Effects of Multiscale Mechanical Pulverization on the Physicochemical and Functional Properties of Black Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Tea Powders at Different Mechanical Pulverizing Scales
2.3. Physicochemical Properties of BTPS at Different Mechanical Pulverizing Scales
2.3.1. Measurement of Particle Size
2.3.2. Analyses of Color Appearance
2.3.3. Determinations of Powder Flowability
2.3.4. Analyses of Functional Compounds
2.4. Functional Properties of BTPS at Different Mechanical Pulverizing Scales
2.4.1. Analyses of Water-Holding Capacity
2.4.2. Measurements of Oil-Holding Capacity
2.4.3. Determinations of Pb2+ Adsorption Capacity
2.4.4. Antioxidant Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of BTPS at Different Mechanical Pulverizing Scales
3.1.1. Particle-Size Distributions
3.1.2. Color Appearance
3.1.3. Powder Flowability
3.1.4. Quantitative Analyses of the Functional Compounds
3.2. Functional Properties of BTPS at Different Mechanical Pulverizing Scales
3.2.1. Water-Holding Capacity
3.2.2. Oil-Holding Capacity
3.2.3. Pb2+ Adsorption Capacity
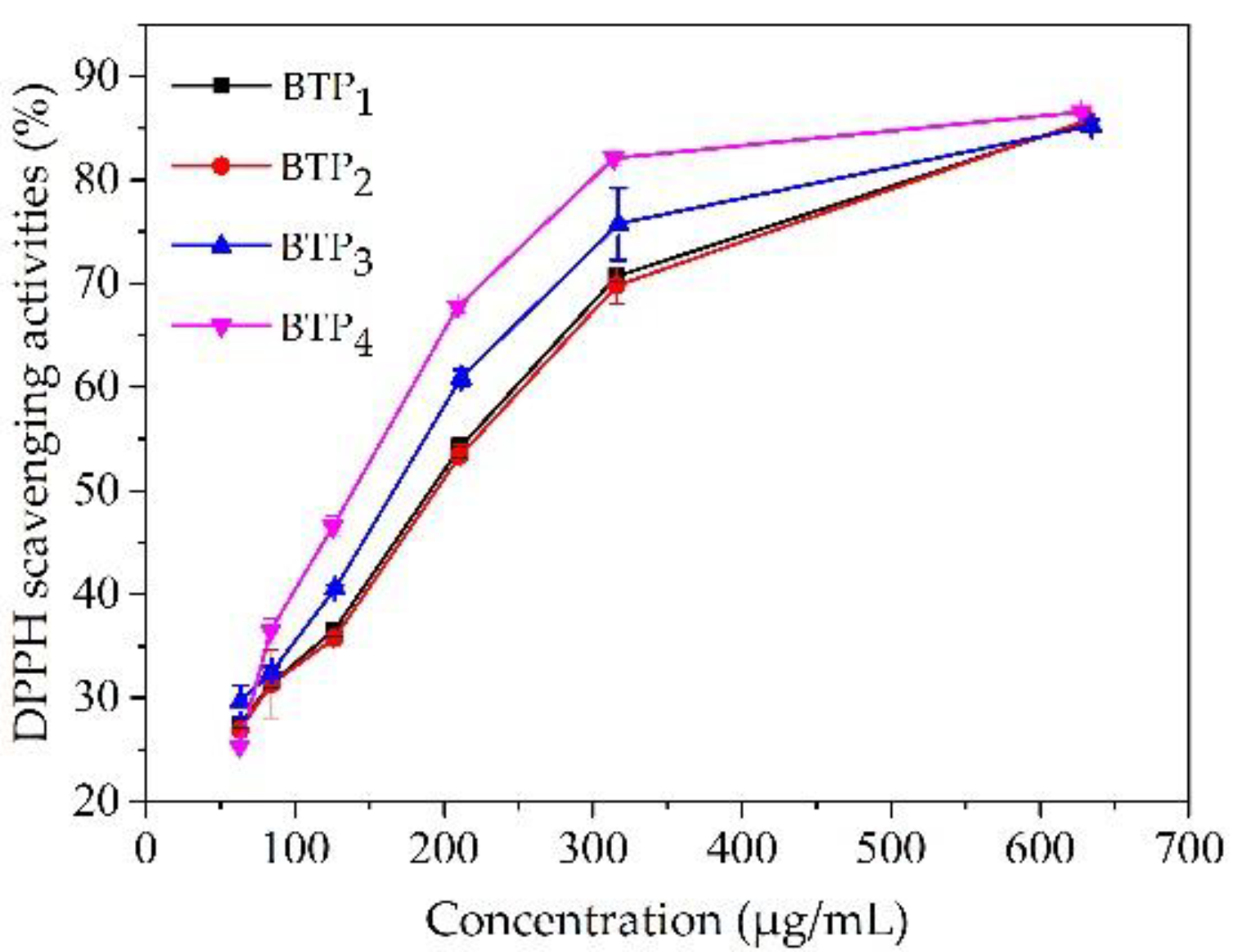
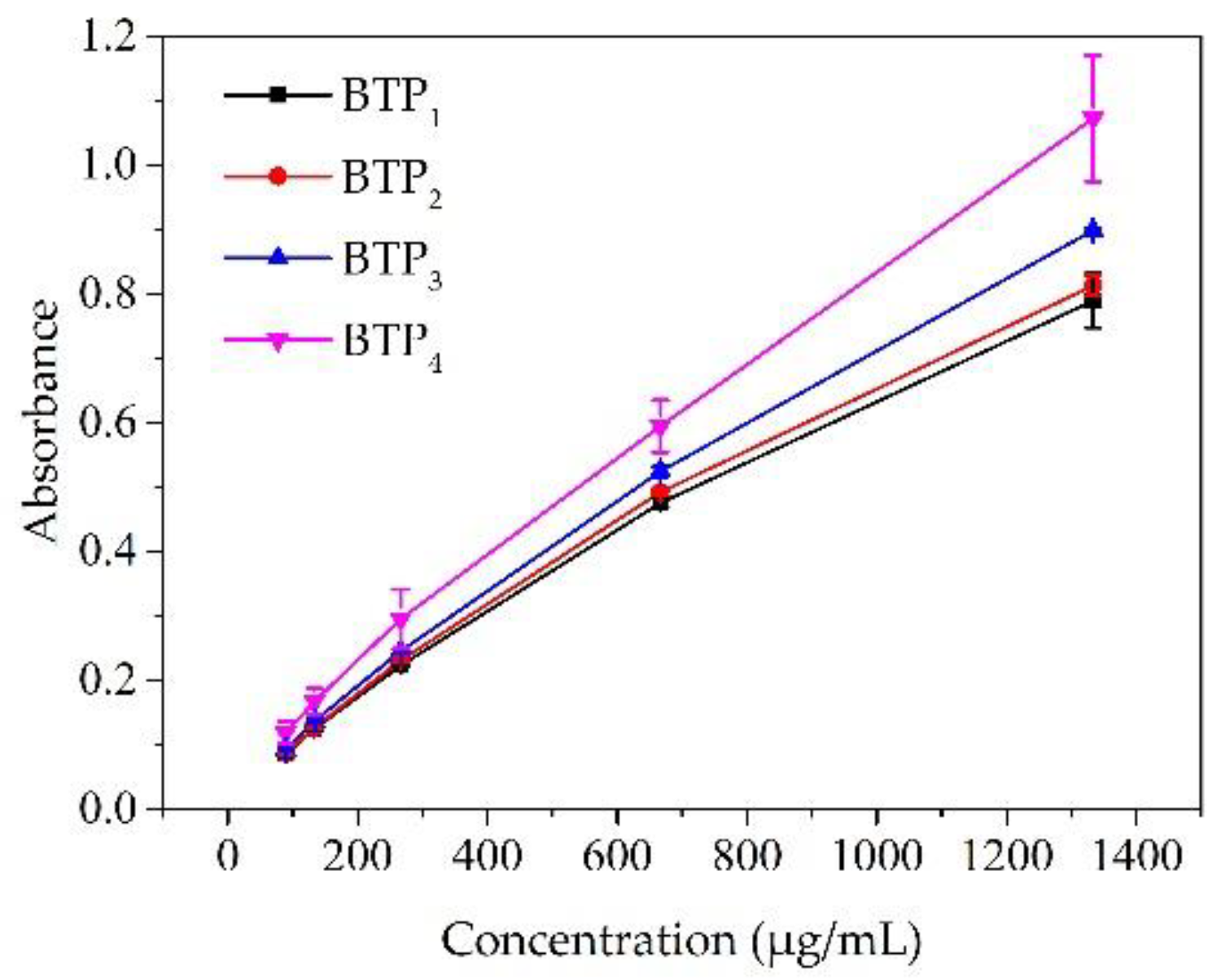
3.2.4. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, X.; Huang, J.; Huang, J.; Wu, W.; Tong, T.; Liu, S.; Zhou, L.; Liu, Z.; Zhang, S. Identification of volatile and odor-active compounds in Hunan black tea by SPME/GC-MS and multivariate analysis. LWT 2022, 164, 113656. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wen, J.; An, K.; Wu, J.; Yu, Y.; Zou, B.; Guo, M. A comparative study of aromatic characterization of Yingde Black Tea infusions in different steeping temperatures. LWT 2021, 143, 110860. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Chen, J.; Xie, J.; Shen, S.; Deng, Y.; Zhu, J.; Yuan, H.; Jiang, Y. Characterization of the key aroma compounds in black teas with different aroma types by using gas chromatography electronic nose, gas chromatography-ion mobility spectrometry, and odor activity value analysis. LWT 2022, 163, 113492. [Google Scholar] [CrossRef]
- Kayama, K.; Wei, R.; Zhang, Y.; Wu, F.; Su, Z.; Dong, J.; Liu, X. Effects of tea powder on the cooking properties, antioxidative potential and volatile profiles of dried noodles. Foods 2022, 11, 858. [Google Scholar] [CrossRef]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Ning, J.; Hou, G.G.; Sun, J.; Wan, X.; Dubat, A. Effect of green tea powder on the quality attributes and antioxidant activity of whole-wheat flour pan bread. LWT 2017, 79, 342–348. [Google Scholar] [CrossRef]
- Shen, X.J.; Han, J.Y.; Ryu, G.H. Effects of the addition of green tea powder on the quality and antioxidant properties of vacuum-puffed and deep-fried Yukwa (rice snacks). LWT 2014, 55, 362–367. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Ni, D. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT 2012, 45, 8–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, W.; Ji, G.; Gao, C.; Chen, X.; Cao, Y.; Han, L. Effects of multiscale-mechanical grinding process on physicochemical properties of black tea particles and their water extracts. Food Bioprod. Process. 2017, 105, 171–178. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Y.; Fan, C.; Han, L. A method for producing superfine black tea powder with enhanced infusion and dispersion property. Food Chem. 2017, 214, 242–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, W.; Cao, Y.; Ji, G.; Gao, C.; Han, L. The effect of ultrafine and coarse grinding on the suspending and precipitating properties of black tea powder particles. J. Food Eng. 2018, 223, 124–131. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Xu, B.; Wang, B. Effects of superfine grinding on the physicochemical properties, antioxidant capacity, and hygroscopicity of Rosa rugosa cv. Plena powders. J. Sci. Food Agric. 2022, 102, 4192–4199. [Google Scholar] [CrossRef]
- ISO 14502-1; Determination of Substances Characteristic of Green and Black Tea—Part 1: Content of Total Polyphenols in Tea—Colorimetric Method Using Folin-Ciocalteu Reagent. International Organization for Standardization: Geneva, Switzerland, 2005.
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- GB 5009.88-2014: Determination of Dietary Fiber Content in Food. 2014. Available online: http://down.foodmate.net/standard/sort/3/47741.html (accessed on 25 August 2022).
- Eastwood, M.A.; Robertson, J.A.; Brydon, W.G.; MacDonald, D. Measurement of water-holding properties of fibre and their faecal bulking ability in man. Br. J. Nutr. 1983, 50, 539–547. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.; Wu, J.; Wang, Z. Functional properties and morphological characters of soluble dietary fibers in different edible parts of Angelica Keiskei. J. Food Sci. 2016, 81, C2189–C2198. [Google Scholar] [CrossRef]
- Ou, S.; Gao, K.; Li, Y. An in vitro study of wheat bran binding capacity for Hg, Cd, and Pb. J. Agric. Food Chem. 1999, 47, 4714–4717. [Google Scholar] [CrossRef]
- Fu, L.; Chen, H.; Dong, P.; Zhang, X.; Zhang, M. Effects of ultrasonic treatment on the physicochemical properties and DPPH radical scavenging activity of polysaccharides from mushroom Inonotus obliquus. J. Food Sci. 2010, 75, C322–C327. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, F.; Wei, X. Characterization and antioxidant activities of polysaccharides from leaves, flowers and seeds of green tea. Carbohydr. Polym. 2012, 88, 146–153. [Google Scholar] [CrossRef]
- Feng, H. Comparative Research on Leaf Tissues for Different Oolong Tea Cultivars in Northern Tea Region of Fujian Province. Masters’ Thesis, Fujian Agriculture and Forestry University, Fujian, China, 2010. [Google Scholar]
- Metzler, D.E. Biochemistry: The Chemical Reactions of Living Cells, 2nd ed.; Academic Press: New York, NY, USA, 2003; pp. 1–46. [Google Scholar]
- Chu, F.; Chen, H.; Sun, D.; He, H.; Ye, Y.; Tong, H. Effects of superfine grinding on the physicochemical properties of Congou black tea. J. Tea Sci. 2017, 37, 616–622. [Google Scholar]
- Long, V.D. Aqueous extraction of black leaf tea. III. Experiments with a stirred column. Int. J. Food Sci. Tech. 1979, 14, 449–462. [Google Scholar] [CrossRef]
- Siliveru, K.; Ambrose, R.K.; Vadlani, P.V. Significance of composition and particle size on the shear flow properties of wheat flour. J. Sci. Food Agric. 2017, 97, 2300–2306. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Shrestha, S. Study on the preparation technology of superfine ground powder of Agrocybe chaxingu Huang. J. Food Eng. 2005, 67, 333–337. [Google Scholar] [CrossRef]
- Huang, X.; Dou, J.; Li, D.; Wang, L. Effects of superfine grinding on properties of sugar beet pulp powders. LWT 2018, 87, 203–209. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.; Pan, M.; Lai, C.; Ho, C. Black tea: Chemical analysis and stability. Food Funct. 2012, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, Y.; Pei, Y.; Gao, W.; Zhang, Z. Effect of process on physicochemical properties of oat bran soluble dietary fiber. J. Food Sci. 2009, 74, C628–C636. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, R.; Li, J. Effect of micronization technology on physicochemical and antioxidant properties of dietary fiber from buckwheat hulls. Biocatal. Agric. Biotechnol. 2014, 3, 30–34. [Google Scholar] [CrossRef]
- Zhu, K.; Huang, S.; Peng, W.; Qian, H.; Zhou, H. Effect of ultrafine grinding on hydration and antioxidant properties of wheat bran dietary fiber. Food Res. Int. 2010, 43, 943–948. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, J. Effect of ultrafine grinding on physicochemical and antioxidant properties of dietary fiber from wine grape pomace. Food Sci. Technol. Int. 2014, 20, 55–62. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Chen, Y. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef]
- Galisteo, M.; Duarte, J.; Zarzuelo, A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008, 19, 71–84. [Google Scholar] [CrossRef]
- Wong, K.; Cheung, P.C.K.; Wu, J. Biochemical and microstructural characteristics of insoluble and soluble dietary fiber prepared from mushroom Sclerotia of Pleurotus tuber-regium, Polyporus rhinocerus, and Wolfiporia cocos. J. Agric. Food Chem. 2003, 51, 7197–7202. [Google Scholar] [CrossRef] [PubMed]
- Lebesi, D.M.; Tzia, C. Use of endoxylanase treated cereal brans for development of dietary fiber enriched cakes. Innov. Food Sci. Emerg. 2012, 13, 207–214. [Google Scholar] [CrossRef]
- Robin, F.; Schuchmann, H.P.; Palzer, S. Dietary fiber in extruded cereals: Limitations and opportunities. Trends Food Sci. Technol. 2012, 28, 23–32. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Sibakov, J.; Lahtinen, P.; Poutanen, K. Wet grinding and microfluidization of wheat bran preparations: Improvement of dispersion stability by structural disintegration. J. Cereal Sci. 2015, 64, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, G.; Xiao, W.; Han, L. 2014 Changes to the physicochemical characteristics of wheat straw by mechanical ultrafine grinding. Cellulose 2014, 21, 3257–3268. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Hemdane, S.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Study of hydration properties of wheat bran as a function of particle size. Food Chem. 2015, 179, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Femenia, A.I.O.F.; Lefebvre, A.C.; Thebaudin, J.Y.; Robertson, J.A.; Bourgeois, C.M. Physical and sensory properties of model foods supplemented with cauliflower fiber. J. Food Sci. 1997, 62, 635–639. [Google Scholar] [CrossRef]
- Simal, S.; Femenía, A.; Llull, P.; Rosselló, C. Dehydration of aloe vera: Simulation of drying curves and evaluation of functional properties. J. Food Eng. 2000, 43, 109–114. [Google Scholar] [CrossRef]
- Benhima, H.; Chiban, M.; Sinan, F.; Seta, P.; Persin, M. Removal of lead and cadmium ions from aqueous solution by adsorption onto micro-particles of dry plants. Colloid Surf. B-Biointerfaces 2008, 61, 10–16. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Zhao, Z.; Huangfu, L.; Dong, L.; Liu, S. Functional groups and antioxidant activities of polysaccharides from five categories of tea. Ind. Crop Prod. 2014, 58, 31–35. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Poutanen, K.; Micard, V. How does wheat grain, bran and aleurone structure impact their nutritional and technological properties? Trends Food Sci. Technol. 2015, 41, 118–134. [Google Scholar] [CrossRef]

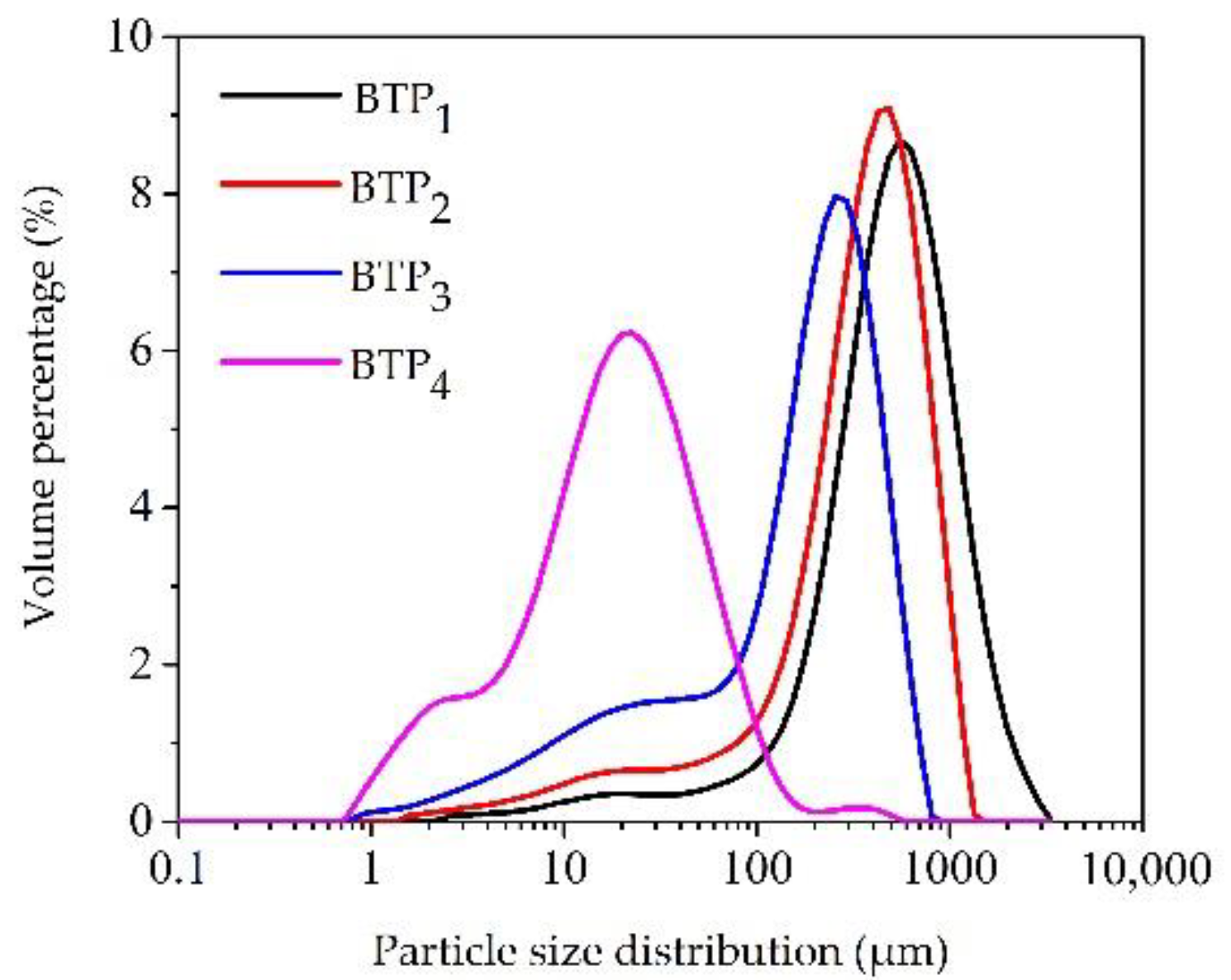
| Tea Sample | Particle Size | Color Appearance | Powder Flowability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| D10 (μm) | D50 (μm) | D90 (μm) | L | a | b | BD (g/cm3) | TD (g/cm3) | RA (°) | |
| BTP1 | 151.33 ± 1.53 d | 512.00 ± 3.46 d | 1203.33 ± 20.82 d | 39.45 ± 0.04 a | 7.72 ± 0.02 b | 20.88 ± 0.07 a | 0.59 ± 0.01 d | 0.74 ± 0.03 a | 38.67 ± 0.55 a |
| BTP2 | 53.90 ± 1.35 c | 366.67 ± 5.69 c | 767.33 ± 11.37 c | 46.05 ± 0.12 b | 8.19 ± 0.08 d | 25.05 ± 0.04 b | 0.56 ± 0.01 c | 0.78 ± 0.00 b | 43.97 ± 1.86 b |
| BTP3 | 13.50 ± 0.36 b | 187.33 ± 1.53 b | 434.00 ± 3.61 b | 50.05 ± 0.17 c | 7.94 ± 0.03 c | 26.55 ± 0.08 c | 0.54 ± 0.00 b | 0.85 ± 0.00 c | 45.90 ± 0.52 c |
| BTP4 | 3.18 ± 0.04 a | 18.53 ± 0.25 a | 60.23 ± 1.54 a | 55.78 ± 0.18 d | 5.97 ± 0.03 a | 28.85 ± 0.07 d | 0.37 ± 0.00 a | 0.90 ± 0.00 d | 46.67 ± 0.42 c |
| Tea Sample | TP (%) | Proteins (%) | SDF (%) | IDF (%) | TDF (%) |
|---|---|---|---|---|---|
| BTP1 | 13.34 ± 0.41 a | 27.75 ± 0.47 a | 3.70 ± 0.44 a | 38.60 ± 0.10 c | 42.30 ± 0.33 b |
| BTP2 | 13.88 ± 0.69 a | 28.09 ± 0.19 a | 4.66 ± 0.02 b | 37.80 ± 0.49 c | 42.45 ± 0.47 b |
| BTP3 | 13.18 ± 0.26 a | 27.88 ± 0.64 a | 6.51 ± 0.09 c | 35.59 ± 0.29 b | 42.10 ± 0.21 b |
| BTP4 | 13.32 ± 0.36 a | 27.63 ± 0.36 a | 10.41 ± 0.22 d | 25.81 ± 0.48 a | 36.21 ± 0.26 a |
| Tea Sample | WHC (g/g) | OHC (g/g) | Pb2+ AC (mg/g) | IC50 (μg/mL) |
|---|---|---|---|---|
| BTP1 | 4.19 ± 0.05 c | 3.74 ± 0.10 a | 1.99 ± 0.04 a | 224.22 ± 1.28 d |
| BTP2 | 4.16 ± 0.09 c | 3.71 ± 0.20 a | 1.98 ± 0.00 a | 214.17 ± 1.32 c |
| BTP3 | 3.66 ± 0.06 b | 3.74 ± 0.20 a | 1.99 ± 0.03 a | 181.72 ± 0.94 b |
| BTP4 | 1.53 ± 0.05 a | 4.08 ± 0.08 b | 1.97 ± 0.10 a | 156.36 ± 4.41 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xiao, W.; Han, L. Effects of Multiscale Mechanical Pulverization on the Physicochemical and Functional Properties of Black Tea. Foods 2022, 11, 2651. https://doi.org/10.3390/foods11172651
Zhang Y, Xiao W, Han L. Effects of Multiscale Mechanical Pulverization on the Physicochemical and Functional Properties of Black Tea. Foods. 2022; 11(17):2651. https://doi.org/10.3390/foods11172651
Chicago/Turabian StyleZhang, Yang, Weihua Xiao, and Lujia Han. 2022. "Effects of Multiscale Mechanical Pulverization on the Physicochemical and Functional Properties of Black Tea" Foods 11, no. 17: 2651. https://doi.org/10.3390/foods11172651
APA StyleZhang, Y., Xiao, W., & Han, L. (2022). Effects of Multiscale Mechanical Pulverization on the Physicochemical and Functional Properties of Black Tea. Foods, 11(17), 2651. https://doi.org/10.3390/foods11172651





