C-Terminal Modification on the Immunomodulatory Activity, Antioxidant Activity, and Structure–Activity Relationship of Pulsed Electric Field (PEF)-Treated Pine Nut Peptide
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. PEF Treatment on Peptides
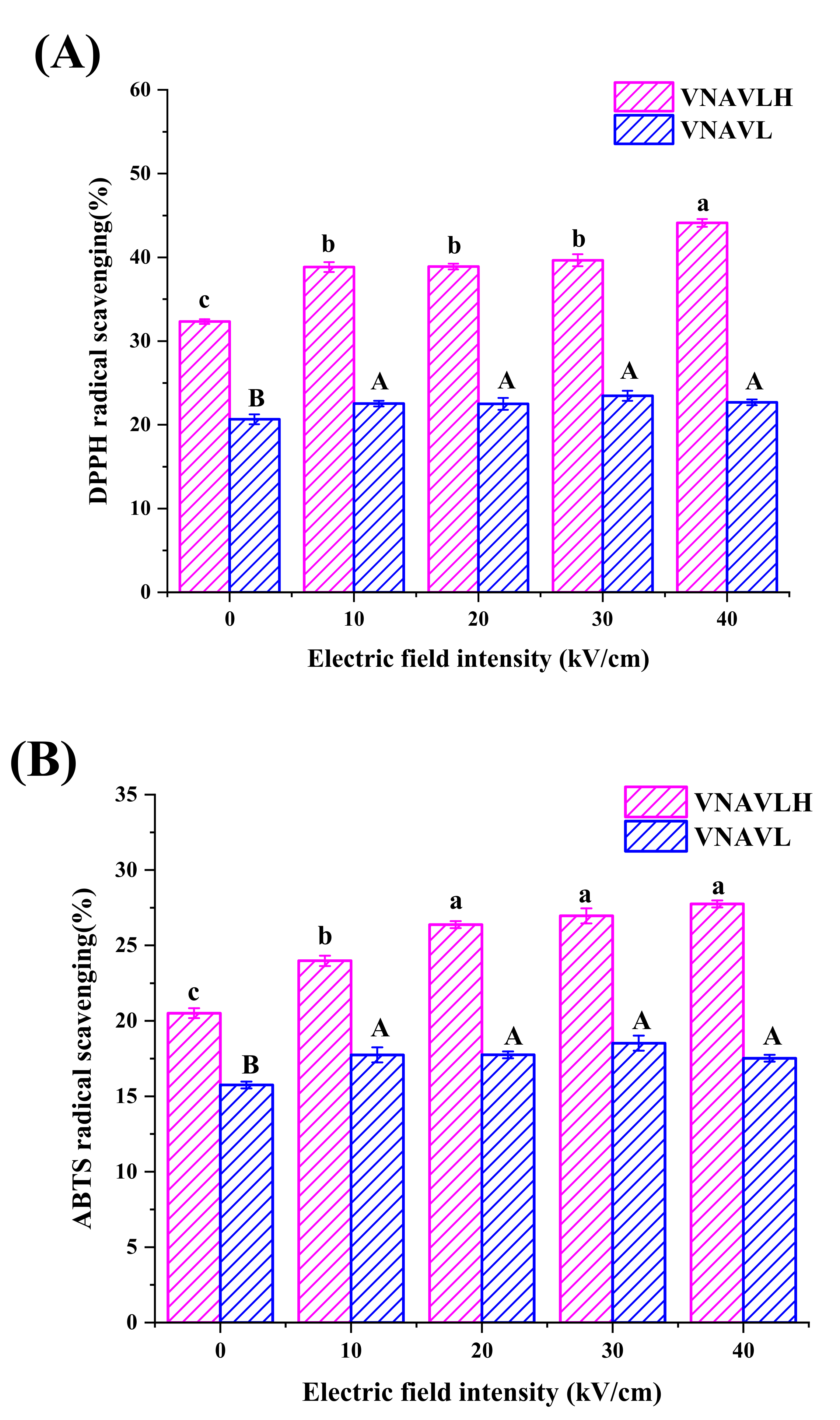
2.3. Determination of DPPH Radical Scavenging
2.4. Determination of ABTS Radical Scavenging
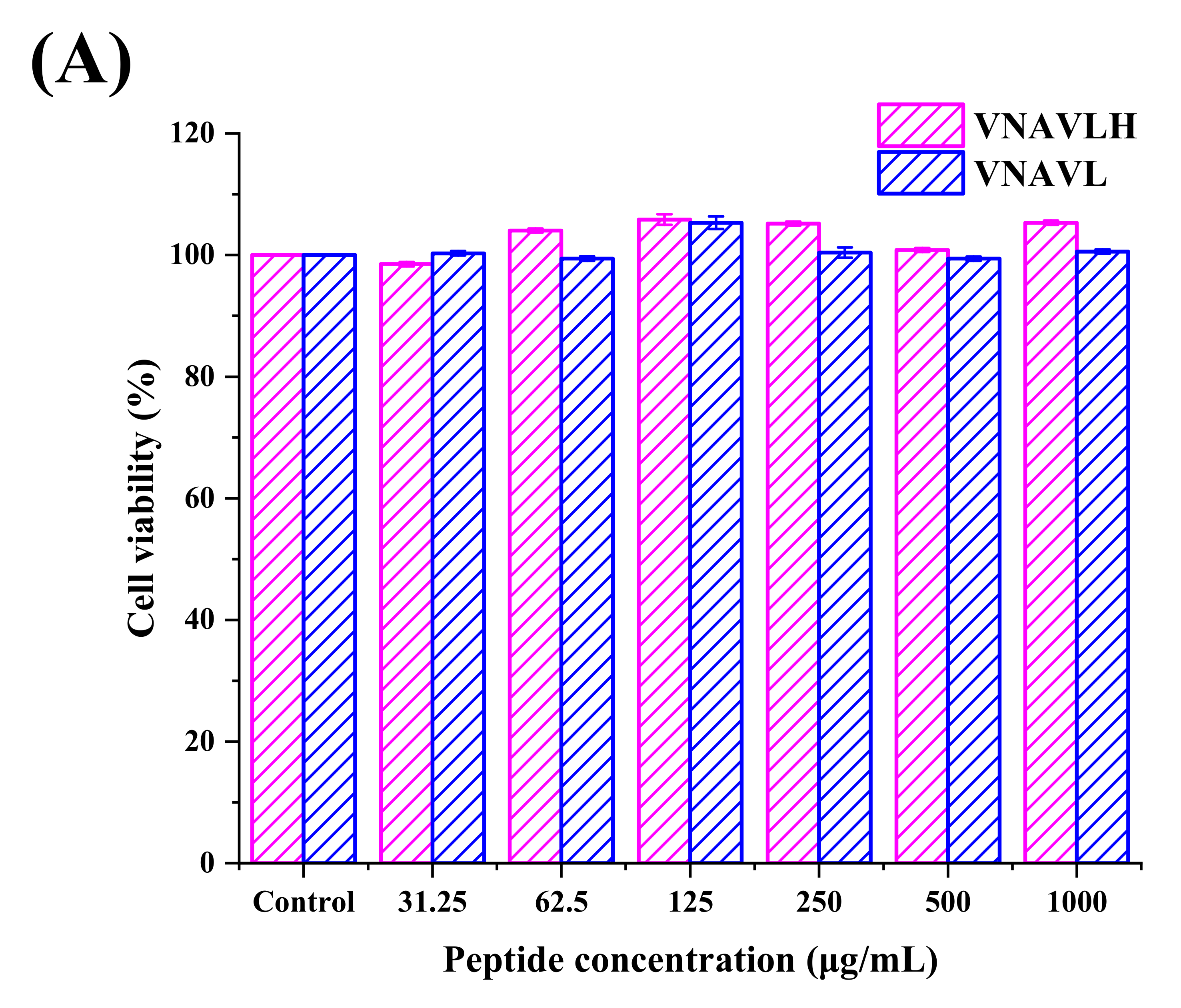
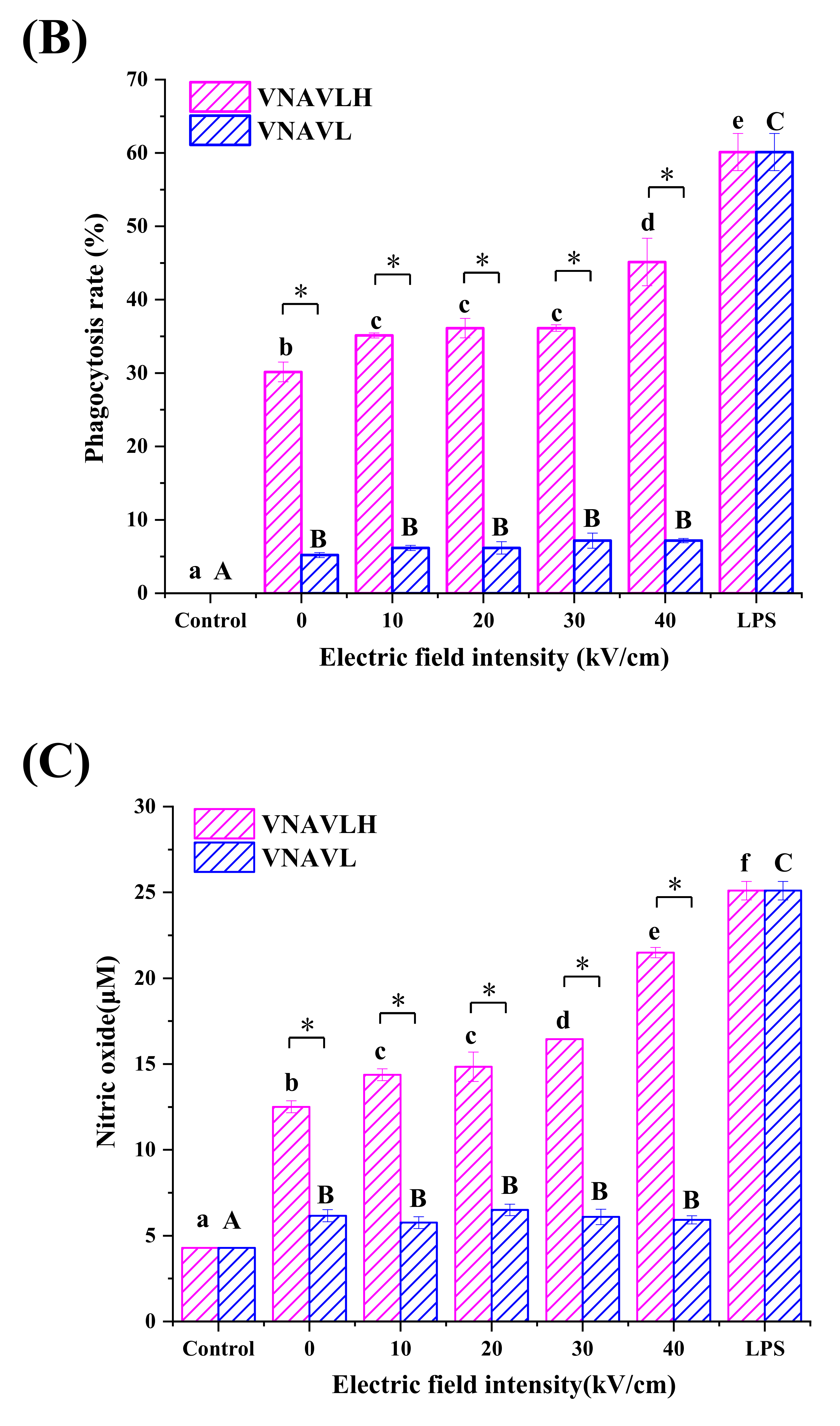
2.5. Determination of Phagocytosis Assay in RAW 264.7 Cells
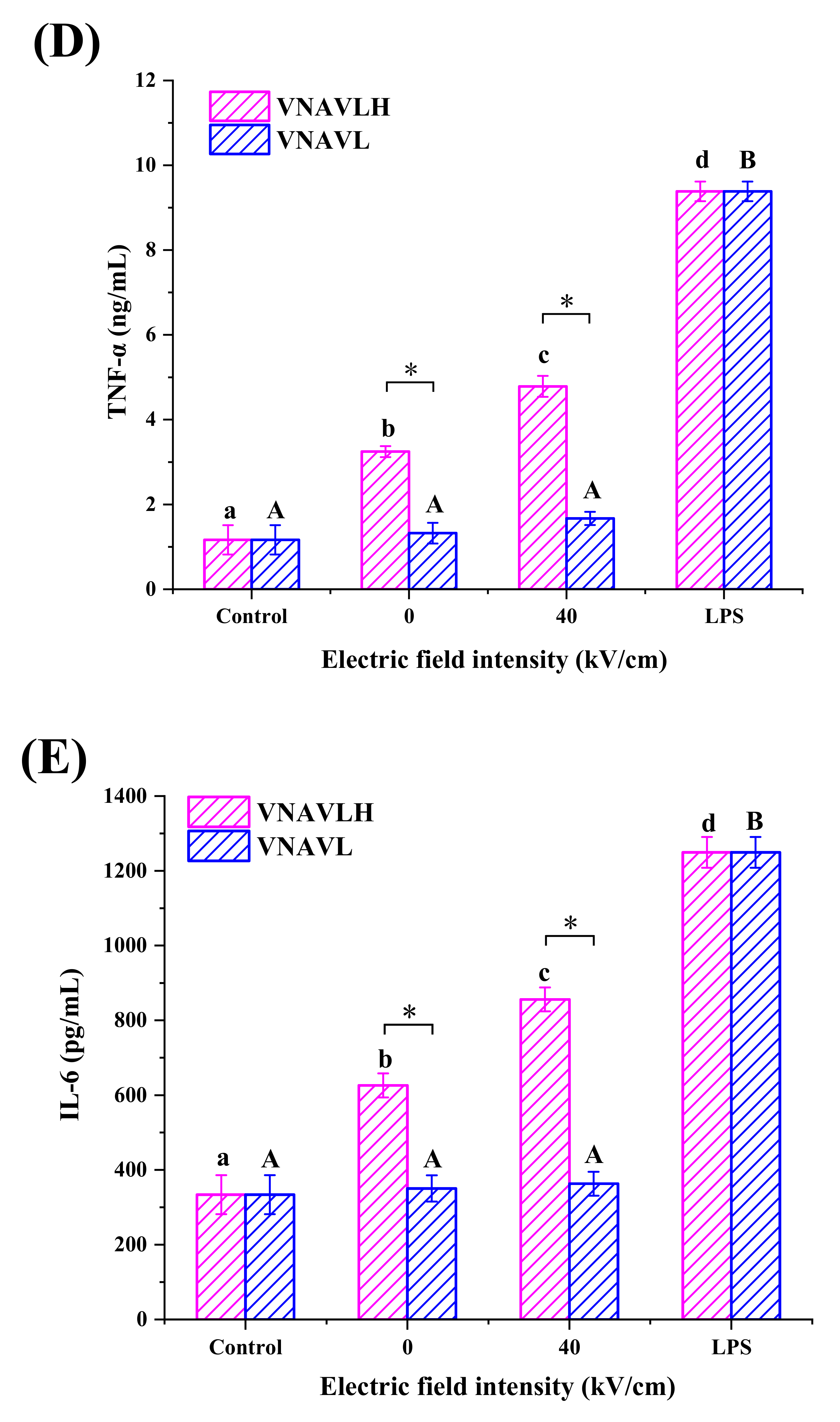
2.6. Determination of NO, TNF-α and IL-6 Production in RAW 264.7 Cells
2.7. CD Spectroscopy Assay
2.8. Molecule Dynamics Simulation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity of Peptides
3.2. Immunomodulatory Activity of Peptides in RAW 264.7
3.3. Effects of PEF Treatment on Secondary Structure of Peptides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Dong, Y.; Bao, Z.; Zhang, S.; Lin, S.; Sun, N. Advances in the activity evaluation and cellular regulation pathways of food-derived antioxidant peptides. Trends Food Sci. Technol. 2022, 122, 171–186. [Google Scholar] [CrossRef]
- Sun, X.; Udenigwe, C. Chemistry and biofunctional significance of bioactive peptide interactions with food and gut components. J. Agric. Food Chem. 2020, 68, 12972–12977. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Hammami, R. Recent insights into structure-function relationships of antimicrobial peptides. J. Food Biochem. 2018, 43, e12546. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Food-derived Bioactive Peptides—Opportunities for Designing Future Foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Tsopmo, A. Reactivity of peptides within the food matrix. J. Food Biochem. 2019, 43, e12489. [Google Scholar] [CrossRef]
- Gusbeth, C.; Frey, W.; Volkmann, H.; Schwartz, T.; Bluhm, H. Pulsed electric field treatment for bacteria reduction and its impact on hospital wastewater. Chemosphere 2009, 75, 228–233. [Google Scholar] [CrossRef]
- Loginova, K.; Vorobiev, E.; Bals, O.; Lebovka, N. Pilot study of countercurrent cold and mild heat extraction of sugar from sugar beets, assisted by pulsed electric fields. J. Food Eng. 2011, 102, 340–347. [Google Scholar] [CrossRef]
- Puertolas, E.; Lopez, N.; Saldana, G.; Alvarez, I.; Raso, J. Evaluation of phenolic extraction during fermentation of red grapes treated by a continuous pulsed electric fields process at pilot-plant scale. J. Food Eng. 2010, 98, 120–125. [Google Scholar] [CrossRef]
- Marczak, F.; Damasceno, L.; Nadia, B.; Eugene, V.; Christelle, B.; Tessaro, C.I.; Marczak, L.D.F.; Vorobiev, E. Effect of pulsed electric fields and high voltage electrical discharges on polyphenol and protein extraction from sesame cake. Innov. Food Sci. Emerg. Technol. 2015, 29, 170–177. [Google Scholar]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Ding, J.; Zhong, L.; Sun, N.; Lin, S. Evaluation of the structure-activity relationship between allergenicity and spatial conformation of ovalbumin treated by pulsed electric field. Food Chem. 2022, 388, 133018. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Gao, Y.; Bao, Z.; Sun, N.; Lin, S. Mechanism of trypsin activation by pulsed electric field treatment revealed based on chemical experiments and molecular dynamics simulations. Food Chem. 2022, 394, 133477. [Google Scholar] [CrossRef]
- Sun, L.; Li, M.; Zhang, S.; Bao, Z.; Lin, S. Mechanism of Ser-Ala-Gly-Pro-Ala-Phe Treatment by Pulsed Electric Field to Improve Ethanol-Induced Gastric Mucosa Injury in Mice. Food Funct. 2022, 13, 6716–6725. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Xing, J.; Lin, S. A possible mechanism for enhancing the antioxidant activity by pulsed electric field on pine nut peptide Glutamine-Tryptophan-Phenylalanine-Histidine. J. Food Biochem. 2019, 43, e12714. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, R.; Zhao, Y.; Zhang, S.; Lin, S. Immunomodulatory Activity Improvement of Pine Nut Peptides by a Pulsed Electric Field and Their Structure–Activity Relationships. J. Agric. Food Chem. 2019, 67, 3796–3810. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, L.; Sun, L.; Yang, Y.; Lin, S. Use of a Combination of the MD Simulations and NMR Spectroscopy to Determine the Regulatory Mechanism of Pulsed Electric Field (PEF) Targeting at C-Terminal Histidine of VNAVLH. Food Chem. 2020, 334, 127554. [Google Scholar] [CrossRef]
- Lin, S.; Jin, Y.; Liu, M.; Yang, Y.; Zhang, M.; Guo, Y.; Jones, G.; Liu, J.; Yin, Y. Research on the preparation of antioxidant peptides derived from egg white with assisting of high-intensity pulsed electric field. Food Chem. 2013, 139, 300–306. [Google Scholar] [CrossRef]
- Lin, S.; Wang, J.; Zhao, P.; Pang, Y.; Ye, H.; Yuan, Y.; Liu, J.; Jones, G. Optimized antioxidant peptides fractions preparation and secondary structure analysis by MIR. Int. J. Biol. Macromol. 2013, 59, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tironi, V.A.; Añón, M.C. Amaranth proteins as a source of antioxidant peptides: Effect of proteolysis. Food Res. Int. 2010, 43, 315–322. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra. J. Funct. Foods 2017, 28, 64–75. [Google Scholar] [CrossRef]
- Devi, K.S.P.; Maiti, T.K. Immunomodulatory and Anti-cancer Properties of Pharmacologically Relevant Mushroom Glycans. Recent Pat. Biotechnol. 2016, 10, 72–78. [Google Scholar]
- Dai, Z.; Di, S.; Zhang, Y.; Sun, Y.; Zeng, X. Immunomodulatory Activity in Vitro and in Vivo of Verbascose from Mung Beans (Phaseolus aureus). J. Agric. Food Chem. 2014, 62, 10727–10735. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]
- Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Berendsen, H. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Berezin, K.V.; Dvoretski, K.N.; Chernavina, M.L.; Likhter, A.M.; Smirnov, V.V.; Shagautdinova, I.T.; Antonova, E.M.; Stepanovich, E.Y.; Dzhalmuhambetova, E.A.; Tuchin, V.V. Molecular modeling of immersion optical clearing of biological tissues. J. Mol. Model. 2018, 24, 45. [Google Scholar] [CrossRef]
- Ranjan, A.; Chauhan, A.; Jindal, T. In-silico and in-vitro evaluation of human acetylcholinesterase inhibition by organophosphates. Environ. Toxicol. Pharmacol. 2018, 57, 131–140. [Google Scholar] [CrossRef]
- Vanga, S.; Wang, J.; Singh, A.; Raghavan, G. Simulations of Temperature and Pressure Unfolding in Soy Allergen Gly m 4 Using Molecular Modeling. J. Agric. Food Chem. 2019, 67, 12547–12557. [Google Scholar] [CrossRef]
- Ao, J.; Li, B. Amino acid composition and antioxidant activities of hydrolysates and peptide fractions from porcine collagen. Food Sci. Technol. Int. 2012, 18, 425–434. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, P.; Chen, G.; Cao, J.; Huang, T.; Chen, K. Macrophage immunomodulatory activity of extracellular polysaccharide (PEP) of Antarctic bacterium Pseudoaltermonas sp.S-5. Int. Immunopharmacol. 2012, 12, 611–617. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, F.; Wang, J.; Jin, Z.; Xu, X. Macrophage immunomodulatory activity of polysaccharides isolated from Glycyrrhiza uralensis fish. Int. Immunopharmacol. 2008, 8, 43–50. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Wang, Z.; Wang, Y.; Xie, M.; Xie, J. Sulfated polysaccharide from Cyclocarya paliurus enhances the immunomodulatory activity of macrophages. Carbohydr. Polym. 2017, 174, 669–676. [Google Scholar] [CrossRef]
- He, P.; Wang, Q.; Zhan, Q.; Pan, L.M.; Zhang, M. Purification and Characterization of Immunomodulatory Peptides from Enzymatic Hydrolysates of Duck Egg Ovalbumin. Food Funct. 2021, 12, 668–681. [Google Scholar] [CrossRef]
- Marlborough, D.I.; Fisher, G.H.; Ryan, J.W. Circular dichroism spectra of some lower homologs of bradykinin potentiating peptide 9α. Arch. Biochem. Biophys. 1981, 210, 43–48. [Google Scholar] [CrossRef]
- Shojapour, M.; Fatemi, F.; Farahmand, S.; Shasaltaneh, M. Investigation of Cyc1 protein structure stability after H53I mutation using computational approaches. J. Mol. Graph. Model. 2021, 105, 107864. [Google Scholar] [CrossRef]
- Yun, S.; Guy, H.R. Stability tests on known and misfolded structures with discrete and all atom molecular dynamics simulations. J. Mol. Graph. Model. 2011, 29, 663–675. [Google Scholar] [CrossRef] [Green Version]




| Secondary Structure | VNAVLH (%) | PEF-VNAVLH (%) | VNAVL (%) | PEF-VNAVL (%) |
|---|---|---|---|---|
| α-Helix | 2.7 ± 0.1 | 4.7 ± 0.1 | - | - |
| β-sheet | 19.9 ± 0.15 | 12.5 ± 0.2 | 35.1 ± 0.25 | 32.9 ± 0.2 |
| Random coil | 77.4 ± 0.25 | 82.8 ± 0.3 | 64.9 ± 0.25 | 67.1 ± 0.2 |
| Secondary Structure | VNAVLH | PEF- VNAVLH | Difference Amount | VNAVL | PEF- VNAVL | Difference Amount |
|---|---|---|---|---|---|---|
| Coil | 4.703 | 4.747 | 0.044↑ | 3.984 | 3.990 | 0.006↑ |
| Bend | 0.196 | 0.204 | 0.008↑ | - | - | - |
| Turn | 0.100 | 0.048 | 0.052↓ | 0.015 | 0.009 | 0.006↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Dong, L.; Bao, Z.; Lin, S. C-Terminal Modification on the Immunomodulatory Activity, Antioxidant Activity, and Structure–Activity Relationship of Pulsed Electric Field (PEF)-Treated Pine Nut Peptide. Foods 2022, 11, 2649. https://doi.org/10.3390/foods11172649
Zhang S, Dong L, Bao Z, Lin S. C-Terminal Modification on the Immunomodulatory Activity, Antioxidant Activity, and Structure–Activity Relationship of Pulsed Electric Field (PEF)-Treated Pine Nut Peptide. Foods. 2022; 11(17):2649. https://doi.org/10.3390/foods11172649
Chicago/Turabian StyleZhang, Shuyu, Liu Dong, Zhijie Bao, and Songyi Lin. 2022. "C-Terminal Modification on the Immunomodulatory Activity, Antioxidant Activity, and Structure–Activity Relationship of Pulsed Electric Field (PEF)-Treated Pine Nut Peptide" Foods 11, no. 17: 2649. https://doi.org/10.3390/foods11172649
APA StyleZhang, S., Dong, L., Bao, Z., & Lin, S. (2022). C-Terminal Modification on the Immunomodulatory Activity, Antioxidant Activity, and Structure–Activity Relationship of Pulsed Electric Field (PEF)-Treated Pine Nut Peptide. Foods, 11(17), 2649. https://doi.org/10.3390/foods11172649






