Optimized Cellulase-Hydrolyzed Deoiled Coconut Cake Powder as Wheat Flour Substitute in Cookies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimentation Sections
2.2.1. One-Factor Experiments for Hydrolyzing DCCP by Celluclast 1.5 L
2.2.2. Experimental Design for the Optimization of the Enzymatic Hydrolyzing Process
2.2.3. Cookie Preparation
2.3. Analytical Methods
2.3.1. Chemical Analysis
2.3.2. Physical Analysis of Cookies
2.3.3. Sensory Evaluation of Cookies
2.4. Statistical Analysis
3. Results and Discussion
3.1. The Influence of Technical Factors on the Fiber Content of DCCP in the Cellulase Treatment
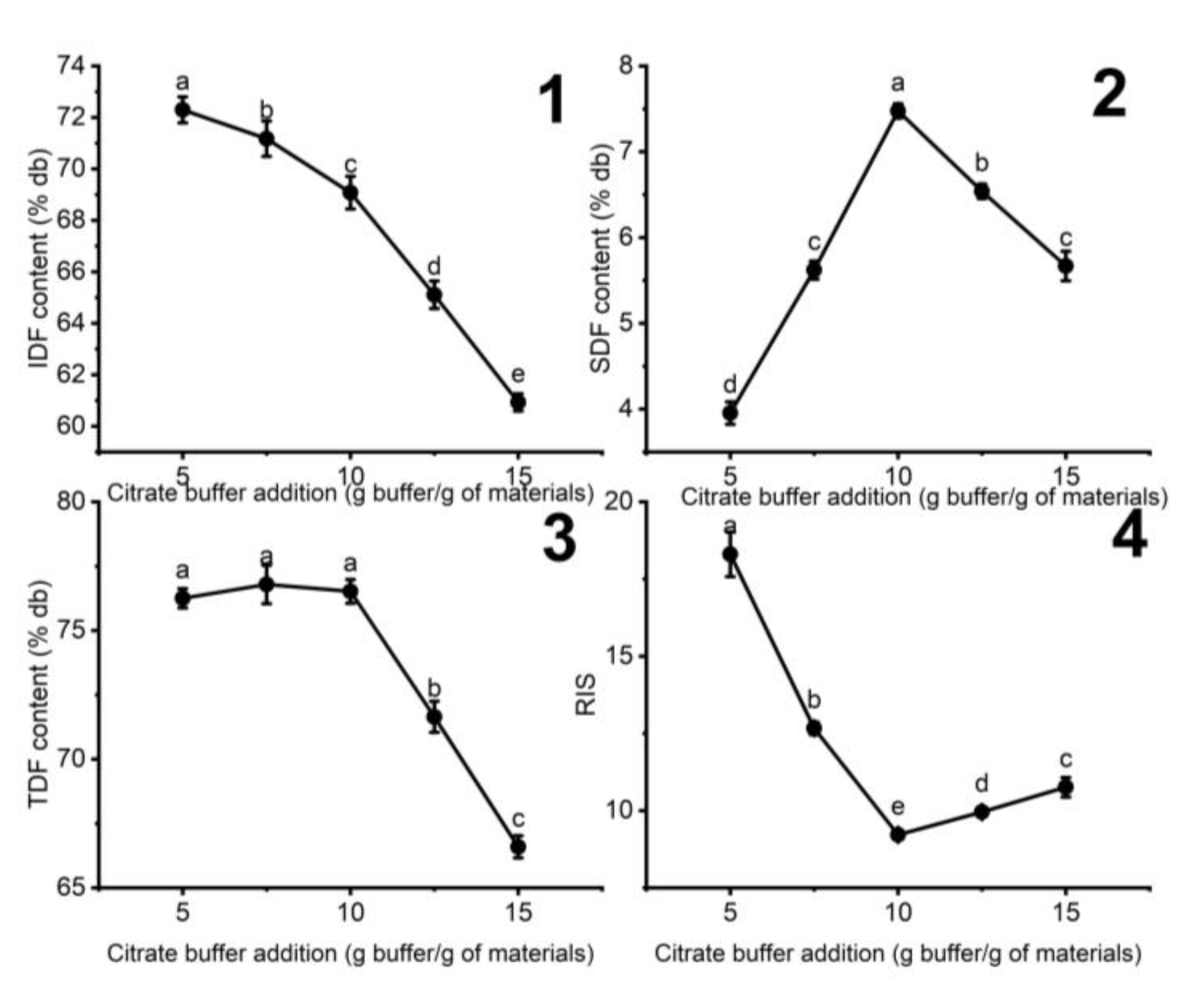
3.1.1. The Influence of the Added Citrate Buffer on the Fiber Content of DCCP
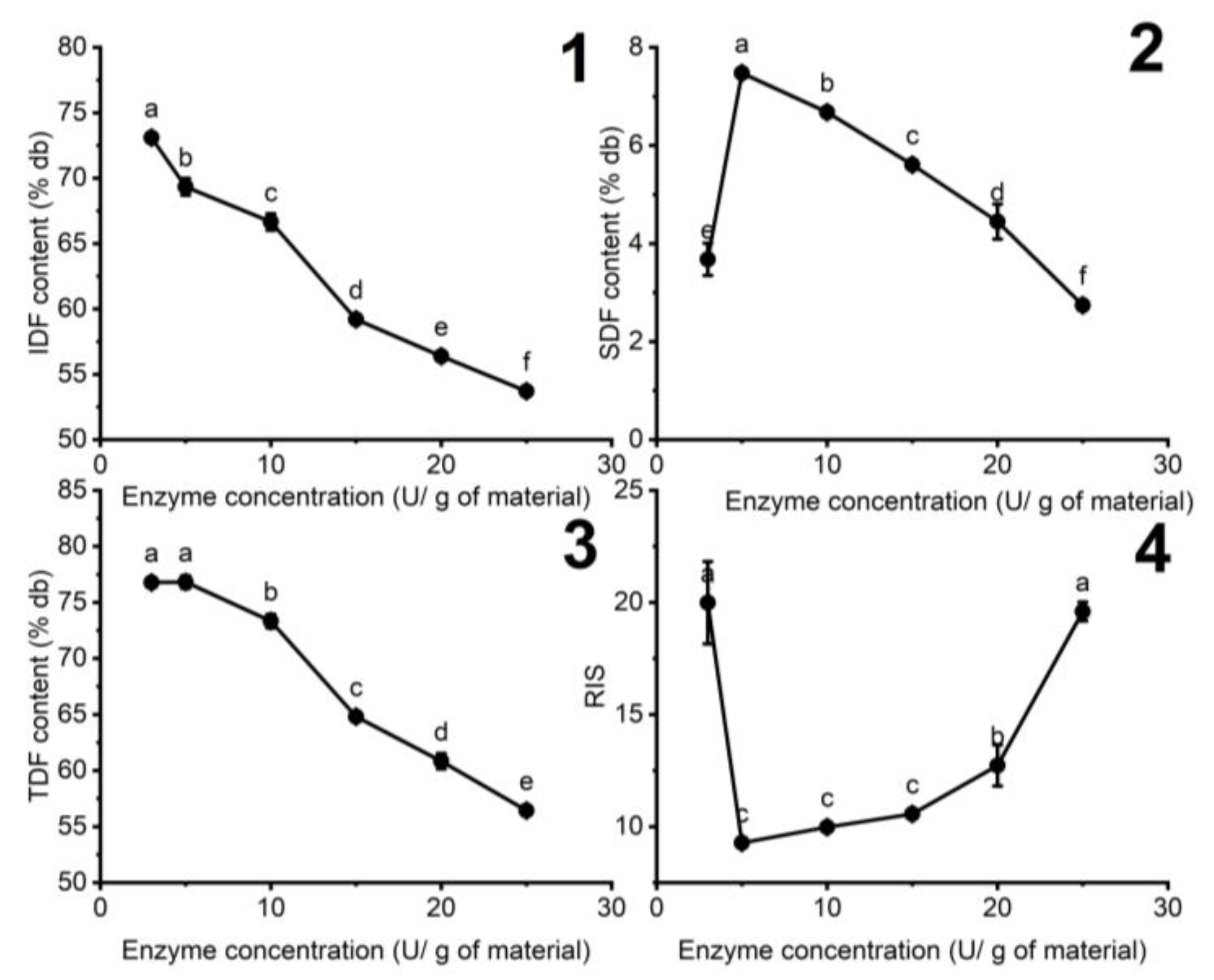
3.1.2. The Influence of Enzyme Concentration on the Fiber Content of DCCP
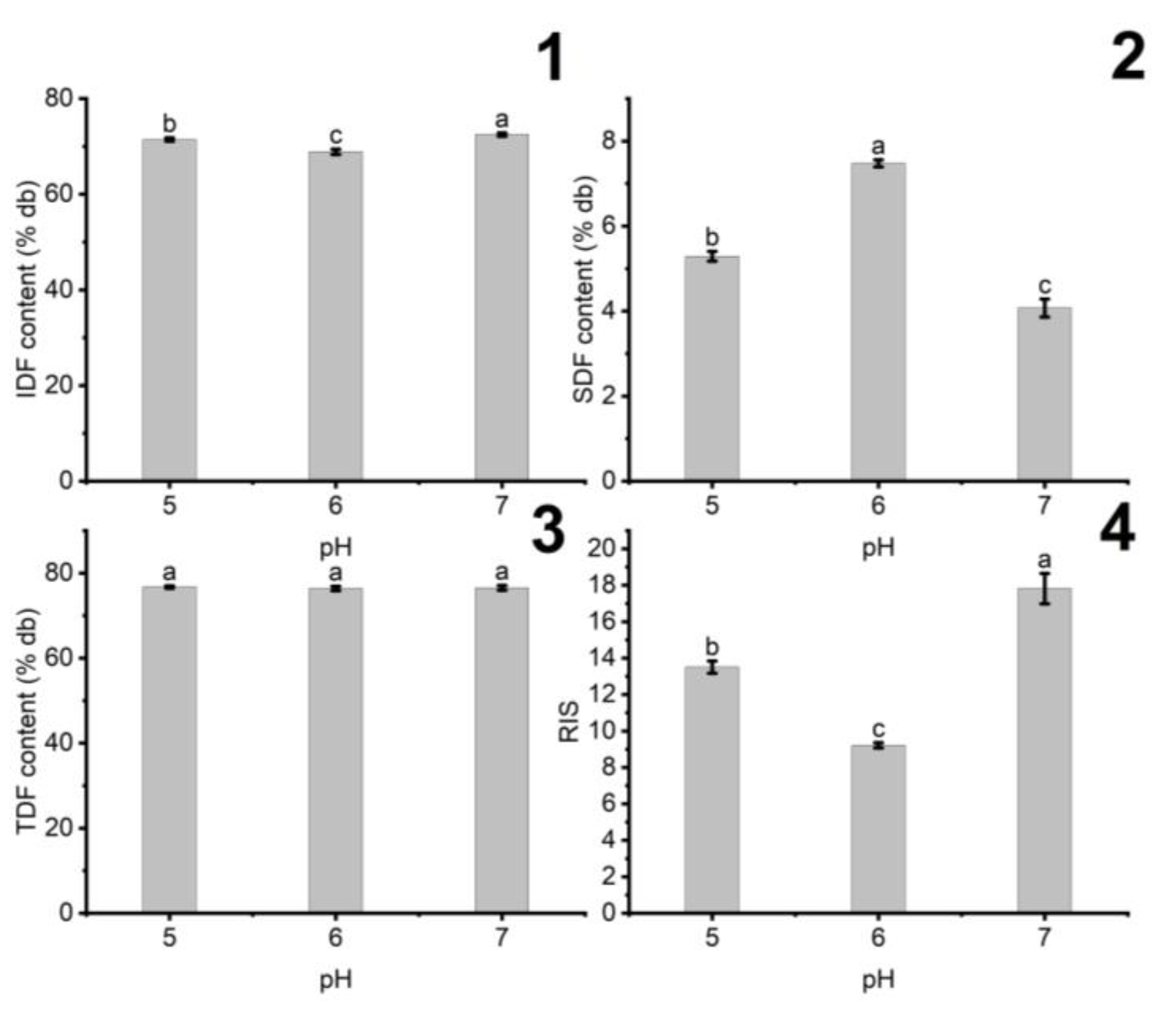
3.1.3. The Influence of pH on the Fiber Content of DCCP
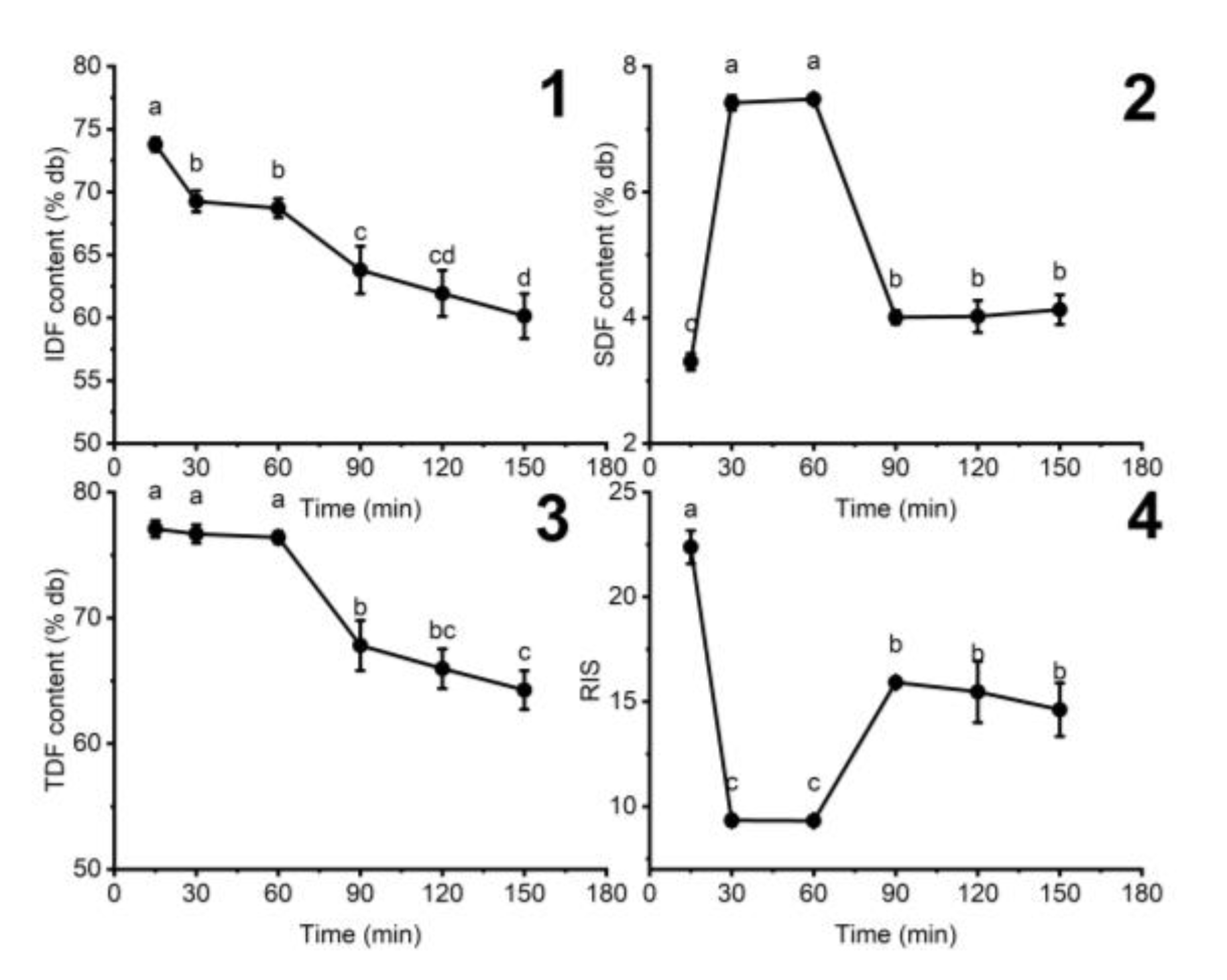
3.1.4. The Influence of Hydrolyzing Time on the Fiber Content of DCCP
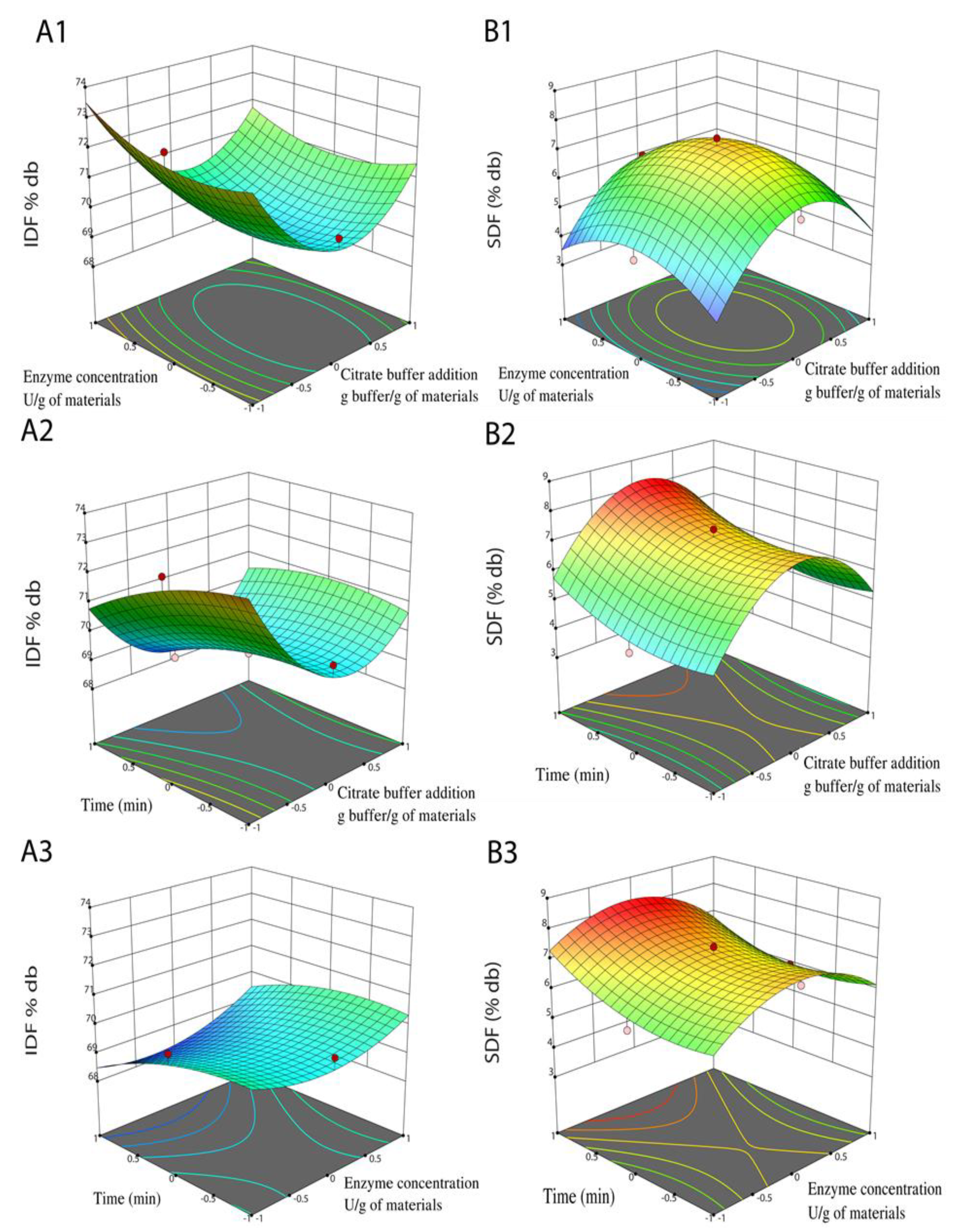
3.2. Optimizing the Cellulase Hydrolysis Process of DCCP
3.2.1. The Regression Models of IDF and SDF
3.2.2. Model Validation
3.3. The Influence of Partial Replacement on Cookie Properties
3.3.1. Chemical Compounds of HDCCP
3.3.2. The Influence of the Partial Replacement of Wheat Flour on the Chemical Composition of Cookie
3.3.3. The Influence of the Partial Replacement of Wheat Flour with DCCP and HDCCP on the Color and Physical Properties of Cookies
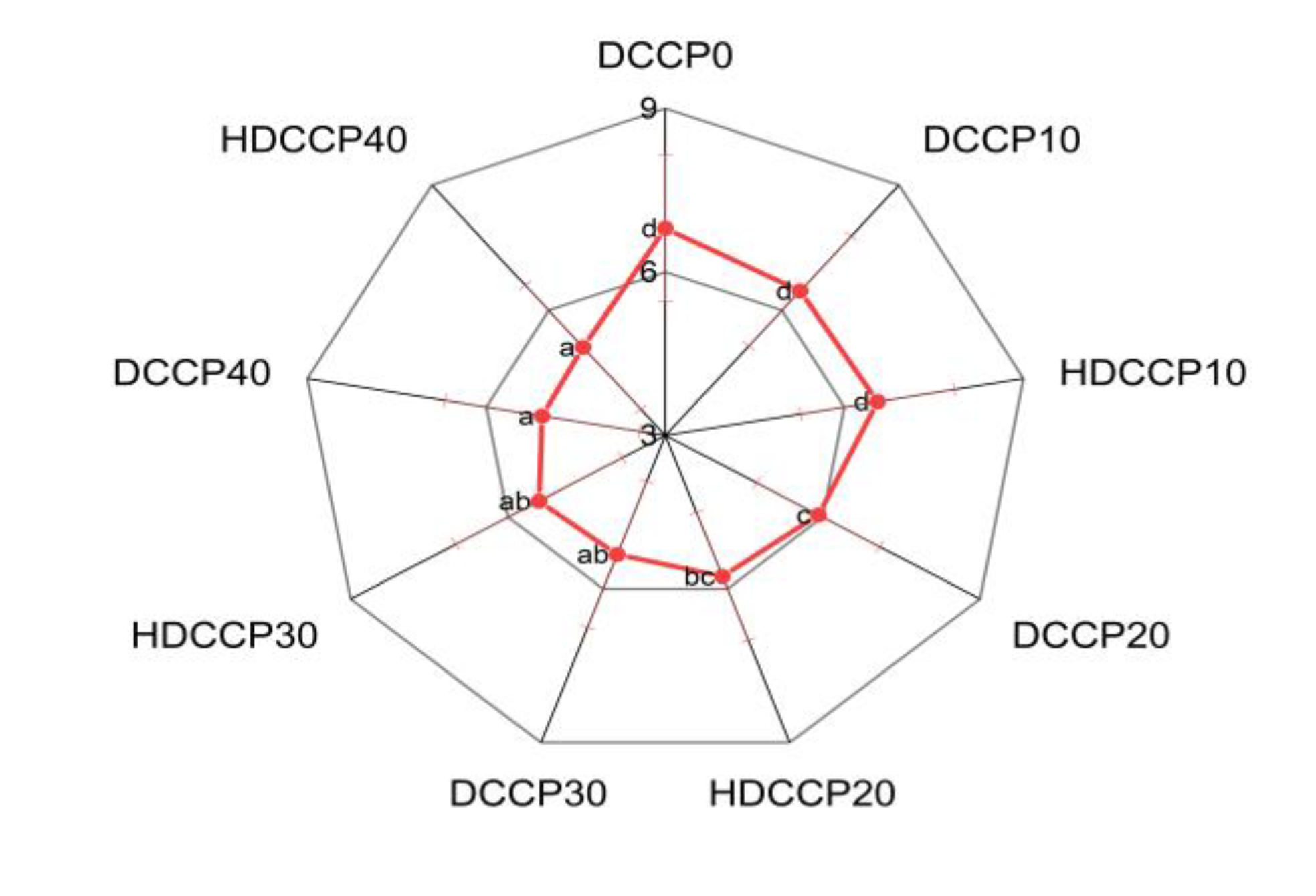
3.3.4. The Influence of the Partial Replacement of Wheat Flour on the Sensory Evaluation of Cookies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/333008/nutrients (accessed on 19 August 2022).
- Available online: https://www.businesswire.com/news/home/20170421005677/en/Global-High-fiber-Biscuit-Market-Drivers-and-Forecasts-by-Technavio (accessed on 18 August 2022).
- Available online: https://www.marketdataforecast.com/market-reports/high-fiber-biscuit-market (accessed on 17 August 2022).
- Murniece, I.; Karklina, D.; Galoburda, R.; Santare, D.; Skrabule, I.; Costa, H.S. Nutritional composition of freshly harvested and stored Latvian potato (Solanum tuberosum L.) varieties depending on traditional cooking methods. J. Food Compos. Anal. 2011, 24, 699–710. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Lu, T.-M.; Lee, C.-C.; Mau, J.-L.; Lin, S.-D. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010, 119, 1090–1095. [Google Scholar] [CrossRef]
- Yang, B.; Guo, M.; Zhao, Z. Incorporation of wheat malt into a cookie recipe and its effect on the physicochemical properties of the corresponding dough and cookies. LWT 2020, 117, 108651. [Google Scholar] [CrossRef]
- Thebaudin, J.Y.; Lefebvre, A.C.; Harrington, M.; Bourgeois, C.M. Dietary fibres: Nutritional and technological interest. Trends Food Sci. Technol. 1997, 8, 41–48. [Google Scholar] [CrossRef]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta Glucan: Health Benefits in Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012, 2012, 851362. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D. Chapter 3—The Interaction Between Insoluble and Soluble Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Samaan, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 35–59. [Google Scholar]
- Bader Ul Ain, H.; Saeed, F.; Khan, M.A.; Niaz, B.; Khan, S.G.; Anjum, F.M.; Tufail, T.; Hussain, S. Comparative study of chemical treatments in combination with extrusion for the partial conversion of wheat and sorghum insoluble fiber into soluble. Food Sci. Nutr. 2019, 7, 2059–2067. [Google Scholar] [CrossRef]
- Steemburgo, T.; Dall’Alba, V.; Almeida, J.C.; Zelmanovitz, T.; Gross, J.L.; de Azevedo, M.J. Intake of soluble fibers has a protective role for the presence of metabolic syndrome in patients with type 2 diabetes. Eur. J. Clin. Nutr. 2009, 63, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mrabet, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Hamza, H.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Sindic, M.; Jiménez-Araujo, A. Enzymatic conversion of date fruit fiber concentrates into a new product enriched in antioxidant soluble fiber. LWT 2017, 75, 727–734. [Google Scholar] [CrossRef]
- Tang, S.; Ma, Y.; Dong, X.; Zhou, H.; He, Y.; Ren, D.; Wang, Q.; Yang, H.; Liu, S.; Wu, L. Enzyme-assisted extraction of fucoidan from Kjellmaniella crassifolia based on kinetic study of enzymatic hydrolysis of algal cellulose. Algal Res. 2022, 66, 102795. [Google Scholar] [CrossRef]
- Napolitano, A.; Lanzuise, S.; Ruocco, M.; Arlotti, G.; Ranieri, R.; Knutsen, S.H.; Lorito, M.; Fogliano, V. Treatment of Cereal Products with a Tailored Preparation of Trichoderma Enzymes Increases the Amount of Soluble Dietary Fiber. J. Agric. Food Chem. 2006, 54, 7863–7869. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, R.; Jeya, G.; Sonali, M.I.; Veenagayathri, K.; Sivamurugan, V. Chapter 4—Chemoenzymatic conversion of biomass for production of value-added products. In Biomass, Biofuels, Biochemicals; Li, H., Saravanamurugan, S., Pandey, A., Elumalai, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 55–74. [Google Scholar]
- Soares, J.F.; Prá, V.D.; Kempka, A.P.; Prestes, R.C.; Tres, M.V.; Kuhn, R.C.; Mazutti, M.A. Chapter 18—Cellulases for Food Applications. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 201–208. [Google Scholar]
- Aktas-Akyildiz, E.; Mattila, O.; Sozer, N.; Poutanen, K.; Koksel, H.; Nordlund, E. Effect of steam explosion on enzymatic hydrolysis and baking quality of wheat bran. J. Cereal Sci. 2017, 78, 25–32. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2019, 95, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.C.; Zhang, Y. Protein Analysis. In Food Analysis; Nielsen, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 315–331. [Google Scholar]
- McCleary, B.V.; DeVries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Champ, M.; Okuma, K. Determination of Insoluble, Soluble, and Total Dietary Fiber (CODEX Definition) by Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study. J. AOAC Int. 2019, 95, 824–844. [Google Scholar] [CrossRef]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y. Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef]
- Chauhan, A.; Saxena, D.C.; Singh, S. Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. LWT—Food Sci. Technol. 2015, 63, 939–945. [Google Scholar] [CrossRef]
- Nguyen, S.N.; Vien, M.D.; Le, T.T.T.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Effects of enzymatic treatment conditions on dietary fibre content of wheat bran and use of cellulase-treated bran in cookie. Int. J. Food Sci. Technol. 2021, 56, 4017–4025. [Google Scholar] [CrossRef]
- Huang, M.; Yang, H. Eucheuma powder as a partial flour replacement and its effect on the properties of sponge cake. LWT 2019, 110, 262–268. [Google Scholar] [CrossRef]
- Liu, H.; Sun, J.; Leu, S.-Y.; Chen, S. Toward a fundamental understanding of cellulase-lignin interactions in the whole slurry enzymatic saccharification process. Biofuels Bioprod. Biorefin. 2016, 10, 648–663. [Google Scholar] [CrossRef]
- Phillips, K.M.; Haytowitz, D.B.; Pehrsson, P.R. Implications of two different methods for analyzing total dietary fiber in foods for food composition databases. J. Food Compos. Anal. 2019, 84, 103253. [Google Scholar] [CrossRef]
- Nelson, D.; Cox, M. Lehninger Principles of Biochemistry, 7th ed.; Macmillan: New York, NY, USA, 2017; p. 3270. [Google Scholar]
- Information on EC 3.2.1.4—Cellulase. Available online: https://www.brenda-enzymes.org/all_enzymes.php?ecno=3.2.1.4&table=Reaction (accessed on 18 March 2020).
- Shewry, P.R. Carbohydrate Chemistry for Food Scientists. J. Cereal Sci. 2008, 48, 879. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Michelle Garcia, G.; Leandro Vinícius Alves, G.; Milla Alves, B.; Daniel, P. Pretreatment of sugarcane bagasse using citric acid and its use in enzymatic hydrolysis. Renew. Energy 2020, 157, 332–341. [Google Scholar] [CrossRef]
- Ziyuan, Z.; Dehua, L.; Xuebing, Z. Conversion of lignocellulose to biofuels and chemicals via sugar platform: An updated review on chemistry and mechanisms of acid hydrolysis of lignocellulose. Renew. Sustain. Energy Rev. 2021, 146, 111169. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. How combinations of dietary fibres can affect physicochemical characteristics of pasta. LWT—Food Sci. Technol. 2015, 61, 41–46. [Google Scholar] [CrossRef]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism; Cengage Learning: Boston, MA, USA, 2012. [Google Scholar]
- Oladunjoye, A.O.; Eziama, S.C.; Aderibigbe, O.R. Proximate composition, physical, sensory and microbial properties of wheat-hog plum bagasse composite cookies. LWT 2021, 141, 111038. [Google Scholar] [CrossRef]
- Pycarelle, S.C.; Delcour, J.A. The role and impact on quality of exogenous and endogenous lipids during sponge cake making. Trends Food Sci. Technol. 2021, 114, 158–166. [Google Scholar] [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Datta, A.K. Biscuit baking: A review. LWT 2020, 131, 109726. [Google Scholar] [CrossRef]
- Jacob, J.; Leelavathi, K. Effect of fat-type on cookie dough and cookie quality. J. Food Eng. 2007, 79, 299–305. [Google Scholar] [CrossRef]
- Lara, E.; Cortés, P.; Briones, V.; Perez, M. Structural and physical modifications of corn biscuits during baking process. LWT—Food Sci. Technol. 2011, 44, 622–630. [Google Scholar] [CrossRef]
| Variables | Units | Responses | ||
|---|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | ||
| X1: added citrate buffer | g buffer/g of materials | 7.5 | 10 | 12.5 |
| X2: enzyme concentration | U/g of materials | 3 | 5 | 10 |
| X3: time | min | 15 | 30 | 60 |
| Independent Factors | IDF%db | SDF%db | |||||
|---|---|---|---|---|---|---|---|
| Run | X1 | X2 | X3 | Forecast Values | Experimental Values | Forecast Values | Experimental Values |
| 1 | −1 | −1 | −1 | 73.4 | 73.1 ± 1.3 | 3.48 | 3.52 ± 0.12 |
| 2 | 0 | 0 | −1 | 69.5 | 69.9 ± 1.2 | 7.26 | 7.09 ± 0.09 |
| 3 | −1 | 1 | −1 | 74.0 | 73.9 ± 1.5 | 3.76 | 3.85 ± 0.07 |
| 4 | 1 | −1 | −1 | 71.4 | 71.3 ± 1.5 | 4.11 | 4.20 ± 0.06 |
| 5 | 1 | 1 | −1 | 71.3 | 71.3 ± 1.5 | 4.39 | 4.03 ± 0.05 |
| 6 | 0 | 0 | 0 | 69.3 | 69.3 ± 1.8 | 7.01 | 7.42 ± 0.17 |
| 7 | 0 | 0 | 0 | 69.3 | 69.3 ± 1.8 | 7.01 | 7.42 ± 0.17 |
| 8 | 0 | 0 | 0 | 69.3 | 69.3 ± 1.8 | 7.01 | 7.42 ± 0.17 |
| 9 | 0 | 1 | 0 | 70.3 | 70.1 ± 1.3 | 5.84 | 5.94 ± 0.14 |
| 10 | −1 | 0 | 0 | 72.3 | 72.8 ± 1.3 | 4.53 | 4.32 ± 0.04 |
| 11 | 1 | 0 | 0 | 71.0 | 70.6 ± 1.2 | 5.15 | 5.24 ± 0.08 |
| 12 | 0 | −1 | 0 | 69.7 | 70.1 ± 1.1 | 5.88 | 5.67 ± 0.03 |
| 13 | −1 | −1 | 1 | 70.8 | 70.7 ± 1.4 | 4.75 | 4.92 ± 0.11 |
| 14 | −1 | 1 | 1 | 72.1 | 72.2 ± 1.2 | 4.37 | 4.44 ± 0.06 |
| 15 | 1 | −1 | 1 | 70.8 | 70.9 ± 1.5 | 5.37 | 5.44 ± 0.13 |
| 16 | 1 | 1 | 1 | 71.4 | 71.7 ± 1.4 | 5.00 | 4.81 ± 0.07 |
| 17 | 0 | 0 | 1 | 68.3 | 68.0 ± 1.7 | 8.19 | 8.03 ± 0.22 |
| Coefficient | IDF | p-Value of IDF | SDF | p-Value of SDF | |
|---|---|---|---|---|---|
| Intercept | Β0 | 69.34 | <0.0001 | 7.17 | <0.0001 |
| Linear | B1 | –0.68 | 0.0007 | 0.27 | 0.0244 |
| B2 | 0.31 | 0.0342 | –0.07 | 0.4908 | |
| B3 | –0.62 | 0.0012 | 0.49 | 0.0012 | |
| Interaction | B12 | –0.18 | 0.2106 | –0.08 | 0.4676 |
| B13 | 0.50 | 0.0069 | 0.00 | 0.9770 | |
| B23 | 0.18 | 0.2143 | –0.16 | 0.1737 | |
| Quadratic | B11 | 2.31 | <0.0001 | –2.21 | <0.0001 |
| B22 | 0.67 | 0.022 | –1.18 | 0.0003 | |
| B33 | –0.42 | 0.1082 | 0.57 | 0.0159 | |
| DF | 9 | 9 | |||
| F-values | 29.27 | 44.30 | |||
| p-values | <0.0001 | <0.0001 | |||
| R2 | 0.9747 | 0.9819 | |||
| R2adjusted | 0.9422 | 0.9587 |
| The Independent Factors of the Hydrolysis Process | |||||||
|---|---|---|---|---|---|---|---|
| An Amount of Added Citrate Buffer g Buffer/g of Materials | Enzyme Concentration U/g of the Materials | Retention Time Min | Dependent Responses | Forecast Values | Experimental Values | Prediction Error% | Rpredicted |
| 10.3 | 3.7 | 60 | IDF%db | 68.21 | 69.17 ± 0.89 | 1.39 | 0.7966 |
| SDF%db | 8.18 | 7.72 ± 0.47 | 5.62 | 0.8963 | |||
| Samples | IDF%db | SDF%db | TDF%db | RIS | Moisture% | Protein%db | Lipid%db | Ash%db |
|---|---|---|---|---|---|---|---|---|
| DCCP0 | 2.3 ± 0.1 a | 1.0 ± 0.2 a | 3.3 ± 0.1 a | 2.3 ± 0.4 a | 3.4 ± 0.1 a | 8.4 ± 0.0 d | 19.9 ± 0.1 b | 1.41 ± 0.01 a |
| DCCP10 | 5.0 ± 0.2 bA | 1.2 ± 0.1 bA | 6.2 ± 0.1 bA | 4.3 ± 0.3 bA | 3.0 ± 0.3 aA | 8.4 ± 0.1 cA | 20.0 ± 0.8 bA | 1.52 ± 0.04 bA |
| DCCP20 | 8.6 ± 0.1 cA | 1.3 ± 0.1 cA | 9.9 ± 0.2 cA | 6.4 ± 0.4 cA | 3.0 ± 0.3 aA | 8.2 ± 0.0 cB | 20.4 ± 0.6 bA | 1.59 ± 0.04 cA |
| DCCP30 | 14.8 ± 0.2 dA | 1.7 ± 0.1 dA | 16.6 ± 0.2 dA | 8.6 ± 0.3 dA | 3.1 ± 0.3 aA | 8.0 ± 0.0 bA | 20.4 ± 0.4 cA | 1.82 ± 0.02 dA |
| DCCP40 | 18.4 ± 0.6 eA | 1.8 ± 0.0 eA | 20.2 ± 0.6 eA | 10.0 ± 0.4 eA | 3.1 ± 0.2 aA | 7.7 ± 0.0 aA | 20.6 ± 0.4 cA | 1.97 ± 0.01 eA |
| HDCCP10 | 4.5 ± 0.2 bB | 1.4 ± 0.1 bB | 5.9 ± 0.1 bA | 3.2 ± 0.3 aB | 3.0 ± 0.3 aA | 8.1 ± 0.1 cB | 19.6 ± 0.1 aA | 1.68 ± 0.01 bB |
| HDCCP20 | 7.3 ± 0.5 cB | 1.7 ± 0.0 cB | 8.9 ± 0.5 cA | 4.3 ± 0.2 bB | 3.0 ± 0.1 aA | 8.1 ± 0.1 cB | 19.7 ± 0.1 aA | 2.69 ± 0.14 cB |
| HDCCP30 | 12.8 ± 0.7 dB | 2.1 ± 0.0 dB | 14.9 ± 0.7 dA | 6.0 ± 0.4 cB | 3.2 ± 0.1 aA | 7.9 ± 0.0 bA | 20.3 ± 0.1 cA | 2.97 ± 0.08 dB |
| Samples | L | a | b | ΔE | Diameter mm | Thickness mm | Spread Factor | Hardness kg |
|---|---|---|---|---|---|---|---|---|
| DCCP0 | 62.2 ± 0.3 a | 4.8 ± 0.2 a | 30.8 ± 0.1 a | 0.0 ± 0.0 a | 34.0 ± 0.0 d | 6.9 ± 0.1 d | 5.00 ± 0.1 a | 3.1 ± 0.3 a |
| DCCP10 | 60.1 ± 0.6 bA | 5.1 ± 0.1 bA | 26.9 ± 0.2 bA | 4.5 ± 0.5 bA | 34.1 ± 0.1 dA | 6.8 ± 0.1 eA | 4.7 ± 0.0 eA | 3.2 ± 0.1 aA |
| DCCP20 | 56.3 ± 0.6 cA | 5.4 ± 0.2 cA | 24.5 ± 0.6 cA | 8.7 ± 0.5 cA | 33.2 ± 0.1 cA | 6.6 ± 0.0 cA | 5.1 ± 0.0 cA | 4.0 ± 0.1 bA |
| DCCP30 | 55.2 ± 0.4 dA | 6.5 ± 0.5 dA | 23.1 ± 0.1 dA | 10.5 ± 0.4 dA | 32.9 ± 0.1 bA | 6.3 ± 0.0 bA | 5.2 ± 0.0 bA | 4.4 ± 0.3 bA |
| DCCP40 | 54.2 ± 0.2 eA | 7.1 ± 0.2 eA | 22.7 ± 0.2 eA | 11.6 ± 0.5 eA | 32.5 ± 0.1 aA | 5.4 ± 0.1 aA | 6.1 ± 0.1 dA | 4.9 ± 0.3 cA |
| HDCCP10 | 57.3 ± 0.3 bB | 5.7 ± 0.6 bB | 24.7 ± 0.5 bB | 7.9 ± 0.6 bA | 34.2 ± 0.2 dA | 6.1 ± 0.1 eB | 5.7 ± 0.0 bB | 2.8 ± 0.1 aB |
| HDCCP20 | 54.3 ± 0.7 cB | 6.8 ± 0.4 cB | 23.7 ± 0.4 cA | 10.8 ± 0.6 cB | 33.5 ± 0.2 bA | 5.8 ± 0.1 cB | 5.8 ± 0.1 cB | 3.1 ± 0.0 bB |
| HDCCP30 | 51.9 ± 0.3 dB | 7.1 ± 0.1 cB | 22.8 ± 0.2 dB | 13.3 ± 0.3 dB | 32.7 ± 0.1 aA | 5.4 ± 0.0 bB | 6.1 ± 0.1 dB | 3.6 ± 0.2 cB |
| HDCCP40 | 47.1 ± 1.1 eB | 8.1 ± 0.7 cB | 21.6 ± 0.4 eB | 18.0 ± 0.9 eB | 32.5 ± 0.2 aA | 5.2 ± 0.0 aB | 6.1 ± 0.1 dA | 3.9 ± 0.2 cB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, T.P.; Duong, N.H.N.; Phan, T.H.; Mai, T.P.; Nguyen, D.Q. Optimized Cellulase-Hydrolyzed Deoiled Coconut Cake Powder as Wheat Flour Substitute in Cookies. Foods 2022, 11, 2709. https://doi.org/10.3390/foods11172709
Vo TP, Duong NHN, Phan TH, Mai TP, Nguyen DQ. Optimized Cellulase-Hydrolyzed Deoiled Coconut Cake Powder as Wheat Flour Substitute in Cookies. Foods. 2022; 11(17):2709. https://doi.org/10.3390/foods11172709
Chicago/Turabian StyleVo, Tan Phat, Nguyen Hong Nhung Duong, Thuy Han Phan, Thanh Phong Mai, and Dinh Quan Nguyen. 2022. "Optimized Cellulase-Hydrolyzed Deoiled Coconut Cake Powder as Wheat Flour Substitute in Cookies" Foods 11, no. 17: 2709. https://doi.org/10.3390/foods11172709
APA StyleVo, T. P., Duong, N. H. N., Phan, T. H., Mai, T. P., & Nguyen, D. Q. (2022). Optimized Cellulase-Hydrolyzed Deoiled Coconut Cake Powder as Wheat Flour Substitute in Cookies. Foods, 11(17), 2709. https://doi.org/10.3390/foods11172709





