molecules 2022, 27, 1056. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Kaczyński, P.; Iwaniuk, P. Analysis of 22 free amino acids in honey from Eastern Europe and Central Asia using LC-MS/MS technique without derivatization step. J. Food Compos. Anal. 2021, 98, 103837. [Google Scholar] [CrossRef]
- Del Rosario Brunetto, M.; Gallignani, M.; Orozco, W.; Clavijo, S.; Delgado, Y.; Ayala, C.; Zambrano, A. RP-HPLC-DAD determination of free amino acids in cocoa samples during fermentation and roasting. Rev. Colomb. Quim. 2020, 49, 11–19. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Seraglio, S.K.T.; Rocha, G.; Balderas, C.B.; Piovezan, M.; Gonzaga, L.V.; Falkenberg, D.d.B.; Fett, R.; de Oliveira, M.A.L.; Costa, A.C.O. Free amino acid determination by GC-MS combined with a chemometric approach for geographical classification of bracatinga honeydew honey (Mimosa scabrella Bentham). Food Control 2017, 78, 383–392. [Google Scholar] [CrossRef]
- Scholz, M.B.d.S.; Quinhone Júnior, A.; Delamuta, B.H.; Nakamura, J.M.; Baudraz, M.C.; Reis, M.O.; Kato, T.; Pedrão, M.R.; Dias, L.F.; dos Santos, D.T.R.; et al. Indication of the geographical origin of honey using its physicochemical characteristics and multivariate analysis. J. Food Sci. Technol. 2020, 57, 1896–1903. [Google Scholar] [CrossRef]
- Cukier, C.; Lea, P.J.; Cañas, R.; Marmagne, A.; Limami, A.M.; Hirel, B. Labeling maize (Zea mays L.) leaves with 15NH4+ and monitoring nitrogen incorporation into amino acids by GC/MS analysis. Curr. Protoc. Plant Biol. 2018, 3, e20073. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Kviesis, J.; Lazdina, D.; Valdovska, A.; Vallejo, F.; Lacis, G. Lignocellulose-degrading enzymes: A biotechnology platform for ferulic acid production from agro-industrial side streams. Foods 2021, 10, 3056. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-waste technology through the enzymatic hydrolysis of agro-industrial by-products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Dulf, F.V.; Podea, P.; Tofană, M. Analysis of fatty acids, amino acids and volatile profile of apple by-products by gas chromatography-mass spectrometry. Molecules 2022, 27, 1987. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, S. Analysis of amino acid and phenolic content in honey by UPLC-ESI-MS/MS. In Honey Analysis; IntechOpen: London, UK, 2017; pp. 79–93. [Google Scholar]
- Cometto, P.M.; Faye, P.F.; Di Paola Naranjo, R.D.; Rubio, M.A.; Aldao, M.A.J. Comparison of free amino acids profile in honey from three Argentinian regions. J. Agric. Food Chem. 2003, 51, 5079–5087. [Google Scholar] [CrossRef] [PubMed]
- Kečkeš, J.; Trifković, J.; Andrić, F.; Jovetić, M.; Tešić, Ž.; Milojković-Opsenica, D. Amino acids profile of Serbian unifloral honeys. J. Sci. Food Agric. 2013, 93, 3368–3376. [Google Scholar] [CrossRef]
- Rybak-Chmielewska, H.; Szczesna, T. Composition and properties of Polish buckwheat honey. In Current Advances in Buckwheat Research; Shinshu University Press: Matsumoto, Japan, 1995; pp. 793–799. [Google Scholar]
- Czipa, N.; Borbély, M.; Gyori, Z. Proline content of different honey types. Acta Aliment. 2012, 41, 26–32. [Google Scholar] [CrossRef]
- Nisbet, C.; Kazak, F.; Ardalı, Y. Determination of quality criteria that allow differentiation between honey adulterated with sugar and pure honey. Biol. Trace Elem. Res. 2018, 186, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M. Honey as source of nitrogen compounds: Aromatic amino acids, free nucleosides and their derivatives. Molecules 2020, 25, 847. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Hu, Y.; Zhou, J.; Chen, L.; Lu, X. Systematic review of the characteristic markers in honey of various botanical, geographic, and entomological origins. ACS Food Sci. Technol. 2022, 2, 206–220. [Google Scholar] [CrossRef]
- Rebane, R.; Herodes, K. Evaluation of the botanical origin of Estonian uni- and polyfloral honeys by amino acid content. J. Agric. Food Chem. 2008, 56, 10716–10720. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Cheng, N.; Xue, X.; Wu, L.; Zheng, J.; Cao, W. Identification of botanical origin of Chinese unifloral honeys by free amino acid profiles and chemometric methods. J. Pharm. Anal. 2017, 7, 317–323. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Vlasiou, M.; Kontakos, S.; Drouza, C.; Kontominas, M.G.; Keramidas, A.D. Geographical discrimination of pine and fir honeys using multivariate analyses of major and minor honey components identified by 1H NMR and HPLC along with physicochemical data. Eur. Food Res. Technol. 2018, 244, 1249–1259. [Google Scholar] [CrossRef]
- Janiszewska, K.; Aniołowska, M.; Nowakowski, P. Free amino acids content of honeys from Poland. Pol. J. Food Nutr. Sci. 2012, 62, 85–89. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Slupsky, C.M.; Ollikka, T.; Kauko, L.; Spevacek, A.R.; Sjövall, O.; Yang, B.; Kallio, H. NMR profiling clarifies the characterization of Finnish honeys of different botanical origins. Food Res. Int. 2016, 86, 83–92. [Google Scholar] [CrossRef]
- Rajindran, N.; Wahab, R.A.; Huda, N.; Julmohammad, N.; Shariff, A.H.M.; Ismail, N.I.; Huyop, F. Physicochemical properties of a new green honey from Banggi. Molecules 2022, 27, 4164. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-chain amino acids: Catabolism in skeletal muscle and implications for muscle and whole-body metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Takeshita, Y.; Takamura, T.; Kita, Y.; Ando, H.; Ueda, T.; Kato, K.; Misu, H.; Sunagozaka, H.; Sakai, Y.; Yamashita, T.; et al. Beneficial effect of branched-chain amino acid supplementation on glycemic control in chronic hepatitis C patients with insulin resistance: Implications for type 2 diabetes. Metabolism 2012, 61, 1388–1394. [Google Scholar] [CrossRef]
- Fouré, A.; Bendahan, D. Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, H.; Li, L.; Chen, F.; Liu, Y.; Zhou, M.; Wang, J.; Jiang, J.; Li, X.; Fan, X.; et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing tropomodulin-3 propionylation in platelets. Circulation 2020, 142, 49–64. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Sugar profiling of honeys for authentication and detection of adulterants using high-performance thin layer chromatography. Molecules 2020, 25, 5289. [Google Scholar] [CrossRef]
- Cwiková, O.; Pavlíková, H.; Ansorgová, A. Detection of honey adulteration using HPLC method. Potravinarstvo 2015, 9, 388–392. [Google Scholar] [CrossRef][Green Version]
- Jiang, L.; Xie, M.; Chen, G.; Qiao, J.; Zhang, H.; Zeng, X. Phenolics and carbohydrates in buckwheat honey regulate the human intestinal microbiota. Evid.-Based Complement. Altern. Med. 2020, 2020, 6432942. [Google Scholar] [CrossRef]
- Amariei, S.; Norocel, L.; Scripcă, L.A. An innovative method for preventing honey crystallization. Innov. Food Sci. Emerg. Technol. 2020, 66, 102481. [Google Scholar] [CrossRef]
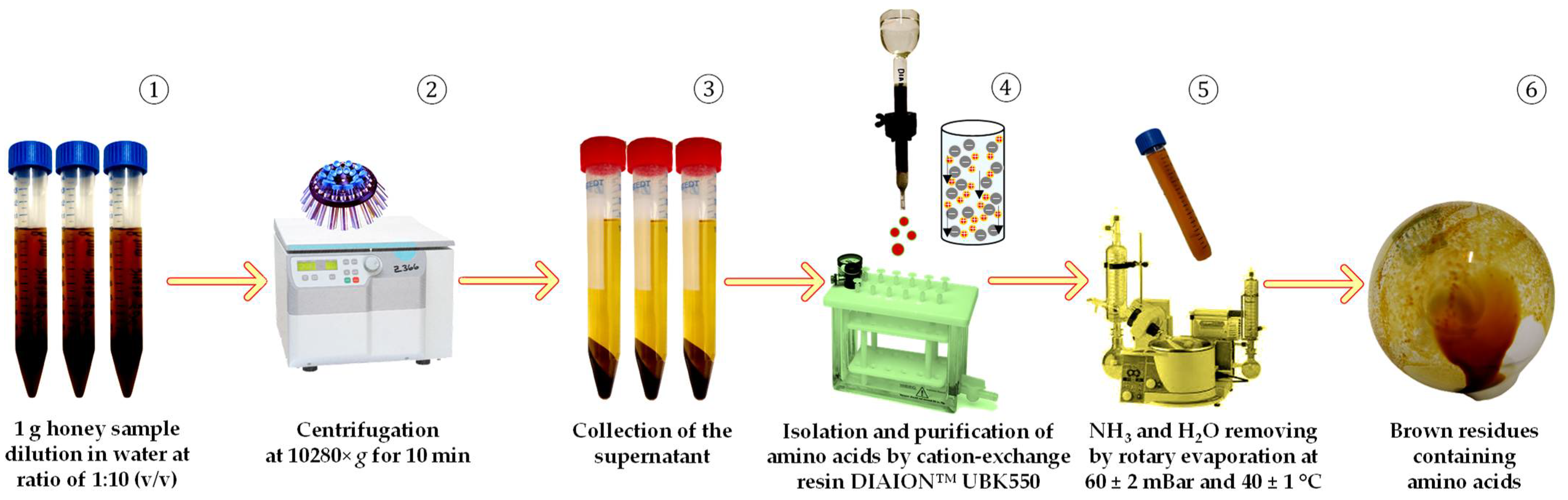
| Honey Type | Origin | Year of Collection | Hive Location |
|---|---|---|---|
| Polyfloral, bee | TJK | 2020 | Tajikistan, Baljuvon (38°18′30.0″ N 69°40′35.0″ E) |
| Polyfloral, bee | LV | 2020 | Latvia, Mazsalaca district, (57°51′47.7″ N 25°01′32.8″ E) |
| Polyfloral (forest), bee | LV | 2019 | Latvia, Liepaja district, Gramzda parish (56°22′00.8″ N 21°36′24.7″ E) |
| Buckwheat, bee | LV | 2019 | Latvia, Liepaja district, Gramzda parish (56°22′00.8″ N 21°36′24.7″ E) |
| Polyfloral, bumblebee | RU | 2021 | Russia, Artybash village (51°47′25.8″ N 87°15′13.7″ E) |
| Optimized MRM Parameters | Parameters of Calibration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acid | Rt, min | Molecular Formula | Ionization Mode | MRM Transitions | Q1 Pre Bias, V | Collision Energy, V | Q3 Pre Bias, V | Dwell Time, Msec | R2 | LOD, ng mL−1 | LOQ, ng mL−1 |
| Cysteine | 2.074 | C6H12N2O4S2 | [M + H]+ | 241.1000→74.0000 | 15.0 | 27.0 | 16.0 | 15.0 | 0.9988 | 5.25 | 15.95 |
| 241.1000→152.0500 | 15.0 | 15.0 | 18.0 | 15.0 | |||||||
| Aspartic acid | 2.107 | C4H7NO4 | [M + H]+ | 134.1000→73.9500 | 14.0 | 15.0 | 16.0 | 15.0 | 0.9992 | 3.50 | 10.60 |
| 134.1000→88.1000 | 20.0 | 11.0 | 20.0 | 15.0 | |||||||
| Serine | 2.113 | C3H7NO3 | [M + H]+ | 105.4000→60.0000 | 17.0 | 12.0 | 20.0 | 34.0 | 0.9989 | 13.79 | 41.78 |
| 105.4000→50.0000 | 18.0 | 19.0 | 21.0 | 34.0 | |||||||
| Glycine | 2.164 | C2H5NO2 | [M + H]+ | 76.3000→30.0500 | 11.0 | 14.0 | 25.0 | 34.0 | 0.9990 | 6.06 | 18.37 |
| Threonine | 2.190 | C4H9NO3 | [M + H]+ | 120.1000→74.0500 120.1000→56.1500 | 13.0 13.0 | 11.0 15.0 | 17.0 23.0 | 15.0 15.0 | 0.9995 | 4.35 | 13.17 |
| Alanine | 2.294 | C3H7NO2 | [M + H]+ | 90.0000→44.1000 90.0000→50.1000 | 10.0 9.0 | 12.0 15.0 | 19.0 21.0 | 34.0 34.0 | 0.9993 | 3.43 | 10.39 |
| Proline | 2.381 | C5H9NO2 | [M + H]+ | 116.0000→70.1500 116.0000→28.0500 | 17.0 16.0 | 16.0 39.0 | 15.0 12.0 | 15.0 15.0 | 0.9997 | 2.33 | 7.05 |
| Histidine | 2.551 | C6H9N3O2 | [M + H]+ | 156.1000→110.1000 156.1000→56.1000 | 22.0 23.0 | 15.0 32.0 | 13.0 13.0 | 15.0 15.0 | 0.9991 | 7.22 | 21.88 |
| Lysine | 2.741 | C6H14N2O2 | [M + H]+ | 147.1000→84.1000 147.1000→130.1000 | 16.0 15.0 | 18.0 24.0 | 19.0 15.0 | 34.0 34.0 | 0.9992 | 6.00 | 18.19 |
| Glutamic acid | 2.747 | C5H9NO4 | [M + H]+ | 147.4000→84.1000 147.4000→56.1000 | 16.0 17.0 | 17.0 30.0 | 19.0 24.0 | 15.0 15.0 | 0.9990 | 7.33 | 22.21 |
| Arginine | 2.930 | C6H14N4O2 | [M + H]+ | 175.1000→70.1000 175.1000→60.1000 | 20.0 19.0 | 23.0 14.0 | 15.0 13.0 | 15.0 15.0 | 0.9992 | 6.72 | 20.37 |
| Valine | 3.538 | C5H11NO2 | [M + H]+ | 118.1000→72.1500 118.1000→55.0500 | 18.0 17.0 | 12.0 22.0 | 15.0 12.0 | 34.0 34.0 | 0.9989 | 5.01 | 15.19 |
| Methionine | 3.900 | C5H11NO2S | [M + H]+ | 150.1000→56.0000 150.1000→104.1000 | 16.0 16.0 | 16.0 12.0 | 12.0 13.0 | 34.0 34.0 | 0.9989 | 5.19 | 15.73 |
| Tyrosine | 4.860 | C9H11NO3 | [M + H]+ | 182.1000→135.9000 182.1000→91.1000 | 27.0 28.0 | 13.0 28.0 | 16.0 21.0 | 34.0 34.0 | 0.9997 | 3.12 | 9.45 |
| Isoleucine | 6.461 | C6H13NO2 | [M + H]+ | 132.1000→86.1000 132.1000→69.0500 | 20.0 19.0 | 11.0 18.0 | 19.0 16.0 | 59.0 59.0 | 0.9992 | 4.79 | 14.52 |
| Leucine | 6.465 | C6H13NO2 | [M + H]+ | 132.3000→86.1500 132.1000→30.0500 | 16.0 20.0 | 12.0 17.0 | 19.0 15.0 | 59.0 59.0 | 0.9997 | 2.22 | 6.72 |
| Phenylalanine | 9.298 | C9H11NO2 | [M + H]+ | 166.1000→120.1000 166.1000→103.1000 | 25.0 18.0 | 14.0 26.0 | 14.0 13.0 | 59.0 59.0 | 0.9996 | 1.48 | 4.48 |
| Tryptophan | 11.037 | C11H12N2O2 | [M + H]+ | 205.0000→188.1500 205.0000→146.1000 | 14.0 22.0 | 11.0 20.0 | 15.0 17.0 | 90.0 90.0 | 0.9998 | 0.04 | 0.14 |
| Amino Acid | Mw | Average Concentration, mg 100 g−1 FW | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Aspartic acid | 133.10 | 0.07 ± 0.00 d | 0.17 ± 0.02 c | 0.17 ± 0.00 c | 0.31 ± 0.00 b | 2.46 ± 0.17 a |
| Cysteine | 240.30 | <LOQ | <LOQ | <LOQ | 0.04 ± 0.00 | <LOQ |
| Serine | 105.09 | 0.16 ± 0.01 e | 0.18 ± 0.08 d | 0.26 ± 0.02 c | 1.29 ± 0.04 a | 0.89 ± 0.03 b |
| Threonine | 119.12 | 0.10 ± 0.00 d | 0.12 ± 0.00 d | 0.16 ± 0.00 c | 1.08 ± 0.02 a | 0.42 ± 0.02 b |
| Glycine | 75.07 | 0.13 ± 0.01 c | 0.13 ± 0.00 c | 0.14 ± 0.01 c | 0.63 ± 0.02 a | 0.30 ± 0.01 b |
| Proline | 115.13 | 26.50 ± 2.97 c | 16.50 ± 4.92 e | 24.38 ± 0.42 d | 41.02 ± 0.23 b | 44.41 ± 0.02 a |
| Alanine | 89.09 | 0.24 ± 0.26 e | 0.55 ± 0.01 d | 0.59 ± 0.01 c | 1.07 ± 0.01 a | 0.74 ± 0.02 b |
| Histidine | 155.15 | 0.14 ± 0.04 a,b | 0.03 ± 0.01 c | 0.11 ± 0.01 b | 0.15 ± 0.01 a | 0.15 ± 0.00 a |
| Lysine | 146.19 | 0.52 ± 0.17 b | 0.29 ± 0.02 c | 0.51 ± 0.02 b | 0.68 ± 0.01 a | 0.12 ± 0.05 d |
| Glutamic acid | 147.13 | 0.51 ± 0.14 b | 0.27 ± 0.02 d | 0.47 ± 0.03 c | 0.65 ± 0.02 a | 0.13 ± 0.06 e |
| Arginine | 174.20 | 0.10 ± 0.03 d | 0.10 ± 0.00 d | 0.27 ± 0.01 b | 0.19 ± 0.01 c | 0.78 ± 0.07 a |
| Valine | 117.15 | 0.39 ± 0.00 d | 0.35 ± 0.03 e | 0.45 ± 0.06 c | 6.30 ± 0.57 a | 1.21 ± 0.02 b |
| Methionine | 149.21 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Tyrosine | 181.19 | 3.31 ± 0.07 b | 0.41 ± 0.04 e | 0.60 ± 0.01 d | 15.73 ± 0.32 a | 0.73 ± 0.23 c |
| Leucine | 131.17 | 0.43 ± 0.01 c | 0.36 ± 0.01 d | 0.38 ± 0.04 d | 12.52 ± 0.10 a | 0.96 ± 0.08 b |
| Isoleucine | 131.17 | 0.45 ± 0.02 c | 0.36 ± 0.04 d | 0.45 ± 0.01 c | 12.31 ± 0.83 a | 1.00 ± 0.08 b |
| Phenylalanine | 165.19 | 7.14 ± 0.42 a | 2.87 ± 0.06 d | 4.78 ± 0.05 b | 4.31 ± 0.03 b | 0.84 ± 0.05 c |
| Tryptophan | 204.23 | 0.00 ± 0.00 | 0.00 ±0.00 | 0.01 ± 0.00 a | 0.00 ± 0.00 | 0.02 ± 0.00 a |
| ∑BCAA | − | 1.27 ± 0.03 c | 1.08 ± 0.08 d | 1.28 ± 0.11 c | 31.13 ± 1.50 a | 3.17 ± 0.18 b |
| ∑total | − | 40.16 ± 4.19 c | 22.69 ± 5.26 e | 33.73 ± 0.70 d | 98.28 ± 2.22 a | 55.16 ± 0.91 b |
| Sugar | RT, min | Average Concentration, g 100 g−1 FW | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Fructose | 7.62 | 36.63 ± 0.23 a | 35.20 ± 2.90 b | 33.05 ± 0.85 c | 36.05 ± 1.16 a | 32.83 ± 1.10 c |
| Glucose | 8.454 | 28.11 ± 0.10 a | 24.74 ± 3.08 b | 24.92 ± 0.64 b | 27.54 ± 0.63 a | 24.05 ± 0.79 b |
| Sucrose | 11.551 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Unknown 1 | 12.447 | 1.49 ± 0.03 a | 1.57 ± 0.12 a | 1.56 ± 0.07 a | 1.26 ± 0.02 b | 1.61 ± 0.04 a |
| Unknown 2 | 13.935 | 1.47 ± 0.16 b | 2.34 ± 0.31 a | 2.32 ± 0.02 a | 0.88 ± 0.04 c | 1.46 ± 0.03 b |
| Unknown 3 | 13.884 | 0.84 ± 0.03 c | 1.60 ± 0.13 a | 1.68 ± 0.01 a | 0.86± 0.07 c | 1.46 ± 0.03 b |
| Unknown 4 | 15.286 | 0.86 ± 0.00 c | 1.25 ± 0.19 b | 1.45 ± 0.07 a | n.d. | 1.24 ± 0.02 b |
| ∑total | − | 69.40 ± 0.55 a | 66.70 ± 6.73 b | 64.98 ± 1.66 c | 66.59 ± 1.92 b | 62.65 ± 2.01 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimins, F.; Cinkmanis, I.; Radenkovs, V.; Augspole, I.; Valdovska, A. Analysis of 18 Free Amino Acids in Honeybee and Bumblebee Honey from Eastern and Northern Europe and Central Asia Using HPLC-ESI-TQ-MS/MS Approach Bypassing Derivatization Step. Foods 2022, 11, 2744. https://doi.org/10.3390/foods11182744
Dimins F, Cinkmanis I, Radenkovs V, Augspole I, Valdovska A. Analysis of 18 Free Amino Acids in Honeybee and Bumblebee Honey from Eastern and Northern Europe and Central Asia Using HPLC-ESI-TQ-MS/MS Approach Bypassing Derivatization Step. Foods. 2022; 11(18):2744. https://doi.org/10.3390/foods11182744
Chicago/Turabian StyleDimins, Fredijs, Ingmars Cinkmanis, Vitalijs Radenkovs, Ingrida Augspole, and Anda Valdovska. 2022. "Analysis of 18 Free Amino Acids in Honeybee and Bumblebee Honey from Eastern and Northern Europe and Central Asia Using HPLC-ESI-TQ-MS/MS Approach Bypassing Derivatization Step" Foods 11, no. 18: 2744. https://doi.org/10.3390/foods11182744
APA StyleDimins, F., Cinkmanis, I., Radenkovs, V., Augspole, I., & Valdovska, A. (2022). Analysis of 18 Free Amino Acids in Honeybee and Bumblebee Honey from Eastern and Northern Europe and Central Asia Using HPLC-ESI-TQ-MS/MS Approach Bypassing Derivatization Step. Foods, 11(18), 2744. https://doi.org/10.3390/foods11182744








