Integrated Strategy for Informative Profiling and Accurate Quantification of Key-Volatiles in Dried Fruits and Nuts: An Industrial Quality Control Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. SPME Devices and ISs Pre-Loading Conditions
2.4. HS-SPME Sampling Conditions: VOCs Profiling
- -
- Average % relative standard deviation (RSD) on analytes’ responses: used to evaluate the repeatability and calculated on all targets for the two replicates of the average point. A %RSD value <20 is usually considered acceptable;
- -
- % Explained variance: usually considered satisfactory if ≥80%, it expresses the fraction of the total variation in response that the model can explain. It is correlated to residuals, showing how each experimental data fits its theoretical position in the model projection [36];
- -
- Coefficient significance: evaluated based on its value and sign, indicating a direct or inverse correlation with the chosen response. This parameter is accompanied by the confidence interval.
2.5. MHS-SPME Principles and Conditions
- exhaustive extraction of target volatiles from calibration standards or certified material covering the actual range of concentrations/amounts of real samples;
- application of the procedure to samples of interest.
2.6. GC-MS System Set-Up and Analytical Conditions
2.7. Data Acquisition and Data Processing
3. Results and Discussion
3.1. Qualitative vs. Quantitative Profiling of Volatiles: Considerations
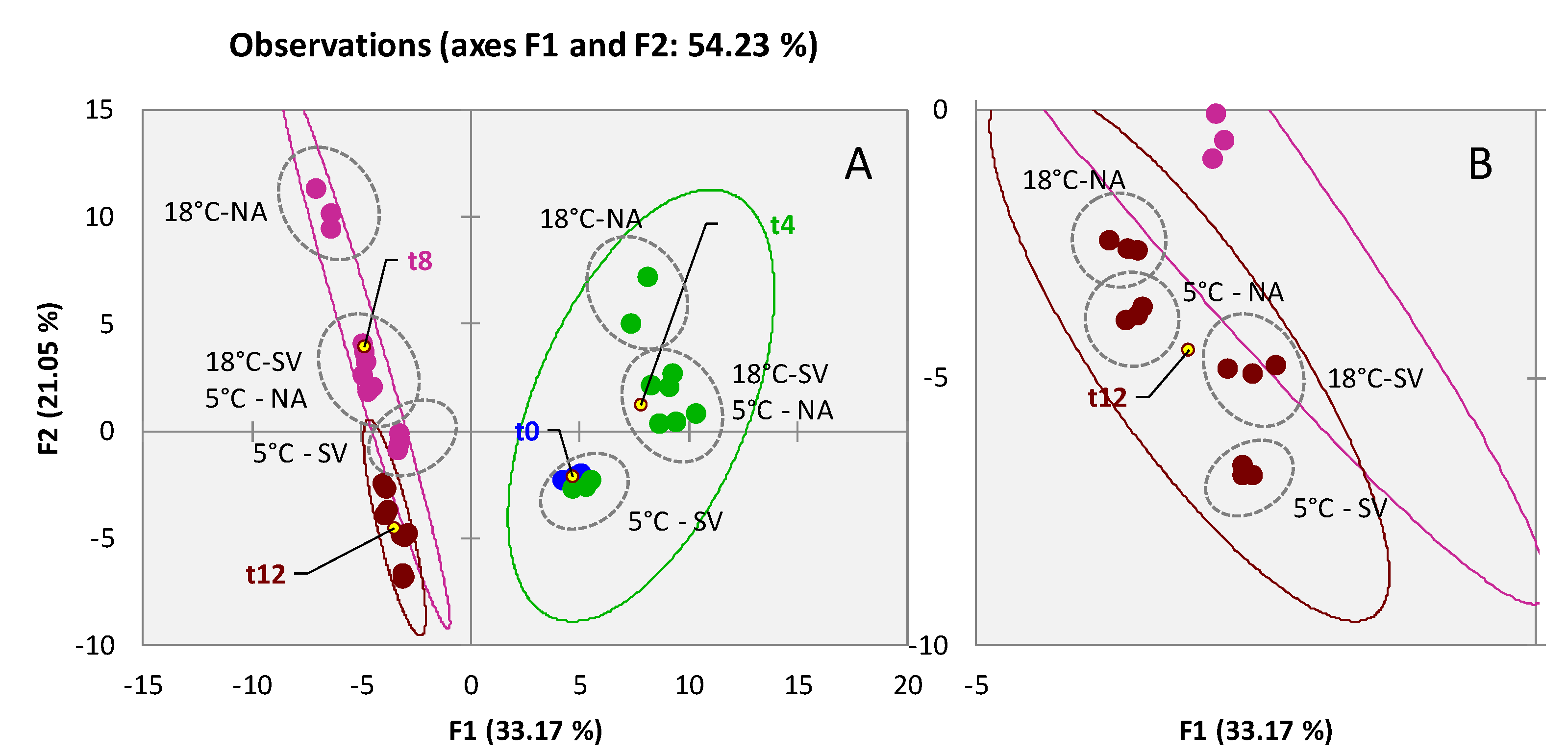
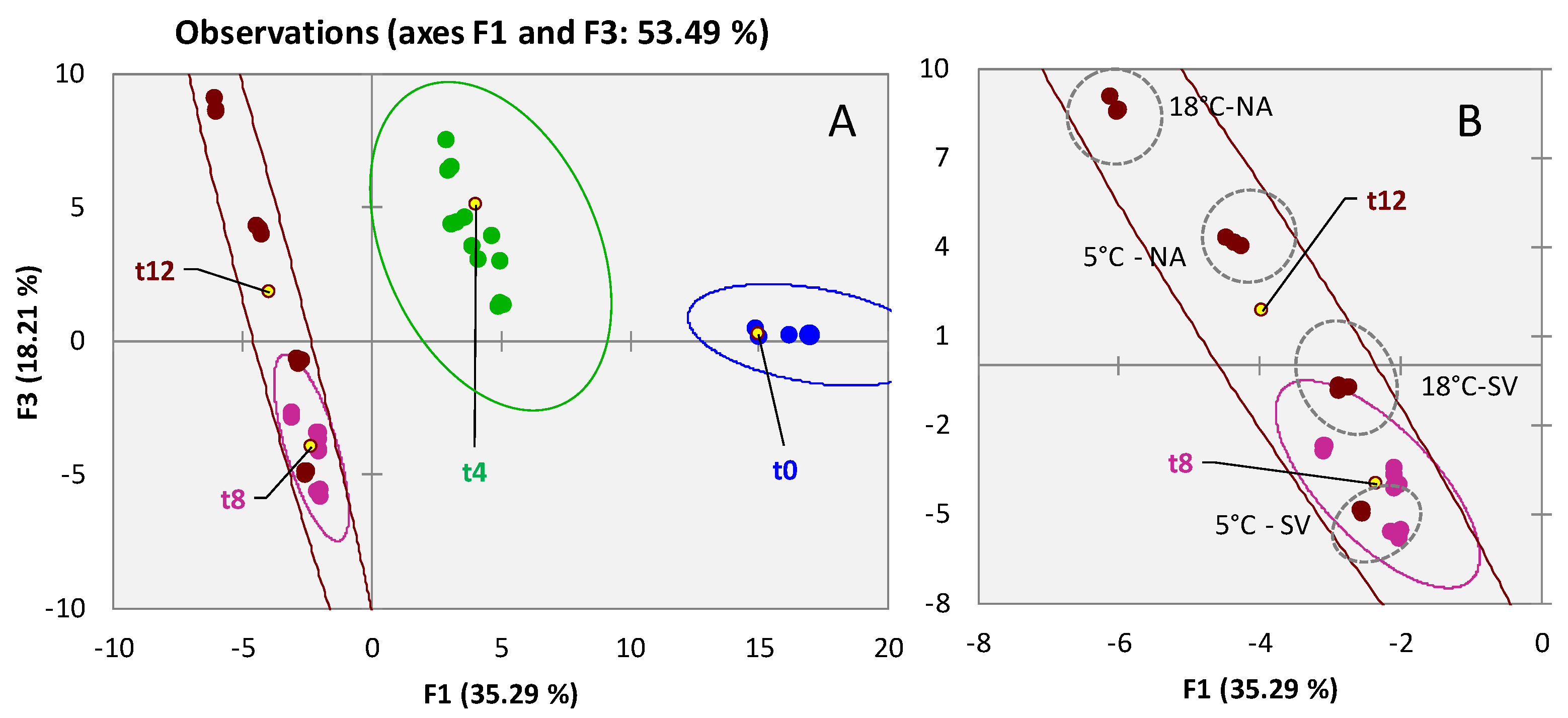
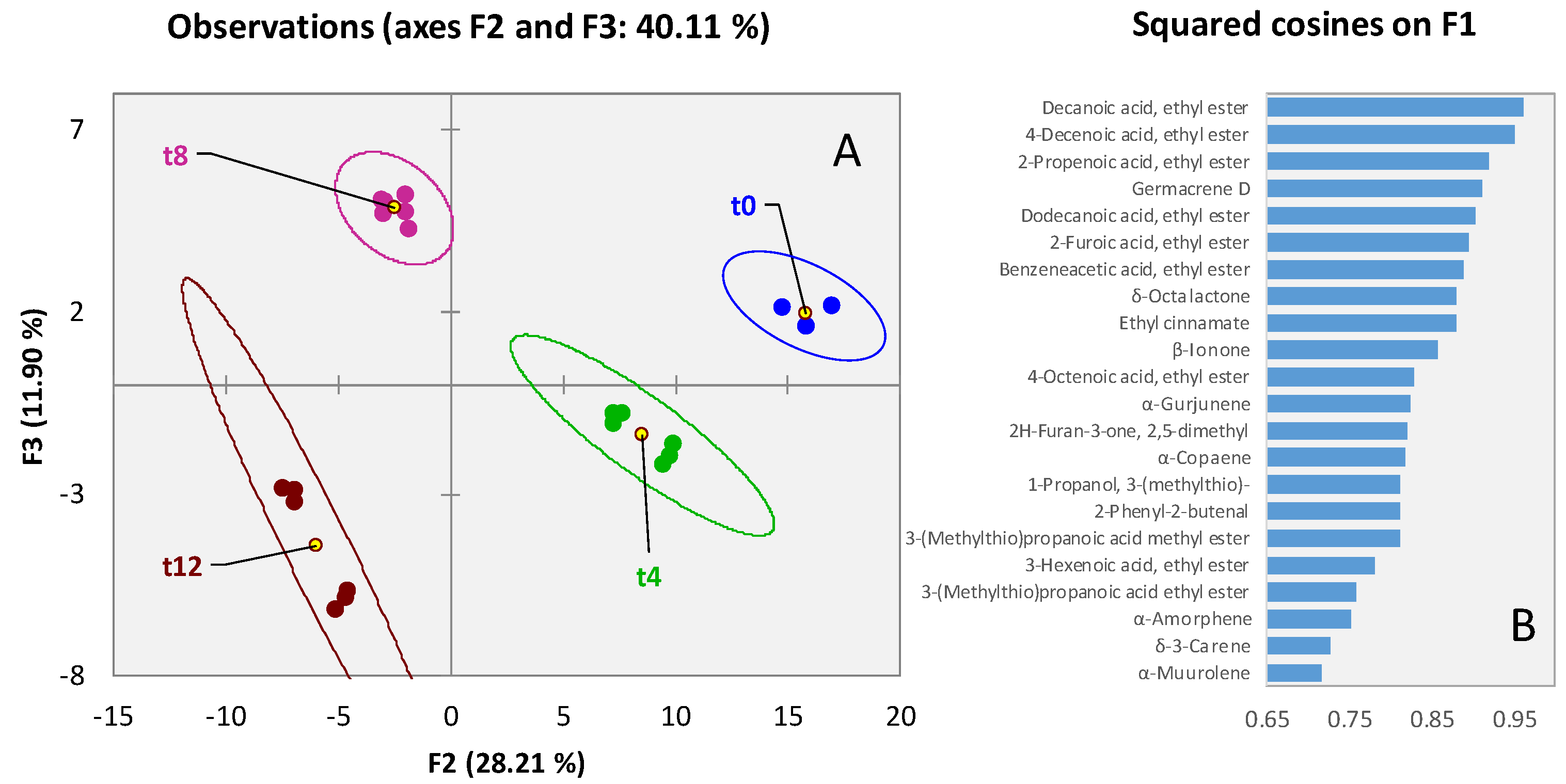
3.2. Qualitative Profiling of Walnut (Juglans regia L. var. Chandler) Volatiles within Shelf-Life
3.3. Qualitative Profiling of Almond (Prunus dulcis (Mill) var. Aldrich) Volatiles within Shelf-Life
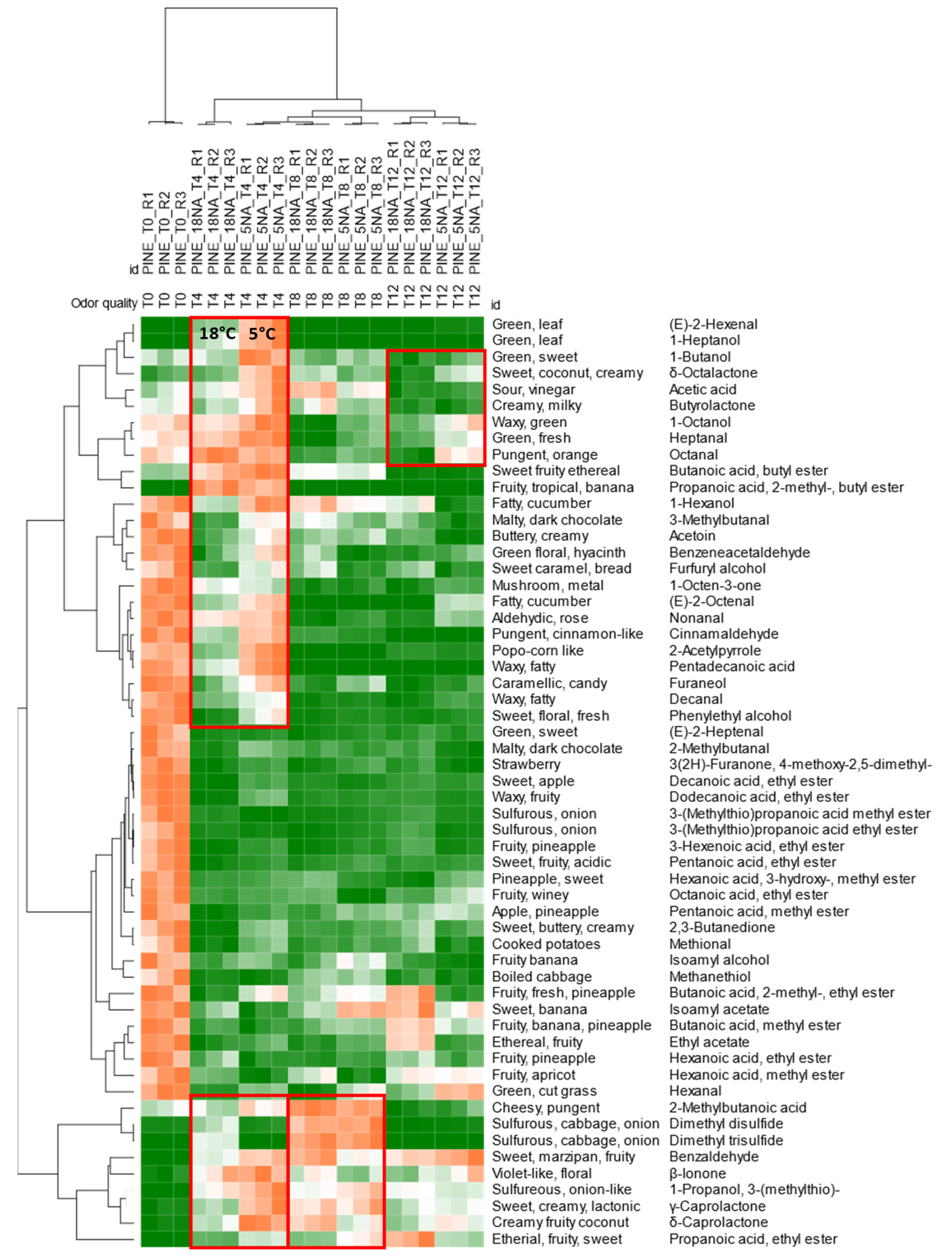
3.4. Qualitative Profiling of Dried Pineapple (Ananas comosus) Volatiles within Shelf-Life
3.5. Quantitative Profiling of Secondary Products of Lipid Oxidation within Shelf-Life
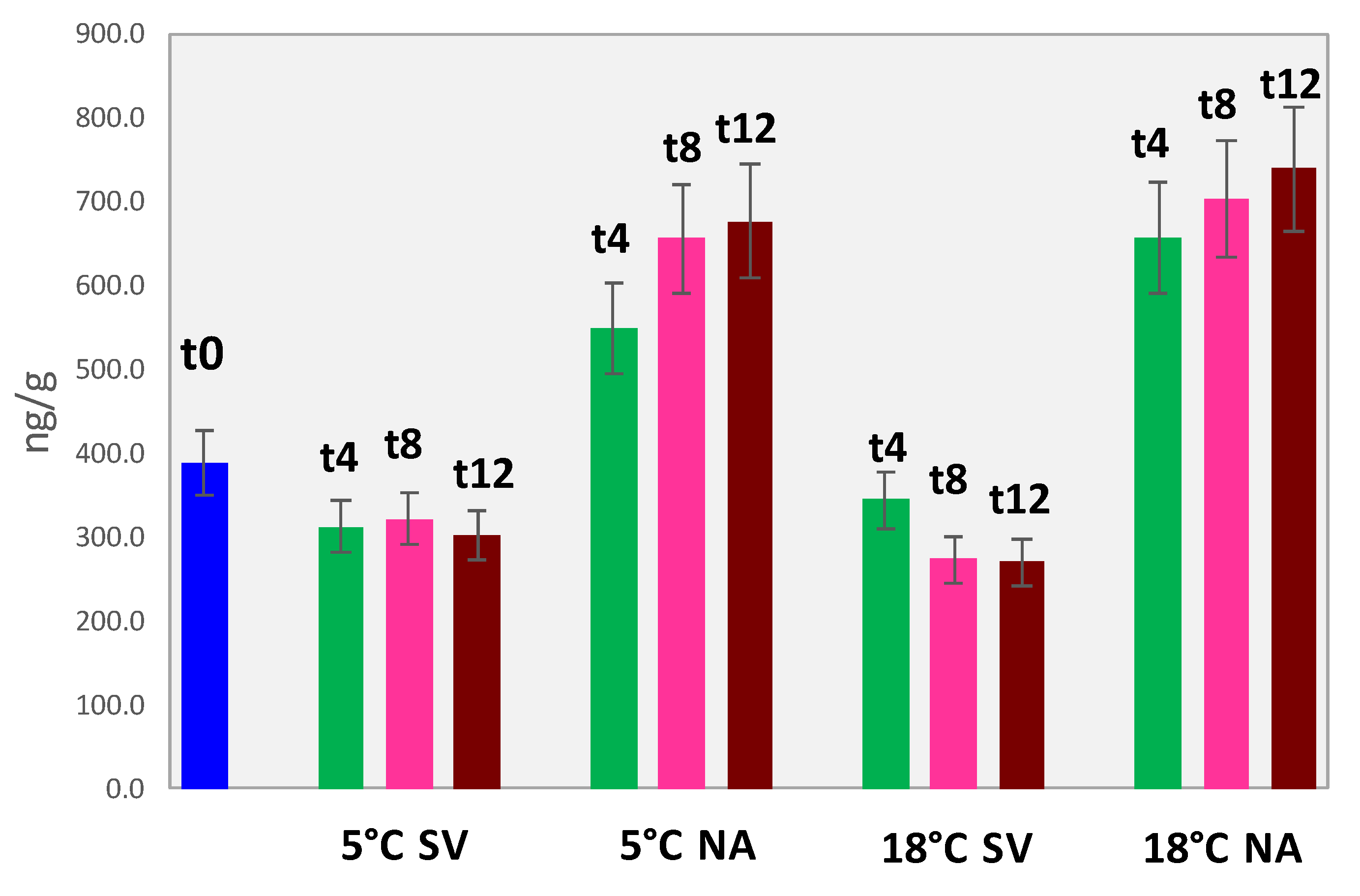
3.5.1. Accurate Quantification of Volatile Lipid Oxidation Products in Walnuts
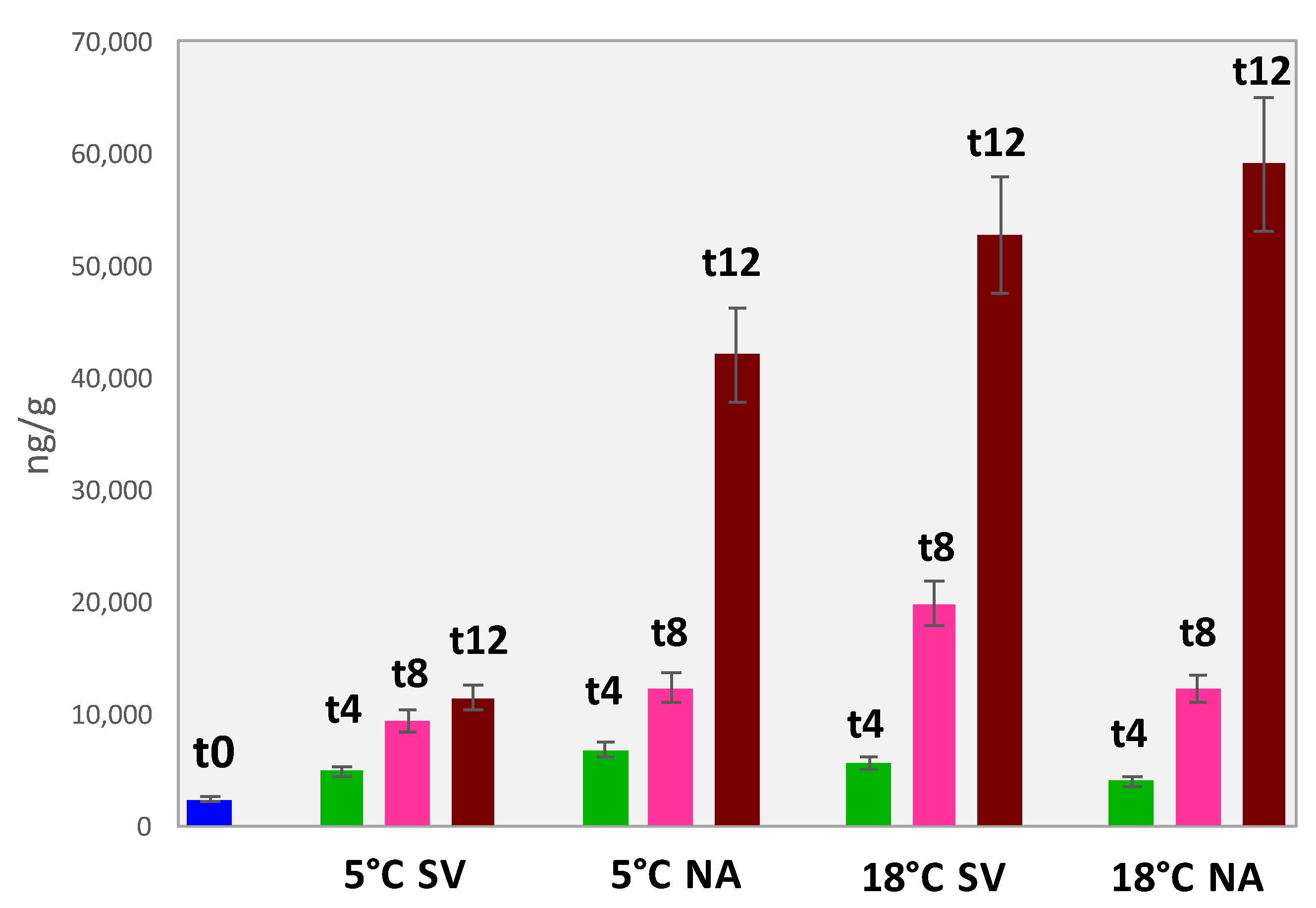
3.5.2. Accurate Quantification of Volatile Lipid Oxidation Products in Almonds
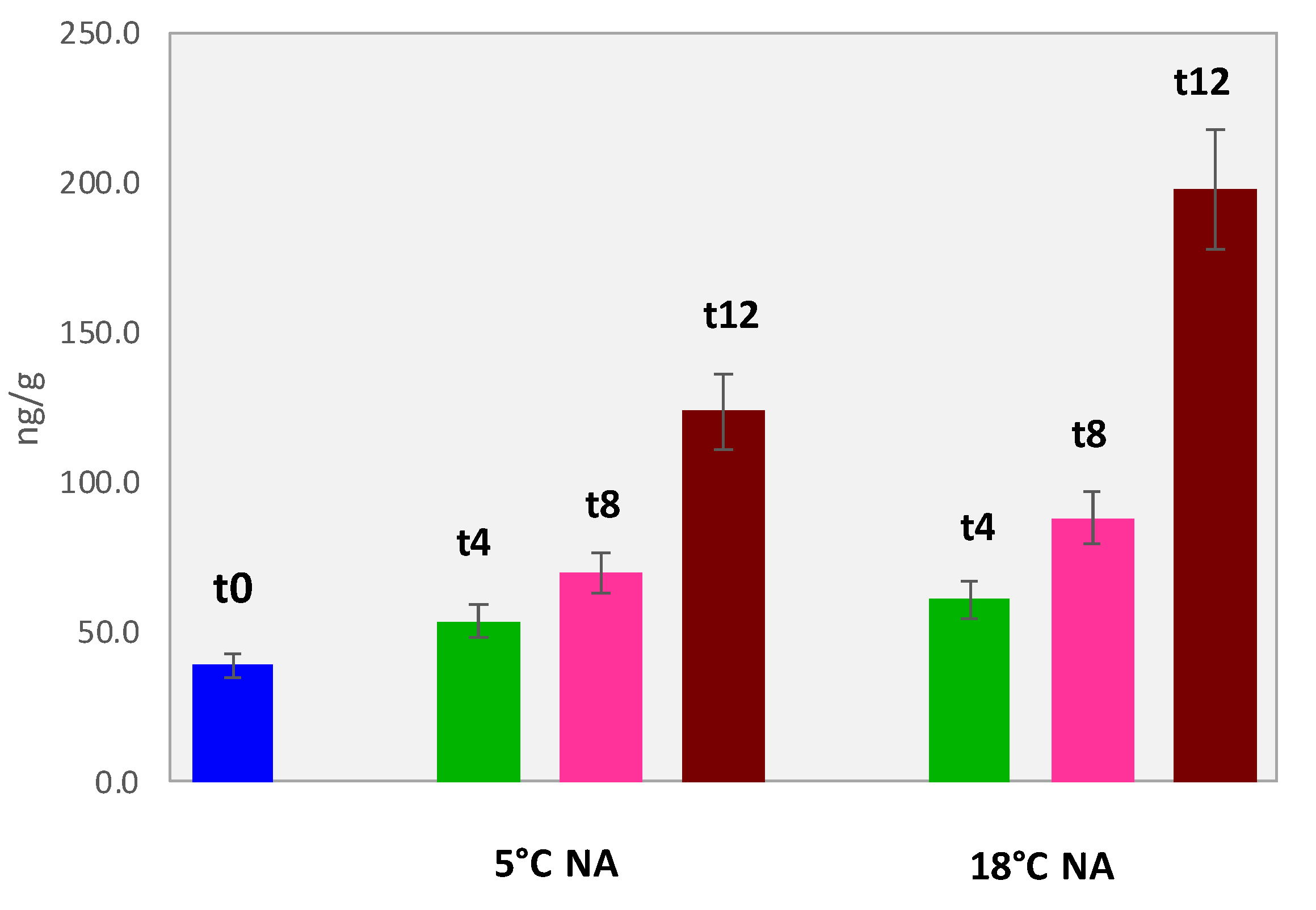
3.5.3. Accurate Quantification of Volatile Lipid Oxidation Products in Dried Pineapples
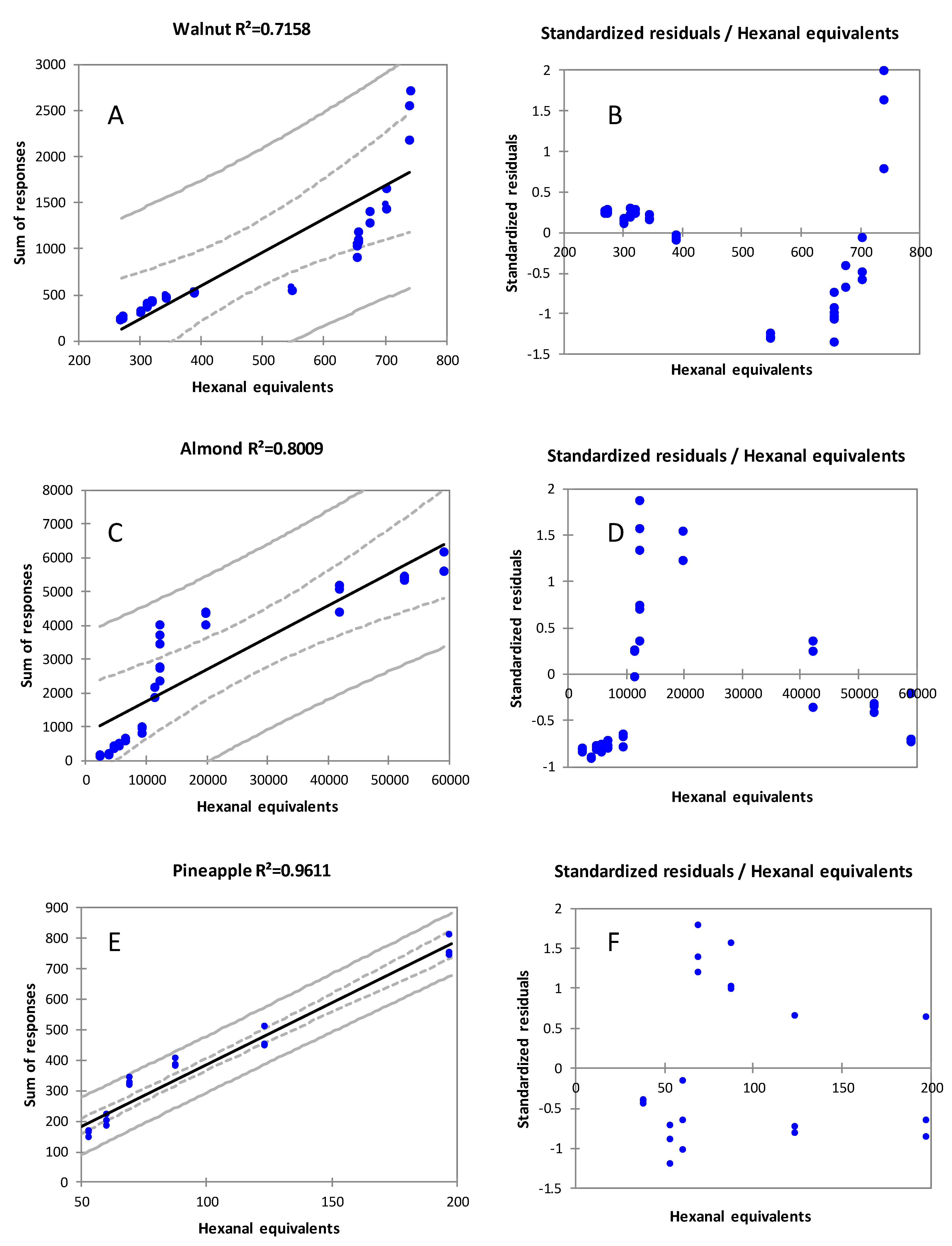
3.6. Quantification Error with Headspace Saturation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gama, T.; Wallace, H.M.; Trueman, S.J.; Hosseini-Bai, S. Quality and shelf life of tree nuts: A review. Sci. Hortic. 2018, 242, 116–126. [Google Scholar] [CrossRef]
- EUROSTAT. Exporting Edible Nuts and Dried Fruits to Europe; CBI: The Hague, The Netherlands, 2017.
- Hernández-Alonso, P.; Camacho-Barcia, L.; Bulló, M.; Salas-Salvadó, J. Nuts and Dried Fruits: An Update of Their Beneficial Effects on Type 2 Diabetes. Nutrients 2017, 9, 673. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Unterweger, H.; Buchbauer, G.; Krist, S. Characterization of volatile compounds and triacylglycerol profiles of nut oils using SPME-GC-MS and MALDI-TOF-MS. Eur. J. Lipid Sci. Technol. 2009, 111, 170–182. [Google Scholar] [CrossRef]
- McNeil, D.; Jackson, D.; Morley-Bunker, M. Edible nuts. In Temperate and Subtropical Fruit Production; CABI: Wallingford, UK, 2017; pp. 294–316. ISBN 9251037485. [Google Scholar]
- KS 2786:2018; The Kenya Bureau of Standards notified the World Trade Organization of the “KS 2786:2018 Dried fruits–Specification”. International Nut and Dried Fruit Council: Reus, Spain, 2018.
- Standard DDP-02; Walnut Kernels-2010 2 NOTE Working Party on Agricultural Quality Standards. United Nations: Geneva, Switzerland, 2002.
- USDA-AMS. United States Standards for Grades of Shelled Walnuts; USDA: Washington, DC, USA, 1968; p. 6.
- USDA-AMS. United States Standards for Grades of Shelled Almonds; USDA: Washington, DC, USA, 1997; p. 7.
- United Nations. Unece Standard Ffv-35; United Nations: Geneva, Switzerland, 2011; p. 7.
- Squara, S.; Stilo, F.; Cialiè Rosso, M.; Liberto, E.; Spigolon, N.; Genova, G.; Castello, G.; Bicchi, C.; Cordero, C. Corylus avellana L. Aroma Blueprint: Potent Odorants Signatures in the Volatilome of High Quality Hazelnuts. Front. Plant Sci. 2022, 13, 1–25. [Google Scholar] [CrossRef]
- Stilo, F.; Cialiè Rosso, M.; Squara, S.; Bicchi, C.; Cordero, C.; Cagliero, C. Corylus avellana L. Natural Signature: Chiral Recognition of Selected Informative Components in the Volatilome of High-Quality Hazelnuts. Front. Plant Sci. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Cialiè Rosso, M.; Stilo, F.; Mascrez, S.; Bicchi, C.; Purcaro, G.; Cordero, C. Shelf-Life Evolution of the Fatty Acid Fingerprint in High-Quality Hazelnuts (Corylus avellana L.) Harvested in Different Geographical Regions. Foods 2021, 10, 685. [Google Scholar] [CrossRef]
- Cialiè Rosso, M.; Liberto, E.; Spigolon, N.; Fontana, M.; Somenzi, M.; Bicchi, C.; Cordero, C. Evolution of potent odorants within the volatile metabolome of high-quality hazelnuts (Corylus avellana L.): Evaluation by comprehensive two-dimensional gas chromatography coupled with mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 3491–3506. [Google Scholar] [CrossRef]
- Seyhan, F.; Ozay, G.; Saklar, S.; Ertaş, E.; Satir, G.; Alasalvar, C. Chemical changes of three native Turkish hazelnut varieties (Corylus avellana L.) during fruit development. Food Chem. 2007, 105, 590–596. [Google Scholar] [CrossRef]
- Kinderlerer, J.L.; Johnson, S. Rancidity in hazelnuts due to volatile aliphatic aldehydes. J. Sci. Food Agric. 1992, 58, 89–93. [Google Scholar] [CrossRef]
- Giraudo, A.; Calvini, R.; Orlandi, G.; Ulrici, A.; Geobaldo, F.; Savorani, F. Development of an automated method for the identification of defective hazelnuts based on RGB image analysis and colourgrams. Food Control 2018, 94, 233–240. [Google Scholar] [CrossRef]
- Stilo, F.; Liberto, E.; Spigolon, N.; Genova, G.; Rosso, G.; Fontana, M.; Reichenbach, S.E.; Bicchi, C.; Cordero, C. An effective chromatographic fingerprinting workflow based on comprehensive two-dimensional gas chromatography—Mass spectrometry to establish volatiles patterns discriminative of spoiled hazelnuts (Corylus avellana L.). Food Chem. 2021, 340, 128135. [Google Scholar] [CrossRef] [PubMed]
- Mehlenbacher, S.A.; Smith, D.C.; Brenner, L.K. Variance Components and Heritability of Nut and Kernel Defects in Hazelnut. Plant Breed. 1993, 110, 144–152. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Maillard reaction and caramelization during hazelnut roasting: A multiresponse kinetic study. Food Chem. 2017, 221, 1911–1922. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Belviso, S.; Dal Bello, B.; Giacosa, S.; Bertolino, M.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Chemical, mechanical and sensory monitoring of hot air- and infrared-roasted hazelnuts (Corylus avellana L.) during nine months of storage. Food Chem. 2017, 217, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Coisson, J.D.; Travaglia, F.; Acquotti, D.; Palla, G.; Palla, L.; Arlorio, M. Application of 1H NMR for the characterisation and authentication of “Tonda Gentile Trilobata” hazelnuts from Piedmont (Italy). Food Chem. 2014, 148, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Standard DDP-04; United Nations Economic Commission for Europe Marketing and Commercial Quality Standard of Hazelnut Kernels. United Nations: Geneva, Switzerland, 2011.
- Ghirardello, D.; Zeppa, G.; Rolle, L.; Gerbi, V.; Contessa, C.; Valentini, N.; Botta, R.; Griseri, G. Effect of different storage conditions on hazelnut quality. Acta Hortic. 2014, 1052, 315–318. [Google Scholar] [CrossRef]
- Locatelli, M.; Coïsson, J.D.; Travaglia, F.; Cereti, E.; Garino, C.; D’Andrea, M.; Martelli, A.; Arlorio, M. Chemotype and genotype chemometrical evaluation applied to authentication and traceability of “tonda Gentile Trilobata” hazelnuts from Piedmont (Italy). Food Chem. 2011, 129, 1865–1873. [Google Scholar] [CrossRef]
- Squara, S.; Caratti, A.; Gavilan, F.O.; Bolzoni, P.; Spigolon, N.; Genova, G.; Castello, G.; González, M.G.B.; Cuadros-Rodriguez, L.; Bicchi, C.; et al. Validation of a high-throughput method for the accurate quantification of secondary products of lipid oxidation in high-quality hazelnuts (Corylus avellana L.): A robust tool for quality assessment. J. Food Compos. Anal. 2022, 114, 104766. [Google Scholar] [CrossRef]
- Ghirardello, D.; Contessa, C.; Valentini, N.; Zeppa, G.; Rolle, L.; Gerbi, V.; Botta, R. Effect of storage conditions on chemical and physical characteristics of hazelnut (Corylus avellana L.). Postharvest Biol. Technol. 2013, 81, 37–43. [Google Scholar] [CrossRef]
- AOCS AOCS Standard Procedure Cd 12c-16. Off. Methods Recom. Pract. AOCS 2017.
- Pollner, G.; Schieberle, P. Characterization of the Key Odorants in Commercial Cold-Pressed Oils from Unpeeled and Peeled Rapeseeds by the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Kiefl, J.; Schieberle, P.; Reichenbach, S.E.; Bicchi, C. Comprehensive two-dimensional gas chromatography and food sensory properties: Potential and challenges. Anal. Bioanal. Chem. 2015, 407, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Stilo, F.; Bicchi, C.; Reichenbach, S.E.; Cordero, C. Comprehensive two-dimensional gas chromatography as a boosting technology in food-omic investigations. J. Sep. Sci. 2021, 44, 1592–1611. [Google Scholar] [CrossRef] [PubMed]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39. [Google Scholar] [CrossRef]
- Cordero, C.; Kiefl, J.; Reichenbach, S.E.; Bicchi, C. Characterization of odorant patterns by comprehensive two-dimensional gas chromatography: A challenge in omic studies. TrAC Trends Anal. Chem. 2019, 113, 364–378. [Google Scholar] [CrossRef]
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, Å.; Pettersen, J.; Bergman, R. Experimental design and optimization. Chemom. Intell. Lab. Syst. 1998, 42, 3–40. [Google Scholar] [CrossRef]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography: Theory and Practice; Wiley-VCH: New York, NY, USA, 2006; ISBN 0471914568. [Google Scholar]
- Cordero, C.; Guglielmetti, A.; Bicchi, C.; Liberto, E.; Baroux, L.; Merle, P.; Tao, Q.; Reichenbach, S.E. Comprehensive two-dimensional gas chromatography coupled with time of flight mass spectrometry featuring tandem ionization: Challenges and opportunities for accurate fingerprinting studies. J. Chromatogr. A 2019, 1597, 132–141. [Google Scholar] [CrossRef]
- Sgorbini, B.; Cagliero, C.; Liberto, E.; Rubiolo, P.; Bicchi, C.; Cordero, C. Strategies for Accurate Quantitation of Volatiles from Foods and Plant-Origin Materials: A Challenging Task. J. Agric. Food Chem. 2019, 67, 1619–1630. [Google Scholar] [CrossRef]
- Nicolotti, L.; Cordero, C.; Cagliero, C.; Liberto, E.; Sgorbini, B.; Rubiolo, P.; Bicchi, C. Quantitative fingerprinting by headspace-Two-dimensional comprehensive gas chromatography-mass spectrometry of solid matrices: Some challenging aspects of the exhaustive assessment of food volatiles. Anal. Chim. Acta 2013, 798, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Ettre, L.S. Theory and practice of multiple headspace extraction. Chromatographia 1991, 32, 505–513. [Google Scholar] [CrossRef]
- Cordero, C.; Zebelo, S.A.; Gnavi, G.; Griglione, A.; Bicchi, C.; Maffei, M.E.; Rubiolo, P. HS-SPME-GC×GC-qMS volatile metabolite profiling of Chrysolina herbacea frass and Mentha spp. leaves. Anal. Bioanal. Chem. 2012, 402, 3017–3018. [Google Scholar] [CrossRef][Green Version]
- Bicchi, C.; Maffei, M. The Plant Volatilome: Methods of Analysis. In Methods in Molecular Biology; Normanly, J., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 918, pp. 289–310. ISBN 978-1-61779-994-5. [Google Scholar]
- Stilo, F.; Borrego, M.d.P.S.; Bicchi, C.; Battaglino, S.; Fernadez, R.M.C.; Morales, M.L.; Reichenbach, S.E.; Mc Curry, J.; Peroni, D.; Cordero, C. Delineating the extra-virgin olive oil aroma blueprint by multiple headspace solid phase microextraction and differential-flow modulated comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2021, 1650, 462232. [Google Scholar] [CrossRef]
- Stilo, F.; Bicchi, C.; Robbat, A.; Reichenbach, S.E.; Cordero, C. Untargeted approaches in food-omics: The potential of comprehensive two-dimensional gas chromatography/mass spectrometry. TrAC Trends Anal. Chem. 2021, 135, 116162. [Google Scholar] [CrossRef]
- Risticevic, S.; Vuckovic, D.; Lord, H.L.; Pawliszyn, J. Solid-Phase Microextraction. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 419–460. ISBN 9780123813749. [Google Scholar]
- Lord, H.L.; Pfannkoch, E.A. Sample Preparation Automation for Gc Injection; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, ISBN 9780123813749. [Google Scholar]
- Ross, C.F. 2.02—Headspace Analysis; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, ISBN 9780123813732. [Google Scholar]
- Yang, C.; Wang, J.; Li, D. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: A review. Anal. Chim. Acta 2013, 799, 8–22. [Google Scholar] [CrossRef]
- Cordero, C.; Schmarr, H.G.; Reichenbach, S.E.; Bicchi, C. Current Developments in Analyzing Food Volatiles by Multidimensional Gas Chromatographic Techniques. J. Agric. Food Chem. 2018, 66, 2226–2236. [Google Scholar] [CrossRef]
- Jiang, R.; Xu, J.; Lin, W.; Wen, S.; Zhu, F.; Luan, T.; Ouyang, G. Investigation of the kinetic process of solid phase microextraction in complex sample. Anal. Chim. Acta 2015, 900, 111–116. [Google Scholar] [CrossRef]
- Brevard, H.; Cantergiani, E.; Cachet, T.; Chaintreau, A.; Demyttenaere, J.; French, L.; Gassenmeier, K.; Joulain, D.; Koenig, T.; Leijs, H.; et al. Guidelines for the quantitative gas chromatography of volatile flavouring substances, from the Working Group on Methods of Analysis of the International Organization of the Flavor Industry (IOFI). Flavour Fragr. J. 2011, 26, 297–299. [Google Scholar] [CrossRef]
- Casadei, E.; Valli, E.; Aparicio-Ruiz, R.; Ortiz-Romero, C.; García-González, D.L.; Vichi, S.; Quintanilla-Casas, B.; Tres, A.; Bendini, A.; Toschi, T.G. Peer inter-laboratory validation study of a harmonized SPME-GC-FID method for the analysis of selected volatile compounds in virgin olive oils. Food Control. 2021, 123, 107823. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; López-Tamames, E. Solid-Phase Microextraction in the Analysis of Virgin Olive Oil Volatile Fraction: Characterization of Virgin Olive Oils from Two Distinct Geographical Areas of Northern Italy. J. Agric. Food Chem. 2003, 51, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Bicchi, C.; Ruosi, M.R.; Cagliero, C.; Cordero, C.; Liberto, E.; Rubiolo, P.; Sgorbini, B. Quantitative analysis of volatiles from solid matrices of vegetable origin by high concentration capacity headspace techniques: Determination of furan in roasted coffee. J. Chromatogr. A 2011, 1218, 753–762. [Google Scholar] [CrossRef]
- Sgorbini, B.; Bicchi, C.; Cagliero, C.; Cordero, C.; Liberto, E.; Rubiolo, P. Herbs and spices: Characterization and quantitation of biologically-active markers for routine quality control by multiple headspace solid-phase microextraction combined with separative or non-separative analysis. J. Chromatogr. A 2015, 1376, 9–17. [Google Scholar] [CrossRef]
- Schieberle, P.; Hofmann, T. Mapping the combinatorial code of food flavors by means of molecular sensory science approach. In Food Flavors: Chemical, Sensory and Technological Properties; Informa UK Limited: London, UK, 2011; pp. 413–438. ISBN 9781439814925. [Google Scholar]
- Cordero, C.; Guglielmetti, A.; Sgorbini, B.; Bicchi, C.; Allegrucci, E.; Gobino, G.; Baroux, L.; Merle, P. Odorants quantitation in high-quality cocoa by multiple headspace solid phase micro-extraction: Adoption of FID-predicted response factors to extend method capabilities and information potential. Anal. Chim. Acta 2019, 1052, 190–201. [Google Scholar] [CrossRef]
- Costa, R.; Tedone, L.; De Grazia, S.; Dugo, P.; Mondello, L. Multiple headspace-solid-phase microextraction: An application to quantification of mushroom volatiles. Anal. Chim. Acta 2013, 770, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tret’yakov, K.V. Retention Data. NIST Mass Spectrometry Data Center. NIST Mass Spectrom. Data Cent. 2007. [Google Scholar]
- McLafferty, F.W. Wiley Registry of Mass Spectral Data, 12th ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Elmore, J.S.; Nisyrios, I.; Mottram, D.S. Analysis of the headspace aroma compounds of walnuts (Juglans regia L.). Flavour Fragr. J. 2005, 20, 501–506. [Google Scholar] [CrossRef]
- MATÉ, J.I.; SALTVEIT, M.E.; KROCHTA, J.M. Peanut and Walnut Rancidity: Effects of Oxygen Concentration and Relative Humidity. J. Food Sci. 1996, 61, 465–469. [Google Scholar] [CrossRef]
- Jensen, P.N.; Sørensen, G.; Engelsen, S.B.; Bertelsen, G. Evaluation of quality changes in walnut kernels (Juglans regia L.) by Vis/NIR spectroscopy. J. Agric. Food Chem. 2001, 49, 5790–5796. [Google Scholar] [CrossRef] [PubMed]
- Grilo, F.S.; Wang, S.C. Walnut (Juglans regia L.) volatile compounds indicate kernel and oil oxidation. Foods 2021, 10, 329. [Google Scholar] [CrossRef]
- Liu, B.; Chang, Y.; Sui, X.; Wang, R.; Liu, Z.; Sun, J.; Chen, H.; Sun, B.; Zhang, N.; Xia, J. Characterization of Predominant Aroma Components in Raw and Roasted Walnut (Juglans regia L.). Food Anal. Methods 2022, 15, 717–727. [Google Scholar] [CrossRef]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the key aroma compounds in pink guawa (Psidium guajawa L.) by means of aroma Re-engineering experiments and Omission Tests. J. Agric. Food Chem. 2009, 57, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Azhu Valappil, Z.; Fan, X.; Zhang, H.Q.; Rouseff, R.L. Impact of Thermal and Nonthermal Processing Technologies on Unfermented Apple Cider Aroma Volatiles. J. Agric. Food Chem. 2009, 57, 924–929. [Google Scholar] [CrossRef]
- Franklin, L.M.; Mitchell, A.E. Review of the Sensory and Chemical Characteristics of Almond (Prunus dulcis) Flavor. J. Agric. Food Chem. 2019, 67, 2743–2753. [Google Scholar] [CrossRef] [PubMed]
- Franklin, L.M.; Chapman, D.M.; King, E.S.; Mau, M.; Huang, G.; Mitchell, A.E. Chemical and Sensory Characterization of Oxidative Changes in Roasted Almonds Undergoing Accelerated Shelf Life. J. Agric. Food Chem. 2017, 65, 2549–2563. [Google Scholar] [CrossRef] [PubMed]
- Raczyk, M.; Kmiecik, D.; Schieberle, P.; Przybylski, R.; Jeleń, H.; Rudzińska, M. Model studies on the formation of volatile compounds generated by a thermal treatment of steryl esters with different fatty acid moieties. Food Res. Int. 2017, 97, 87–94. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Detection of monohydroperoxides with unconjugated diene systems as minor products of the autoxidation of methyl linoleate. Z. Lebensm. Unters. Forsch. 1981, 173, 199–203. [Google Scholar] [CrossRef]
- Erten, E.S.; Cadwallader, K.R. Identification of predominant aroma components of raw, dry roasted and oil roasted almonds. Food Chem. 2017, 217, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calderón, M.; Rojas-Graü, M.A.; Martín-Belloso, O. Aroma Profile and Volatiles Odor Activity Along Gold Cultivar Pineapple Flesh. J. Food Sci. 2010, 75, S506–S512. [Google Scholar] [CrossRef] [PubMed]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef] [PubMed]
- Steingass, C.B.; Jutzi, M.; Müller, J.; Carle, R.; Schmarr, H.G. Ripening-dependent metabolic changes in the volatiles of pineapple (Ananas comosus (L.) Merr.) fruit: II. Multivariate statistical profiling of pineapple aroma compounds based on comprehensive two-dimensional gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2609–2624. [Google Scholar] [CrossRef] [PubMed]
- Steingass, C.B.; Langen, J.; Carle, R.; Schmarr, H.G. Authentication of pineapple (Ananas comosus [L.] Merr.) fruit maturity stages by quantitative analysis of γ- and δ-lactones using headspace solid-phase microextraction and chirospecific gas chromatography-selected ion monitoring mass spectrometry (HS-SPME. Food Chem. 2014, 168, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.M.P.; Pedroso, M.P.; Augusto, F.; Silva, M.A. Volatiles identification in pineapple submitted to drying in an ethanolic atmosphere. Dry. Technol. 2009, 27, 248–257. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9783540699330. [Google Scholar]
- Nukuntornprakit, O.; Chanjirakul, K.; van Doorn, W.G.; Siriphanich, J. Chilling injury in pineapple fruit: Fatty acid composition and antioxidant metabolism. Postharvest Biol. Technol. 2015, 99, 20–26. [Google Scholar] [CrossRef]
- Kaewtathip, T.; Charoenrein, S. Changes in volatile aroma compounds of pineapple (Ananas comosus) during freezing and thawing. Int. J. Food Sci. Technol. 2012, 47, 985–990. [Google Scholar] [CrossRef]
- Neugebauer, A.; Granvogl, M.; Schieberle, P. Characterization of the Key Odorants in High-Quality Extra Virgin Olive Oils and Certified Off-Flavor Oils to Elucidate Aroma Compounds Causing a Rancid Off-Flavor. J. Agric. Food Chem. 2020, 68, 5927–5937. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ISO International Organization for Standardization: Geneva, Switzerland, 2017.
| Storage | ||||||
|---|---|---|---|---|---|---|
| Sample Type | Acronym | Shelf-Life | Temperature | Atmosphere | Subsamples | N° Samples |
| Walnut | WAL | 0 months—t0 | - | - | R1, R2, R3 | 3 |
| Walnut | WAL | 4 months—t4 | 5 °C | NA, SV | R1, R2, R3 | 6 |
| Walnut | WAL | 4 months—t4 | 18 °C | NA, SV | R1, R2, R3 | 6 |
| Walnut | WAL | 8 months—t8 | 5 °C | NA, SV | R1, R2, R3 | 6 |
| Walnut | WAL | 8 months—t8 | 18 °C | NA, SV | R1, R2, R3 | 6 |
| Walnut | WAL | 12 months—t12 | 5 °C | NA, SV | R1, R2, R3 | 6 |
| Walnut | WAL | 12 months—t12 | 18 °C | NA, SV | R1, R2, R3 | 6 |
| Almond | ALM | 0 months—t0 | - | - | R1, R2, R3 | 3 |
| Almond | ALM | 4 months—t4 | 5 °C | NA, SV | R1, R2, R3 | 6 |
| Almond | ALM | 4 months—t4 | 18 °C | NA, SV | R1, R2, R3 | 6 |
| Almond | ALM | 8 months—t8 | 5 °C | NA, SV | R1, R2, R3 | 6 |
| Almond | ALM | 8 months—t8 | 18 °C | NA, SV | R1, R2, R3 | 6 |
| Almond | ALM | 12 months—t12 | 5 °C | NA, SV | R1, R2, R3 | 6 |
| Almond | ALM | 12 months—t12 | 18 °C | NA, SV | R1, R2, R3 | 6 |
| Pineapple | PINE | 0 months—t0 | - | - | R1, R2, R3 | 3 |
| Pineapple | PINE | 4 months—t4 | 5 °C | NA | R1, R2, R3 | 3 |
| Pineapple | PINE | 4 months—t4 | 18 °C | NA | R1, R2, R3 | 3 |
| Pineapple | PINE | 8 months—t8 | 5 °C | NA | R1, R2, R3 | 3 |
| Pineapple | PINE | 8 months—t8 | 18 °C | NA | R1, R2, R3 | 3 |
| Pineapple | PINE | 12 months—t12 | 5 °C | NA | R1, R2, R3 | 3 |
| Pineapple | PINE | 12 months—t12 | 18 °C | NA | R1, R2, R3 | 3 |
| Exp # | Amount (g) | Time (min) | Temperature (°C) |
|---|---|---|---|
| 1 | −1 (0.5) | −1 (30) | −1 (30) |
| 2 | −1 (0.5) | −1 (30) | +1 (60) |
| 3 | −1 (0.5) | 0 (45) | 0 (45) |
| 4 | −1 (0.5) | +1 (60) | −1 (30) |
| 5 | −1 (0.5) | +1 (60) | +1 (60) |
| 6 | 0 (1.75) | -α (20) | 0 (45) |
| 7 | 0 (1.75) | 0 (45) | −1 (30) |
| 8 | 0 (1.75) | 0 (45) | 0 (45) |
| 9 | 0 (1.75) | 0 (45) | 0 (45) |
| 10 | 0 (1.75) | 0 (45) | +α (70) |
| 11 | 0 (1.75) | +α (70) | 0 (45) |
| 12 | + α (3.85) | 0 (45) | 0 (45) |
| 13 | +1 (3) | −1 (30) | −1 (30) |
| 14 | +1 (3) | −1 (30) | +1 (60) |
| 15 | +1 (3) | +1 (60) | −1 (30) |
| 16 | +1 (3) | +1 (60) | +1 (60) |
| Sample | Amount | Temperature | Time |
|---|---|---|---|
| Walnut | 1.75 g | 40 °C | 60 min |
| Almond | 1.75 g | 50 °C | 45 min |
| Pineapple | 1.75 g | 60 °C | 45 min |
| Amount ng/g (Averaged over 3 Replicates/3 Batches ± Absolute Uncertainty) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Hexanal | Heptanal | (E)-2-Heptenal | Octanal | (E)-2-Octenal | Nonanal | Decanal | Hexanal Eq. | |||||||
| WAL_T0 | 377.8 | ±34.0 | ≤LOD- | 3.3 | ±0.6 | 1.5 | ±0.1 | 3.6 | ±0.4 | 1.2 | ±0.1 | 5.6 | ±0.5 | 389.2 | |
| WAL_5NA_T4 | 532.5 | ±47.9 | ≤LOD | 4.4 | ±0.8 | 3.4 | ±0.3 | 4.8 | ±0.5 | 3.9 | ±0.4 | 5.3 | ±0.5 | 549.0 | |
| WAL_5NA_T8 | 589.5 | ±53.1 | 2.7 | ±0.3 | ≤LOD | 25.9 | ±2.5 | 36.1 | ±3.5 | 8.2 | ±0.8 | 13.8 | ±1.4 | 655.4 | |
| WAL_5NA_T12 | 607.6 | ±54.7 | 1.0 | ±0.1 | ≤LOD | 19.7 | ±1.9 | 31.1 | ±3.1 | 26.5 | ±2.6 | 13.7 | ±1.3 | 676.1 | |
| WAL_5SV_T4 | 301.9 | ±27.2 | ≤LOD | 1.2 | ±0.2 | 1.7 | ±0.2 | 4.6 | ±0.5 | 1.0 | ±0.1 | 4.7 | ±0.5 | 311.7 | |
| WAL_5SV_T8 | 277.0 | ±24.9 | 1.6 | ±0.2 | ≤LOD | 9.5 | ±0.9 | 28.6 | ±2.8 | 2.3 | ±0.2 | 18.0 | ±1.8 | 321.7 | |
| WAL_5SV_T12 | 260.9 | ±23.5 | 1.2 | ±0.1 | ≤LOD | 8.0 | ±0.8 | 30.7 | ±3.0 | 3.5 | ±0.3 | 11.0 | ±1.1 | 302.1 | |
| WAL_18NA_T4 | 565.4 | ±50.9 | ≤LOD | 21.6 | ±3.9 | 15.5 | ±1.5 | 22.8 | ±2.2 | 16.4 | ±1.6 | 47.2 | ±4.6 | 656.7 | |
| WAL_18NA_T8 | 650.0 | ±58.5 | 1.9 | ±0.2 | ≤LOD | 11.4 | ±1.1 | 30.0 | ±2.9 | 8.9 | ±0.9 | 19.1 | ±1.9 | 702.9 | |
| WAL_18NA_T12 | 672.5 | ±60.5 | 3.0 | ±0.3 | ≤LOD | 24.0 | ±2.4 | 32.8 | ±3.2 | 15.8 | ±1.5 | 13.3 | ±1.3 | 739.6 | |
| WAL_18SV_T4 | 320.8 | ±28.9 | ≤LOD | 2.6 | ±0.5 | 1.0 | ±0.1 | 11.5 | ±1.1 | 10.9 | ±1.1 | 5.1 | ±0.5 | 344.0 | |
| WAL_18SV_T8 | 235.8 | ±21.2 | 2.0 | ±0.2 | ≤LOD | 5.8 | ±0.6 | 27.1 | ±2.7 | 2.0 | ±0.2 | 13.4 | ±1.3 | 273.6 | |
| WAL_18SV_T12 | 224.0 | ±20.2 | 1.4 | ±0.1 | ≤LOD | 4.4 | ±0.4 | 34.5 | ±3.4 | 8.0 | ±0.8 | 12.3 | ±1.2 | 269.7 | |
| Amount ng/g (Averaged over 3 Replicates/3 Batches ± Absolute Uncertainty) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Hexanal | Octanal | (E)-2-Octenal | Nonanal | Decanal | Hexanal Eq. | |||||
| ALM_T0 | 2345.6 | ±187.6 | 10.8 | ±0.9 | 1.0 | ±0.1 | 10.6 | ±0.8 | 1.4 | ±0.1 | 2363.2 |
| ALM_5NA_T4 | 6724.8 | ±538.0 | 13.6 | ±1.1 | 2.4 | ±0.2 | 16.0 | ±1.3 | 5.6 | ±0.4 | 6752.2 |
| ALM_5NA_T8 | 12303.6 | ±984.3 | 21.7 | ±1.7 | 10.0 | ±0.8 | 32.6 | ±2.6 | 2.5 | ±0.2 | 12353.1 |
| ALM_5NA_T12 | 41888.8 | ±3351.1 | 52.9 | ±4.2 | 43.3 | ±3.5 | 99.6 | ±8.0 | 4.2 | ±0.3 | 42037.4 |
| ALM_5SV_T4 | 4843.5 | ±387.5 | 16.5 | ±1.3 | 1.9 | ±0.2 | 11.9 | ±1.0 | 5.8 | ±0.5 | 4870.0 |
| ALM_5SV_T8 | 9319.7 | ±12.5 | 10.5 | ±13.9 | 1.9 | ±0.2 | 13.88 | ±1.1 | 1.93 | ±0.2 | 9340.5 |
| ALM_5SV_T12 | 11423.1 | ±12.5 | 57.7 | ±13.9 | 17.2 | ±1.4 | 17.5 | ±1.4 | 1.8 | ±0.1 | 11454.7 |
| ALM_18NA_T4 | 3982.7 | ±318.6 | 13.6 | ±1.1 | 1.4 | ±0.1 | 14.7 | ±1.2 | 6.4 | ±0.5 | 4008.9 |
| ALM_18NA_T8 | 12245.0 | ±979.6 | 16.3 | ±1.3 | 10.0 | ±0.8 | 64.6 | ±5.2 | 3.7 | ±0.3 | 12313.5 |
| ALM_18NA_T12 | 58902.4 | ±4712.2 | 84.1 | ±6.7 | 57.8 | ±4.6 | 104.3 | ±8.3 | 5.4 | ±0.4 | 59090.9 |
| ALM_18SV_T4 | 5655.0 | ±452.4 | 13.6 | ±1.1 | 2.4 | ±0.2 | 12.3 | ±1.0 | 4.1 | ±0.3 | 5678.8 |
| ALM_18SV_T8 | 19775.5 | ±1582.0 | 18.9 | ±1.5 | 14.5 | ±1.2 | 37.4 | ±3.0 | 2.7 | ±0.2 | 19829.8 |
| ALM_18SV_T12 | 52526.2 | ±4202.1 | 75.2 | ±6.0 | 58.6 | ±4.7 | 100.9 | ±8.1 | 10.3 | ±0.8 | 52709.2 |
| Amount ng/g (Averaged over 3 Replicates/3 Batches ± Absolute Uncertainty) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Hexanal | Heptanal | (E)-2-Heptenal | Octanal | Nonanal | Decanal | Hexanal Eq. | ||||||
| PINE_T0 | 8.4 | ±0.8 | 1.0 | ±0.1 | ≤LOD | 7.5 | ±0.7 | 18.7 | ±1.8 | 16.6 | ±1.6 | 39.1 | |
| PINE_5NA_T4 | 10.2 | ±1.0 | 1.2 | ±0.1 | ≤LOD | 8.7 | ±0.8 | 19.1 | ±1.8 | 34.4 | ±3.2 | 53.6 | |
| PINE_5NA_T8 | 15.9 | ±1.5 | 0.9 | ±0.1 | 2.4 | ±0.2 | 13.2 | ±1.2 | 19.7 | ±1.8 | 41.9 | ±3.9 | 69.8 |
| PINE_5NA_T12 | 67.1 | ±6.3 | 2.8 | ±0.3 | 1.2 | ±0.1 | 14.8 | ±1.4 | 32.4 | ±3.0 | 29.1 | ±2.7 | 123.7 |
| PINE_18NA_T4 | 12.6 | ±1.2 | 1.3 | ±0.1 | ≤LOD | 10.1 | ±0.9 | 21.9 | ±2.0 | 37.3 | ±3.5 | 61.0 | |
| PINE_18NA_T8 | 25.3 | ±2.4 | 0.7 | ±0.1 | 2.0 | ±0.2 | 15.0 | ±1.4 | 20.6 | ±1.9 | 53.4 | ±5.0 | 88.1 |
| PINE_18NA_T12 | 76.0 | ±7.1 | 7.9 | ±0.7 | 3.4 | ±0.3 | 16.5 | ±1.5 | 94.2 | ±8.8 | 50.4 | ±4.7 | 197.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caratti, A.; Squara, S.; Stilo, F.; Battaglino, S.; Liberto, E.; Cincera, I.; Genova, G.; Spigolon, N.; Bicchi, C.; Cordero, C. Integrated Strategy for Informative Profiling and Accurate Quantification of Key-Volatiles in Dried Fruits and Nuts: An Industrial Quality Control Perspective. Foods 2022, 11, 3111. https://doi.org/10.3390/foods11193111
Caratti A, Squara S, Stilo F, Battaglino S, Liberto E, Cincera I, Genova G, Spigolon N, Bicchi C, Cordero C. Integrated Strategy for Informative Profiling and Accurate Quantification of Key-Volatiles in Dried Fruits and Nuts: An Industrial Quality Control Perspective. Foods. 2022; 11(19):3111. https://doi.org/10.3390/foods11193111
Chicago/Turabian StyleCaratti, Andrea, Simone Squara, Federico Stilo, Sonia Battaglino, Erica Liberto, Irene Cincera, Giuseppe Genova, Nicola Spigolon, Carlo Bicchi, and Chiara Cordero. 2022. "Integrated Strategy for Informative Profiling and Accurate Quantification of Key-Volatiles in Dried Fruits and Nuts: An Industrial Quality Control Perspective" Foods 11, no. 19: 3111. https://doi.org/10.3390/foods11193111
APA StyleCaratti, A., Squara, S., Stilo, F., Battaglino, S., Liberto, E., Cincera, I., Genova, G., Spigolon, N., Bicchi, C., & Cordero, C. (2022). Integrated Strategy for Informative Profiling and Accurate Quantification of Key-Volatiles in Dried Fruits and Nuts: An Industrial Quality Control Perspective. Foods, 11(19), 3111. https://doi.org/10.3390/foods11193111







