A Rapid Fluorescence Sensor for the Direct Quantification of Rongalite in Foodstuffs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Characterization of the Fluorescence Sensing Platform
2.3. Dopamine and Resorcinol Concentration-Response
2.4. Reaction Conditions Optimization
2.4.1. pH Values Optimization
2.4.2. Carbonate Concentration Optimization
2.4.3. Reaction Time Optimization
2.5. Fluorescence Sensing Platform Development
2.6. Sample Preparation and Method Validation
2.6.1. Sample Preparation
2.6.2. Limit of Detection
2.6.3. Method Validation
3. Results and Discussion
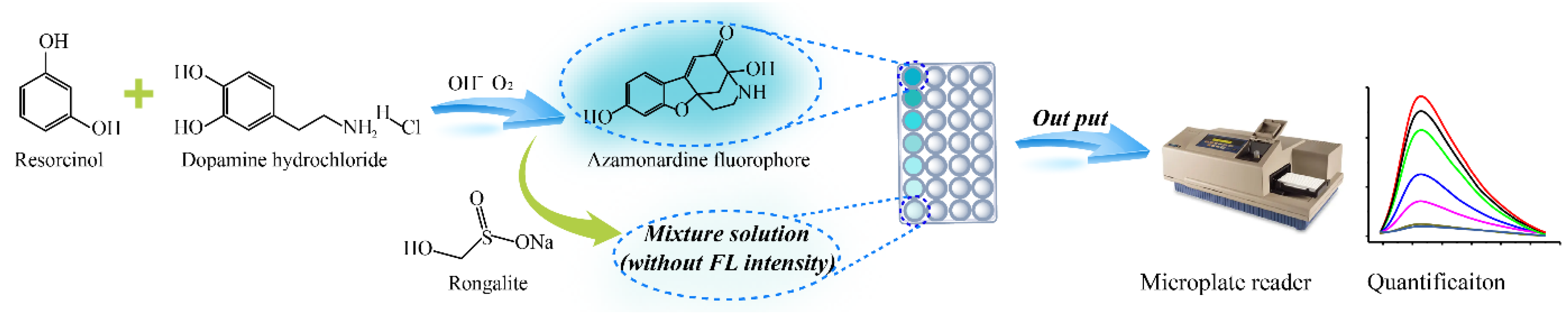
3.1. Assay Principle for the Fluorescence Sensing Platform
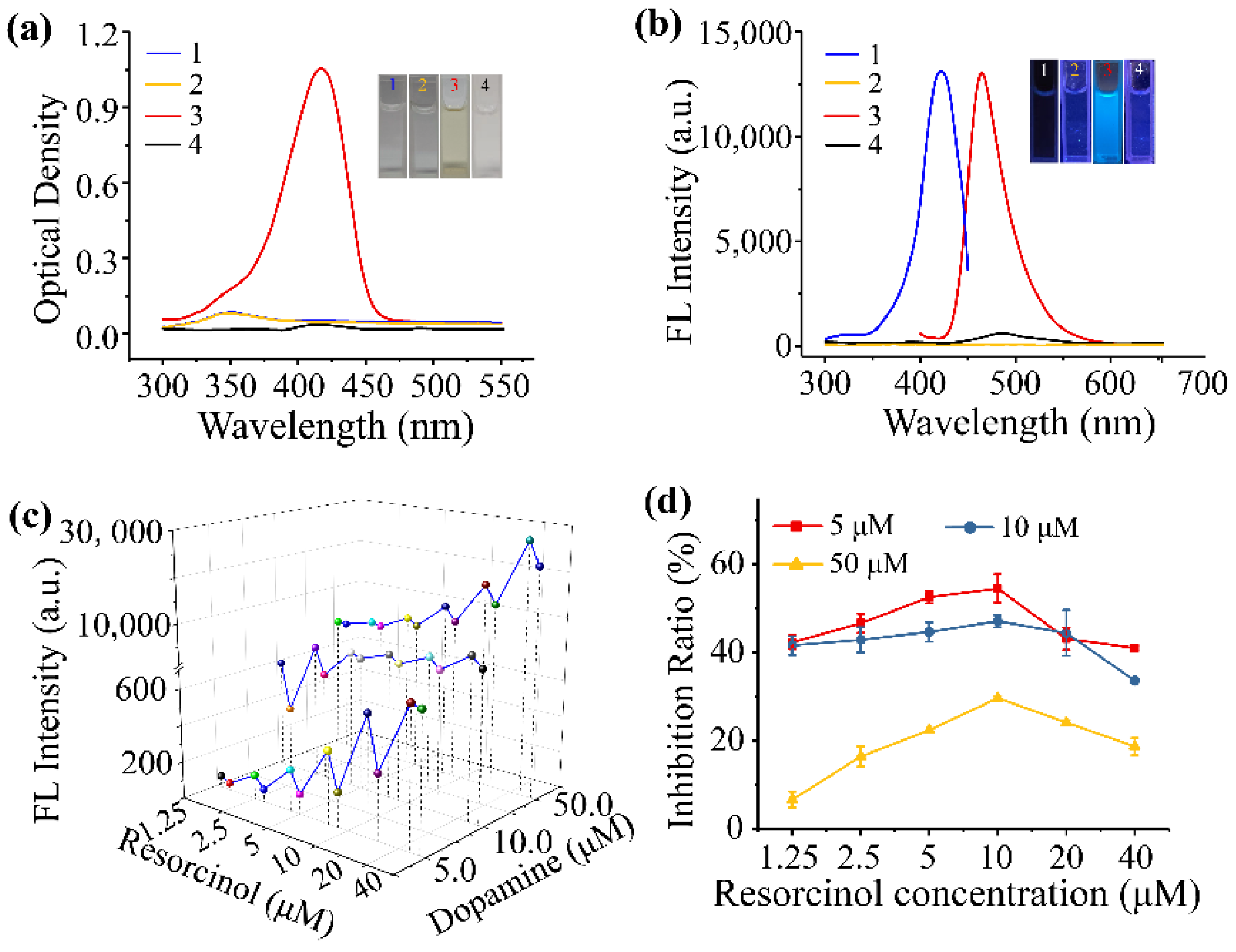
3.2. Dopamine and Resorcinol Concentration–Response
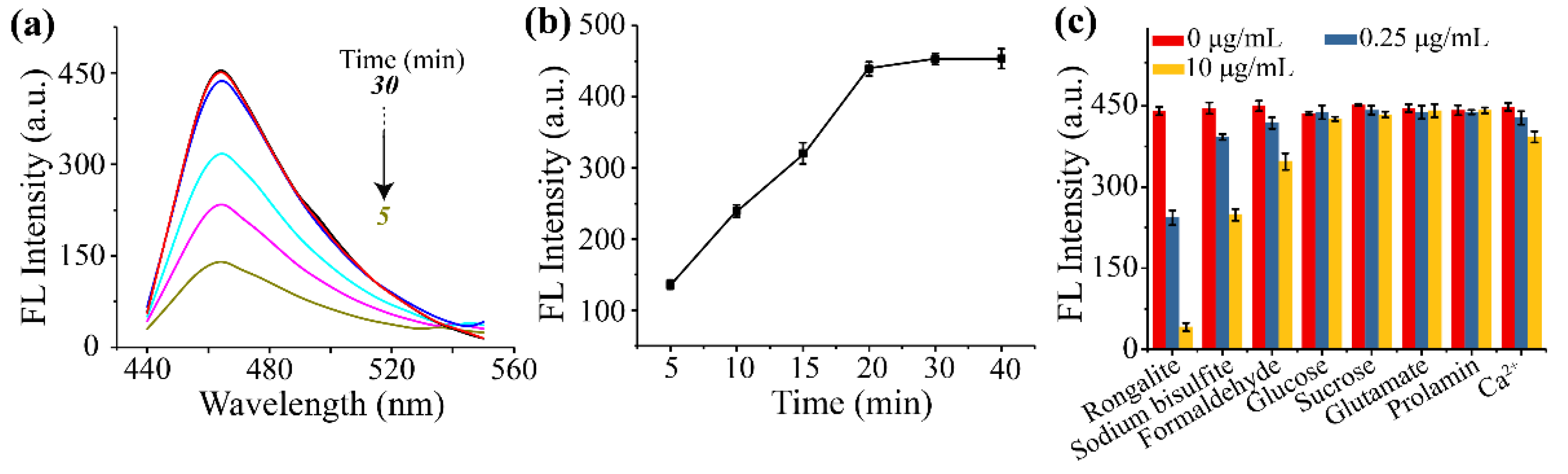
3.3. Optimization of Reaction pH, Carbonate Concentration and Response Time
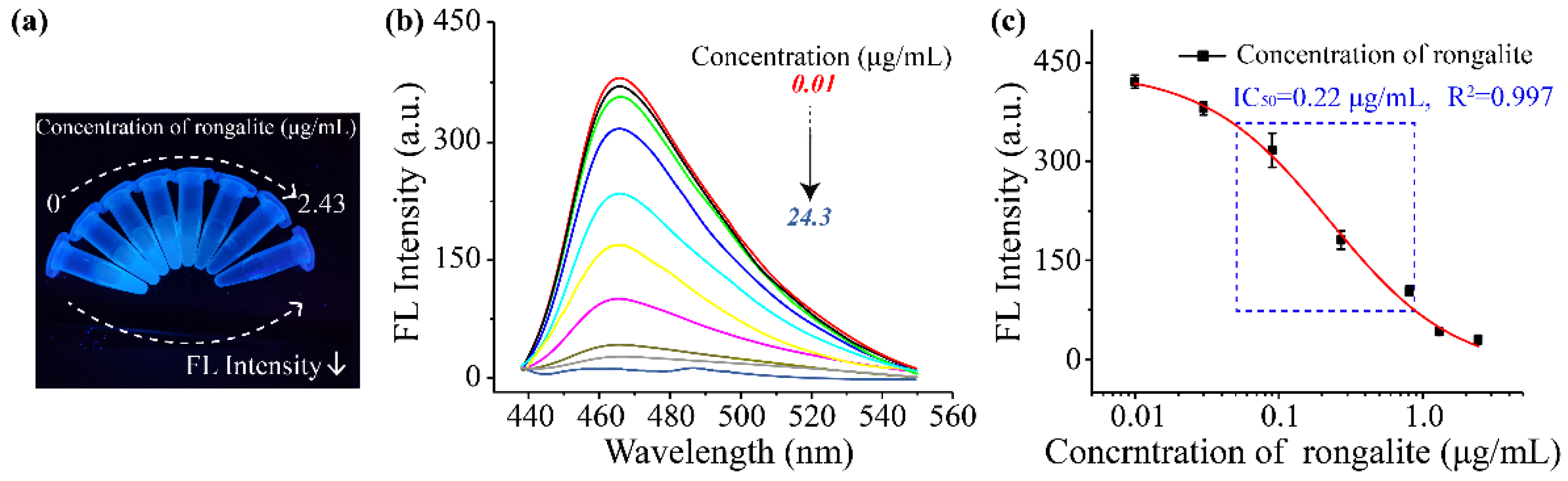
3.4. Development of Fluorescence Sensor
3.5. Accuracy of the Fluorescence Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, R. New dimensions in rongalite chemistry: The land of opportunities in organic synthesis and material sciences. ChemistrySelect 2020, 5, 10795–10815. [Google Scholar] [CrossRef]
- Aravindu, K.; Cloutet, E.; Brochon, C.; Hadziioannou, G.; Vignolle, J.; Robert, F.; Taton, D.; Landais, Y. Poly(arylene vinylene) synthesis via a precursor step-growth polymerization route involving the ramberg-bäcklund reaction as a key post-chemical modification step. Macromolecules 2018, 51, 5852–5862. [Google Scholar] [CrossRef]
- Yemata, T.A.; Zheng, Y.; Ko Kyaw, A.K.; Wang, X.; Song, J.; Chin, W.S.; Xu, J. Sodium formaldehyde sulfoxylate, an ionic-type, water-soluble reducing reagent to effectively improve seebeck coefficient of PEDOT:PSS film. Org. Electron. 2020, 81, 105682. [Google Scholar] [CrossRef]
- Kotha, S.; Khedkar, P. Rongalite: A useful green reagent in organic synthesis. Chem. Rev. 2012, 112, 1650–1680. [Google Scholar] [CrossRef]
- Makarov, S.V.; Horvath, A.K.; Silaghi-dumitrescu, R.; Gao, Q. Reactivity of small oxoacids of sulfur. Molecules 2019, 24, 2768. [Google Scholar] [CrossRef]
- Kamps, J.J.A.G.; Hopkinson, R.J.; Schofield, C.J.; Claridge, T.D.W. How formaldehyde reacts with amino acids. Commun. Chem. 2019, 2, 126. [Google Scholar] [CrossRef]
- Nowshad, F.; Islam, M.N.; Khan, M.S. Concentration and formation behavior of naturally occurring formaldehyde in foods. Agric. Food Secur. 2018, 7, 17. [Google Scholar] [CrossRef]
- Makarov, S.V.; Horvath, A.K.; Silaghi-dumitrescu, R.; Gao, Q. Sodium Dithionite, Rongalite and Thiourea Oxides: Chemistry and Application; World Scientific: London, UK, 2016. [Google Scholar]
- Abdussalam, A.; Yuan, F.; Ma, X.; Du, F.; Zholudov, Y.T.; Zafar, M.N.; Xu, G. Tris(2,2′-bipyridine)ruthenium(II) electrochemiluminescence using rongalite as coreactant and its application in detection of foodstuff adulteration. J. Electroanal. Chem. 2020, 872, 114649. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Peng, X.; Hu, C.; Huang, J.; He, H. Uncertainty evaluation of high performance liquid chromatographic determination of sodium formaldehyde sulfoxylate (rongalite) in wheat powder and rice powder. Food Sci. 2020, 30, 205–208. [Google Scholar]
- de Carvalho, L.M.; Schwedt, G. Sulfur speciation by capillary zone electrophoresis. J. Chromatogr. A 2005, 1099, 185–190. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, F.; Huang, L.; Wang, L. Electrochemical nonenzymatic sensor based on cetyltrimethylammonium bromide and chitosan functionalized carbon nanotube modified glassy carbon electrode for the determination of hydroxymethanesulfinate in the presence of sulfite in foods. Food Chem. 2018, 259, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Mu, T.; Wu, Y.; Yu, X.; Song, J.; Zhou, J.; Yang, H. Determination of sodium formaldehyde sulfoxylate residues in wheat flour and bean starch noodles by capillary electrophoresis-contactless conductivity detection. Food Sci. 2015, 36, 216–219. [Google Scholar]
- Zhang, Y.; Pan, X.; Zhang, L. Determination of sodium formaldehyde sulfoxylate in food by high-performance liquid chromatography. J. Food Saf. Qual. 2017, 8, 1470–1474. [Google Scholar]
- Li, J.; Jing, L.; Song, Y.; Zhang, J.; Chen, Q.; Wang, B.; Xia, X.; Han, Q. Rapid detection of rongalite via a sandwich lateral flow strip assay using a pair of aptamers. Nanoscale Res. Lett. 2018, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Li, J.; Qin, M.; Song, Y.; Zhang, J.; Chen, Q.; Xia, X.; Han, Q. Development and characterization of sandwich-type enzyme-linked aptamer assay for the detection of rongalite in food. Anal. Biochem. 2018, 563, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Goshisht, M.K.; Tripathi, N. Fluorescence-based sensors as an emerging tool for anion detection: Mechanism, sensory materials and applications. J. Mater. Chem. C 2021, 9, 982–985. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent organic polymers and frameworks for fluorescence-based sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Guo, L.; Wang, Y.; Feng, L. Intelligent hybrid hydrogels for rapidin situ detection and photothermal therapy of bacterial infection. ACS Appl. Mater. Inter. 2020, 12, 39685–39694. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, Y.; Zhang, X.; Wang, S. Modulating an in situ fluorogenic reaction for the label-free ratiometric detection of biothiols. Analyst 2019, 144, 4520–4525. [Google Scholar] [CrossRef]
- Acuña, A.U.; Álvarez-Pérez, M.; Liras, M.; Coto, P.B.; Amat-Guerri, F. Synthesis and photophysics of novel biocompatible fluorescent oxocines and azocines in aqueous solution. Phys. Chem. Chem. Phys. 2013, 15, 16704. [Google Scholar] [CrossRef]
- El Sayed, S.; Pascual, L.; Licchelli, M.; Martínez-Máñez, R.; Gil, S.; Costero, A.M.; Sancenón, F. Chromogenic detection of aqueous formaldehyde using functionalized silica nanoparticles. ACS Appl. Mater. Inter. 2016, 8, 14318–14322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, S.; Lu, S.; Liu, G.; Sun, J.; Yang, X. Fluorometric and colorimetric dual-readout immunoassay based on an alkaline phosphatase-triggered reaction. Anal. Chem. 2019, 91, 7828–7834. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Lu, S.; Bao, X.; Sun, J.; Yang, X. An enzyme cascade-triggered fluorogenic and chromogenic reaction applied in enzyme activity assay and immunoassay. Anal. Chem. 2018, 90, 7754–7760. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, G.; Sun, J.; Wang, Q.; Li, Z.; Yang, X. Dual-readout tyrosinase activity assay facilitated by a chromo-fluorogenic reaction between catechols and naphthoresorcin. Anal. Chem. 2020, 92, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; He, Q.; Zhong, Y.; Pan, J.; Lao, Z.; Lin, M.; Wang, T.; Cui, X.; Ding, J.; Zhao, S. An ultrasensitive colorimetric assay based on a multi-amplification strategy employing Pt/IrO2@SA@HRP nanoflowers for the detection of progesterone in saliva samples. Anal. Methods-Uk 2021, 13, 1164–1171. [Google Scholar] [CrossRef]
- Qie, Z.; Liu, Q.; Yan, W.; Gao, Z.; Meng, W.; Xiao, R.; Wang, S. Universal and ultrasensitive immunochromatographic assay by using an antigen as a bifunctional element and antialbumin antibody on a test line. Anal. Chem. 2019, 91, 9530–9537. [Google Scholar] [CrossRef]
- Wang, L.; Wen, L.; Zhao, L.; Chao, J.; Tao, F.; Wang, F.; Li, C. Development of fluorescence sensor and test paper based on molecularly imprinted carbon quantum dots for spiked detection of domoic acid in shellfish and lake water. Anal. Chim. Acta 2022, 1197, 339515. [Google Scholar] [CrossRef] [PubMed]
- Jayan, H.; Pu, H.; Sun, D. Recent development in rapid detection techniques for microorganism activities in food matrices using bio-recognition: A review. Trends Food Sci. Tech. 2020, 95, 233–246. [Google Scholar] [CrossRef]
- Wu, H.; Qian, C.; Wang, R.; Wu, C.; Wang, Z.; Wang, L.; Zhang, M.; Ye, Z.; Zhang, F.; He, J.; et al. Identification of pork in raw meat or cooked meatballs within 20 min using rapid PCR coupled with visual detection. Food Control 2020, 109, 106905. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Jiao, S.; Zhao, Y.; Guo, Y.; Wang, M.; Zhu, G. Quantum-dot-based lateral flow immunoassay for detection of neonicotinoid residues in tea leaves. J. Agric. Food Chem. 2017, 65, 10107–10114. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Li, S.; Zhao, Y.; Chen, D.; Wu, Y. Simultaneous determination of neonicotinoids and fipronils in tea using a modified QuEChERS method and liquid chromatography-high resolution mass spectrometry. Food Chem. 2020, 329, 127159. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, B.; Dou, L.; Yu, W.; Yu, X.; Wen, K.; Ke, Y.; Shen, J.; Wang, Z. Fluorescent lateral flow immunoassay for highly sensitive detection of eight anticoagulant rodenticides based on cadmium-free quantum dot-encapsulated nanospheres. Sensor. Actuat. B–Chem. 2020, 324, 128771. [Google Scholar] [CrossRef]
- Tian, L.; Guo, H.; Li, J.; Yan, L.; Zhu, E.; Liu, X.; Li, K. Fabrication of a near-infrared excitation surface molecular imprinting ratiometric fluorescent probe for sensitive and rapid detecting perfluorooctane sulfonate in complex matrix. J. Hazard. Mater. 2021, 413, 125353. [Google Scholar] [CrossRef] [PubMed]

| Samples | Spiked | Intra-Assay | Inter-Assay | ||
|---|---|---|---|---|---|
| (μg/g) | Recovery | CV 1 | Recovery | CV 1 | |
| (%) | (%) | (%) | (%) | ||
| Vermicelli | 1 | 80.7 | 12.6 | 82.3 | 12.1 |
| 2 | 88.4 | 8.1 | 85.3 | 10.1 | |
| 4 | 92.5 | 7.3 | 90.6 | 6.9 | |
| Wheat powder | 1 | 86.9 | 7.5 | 84.1 | 9.7 |
| 2 | 95.3 | 10.4 | 88.7 | 8.3 | |
| 4 | 102.1 | 6.9 | 95.8 | 5.4 | |
| Rice powder | 1 | 89.6 | 9.1 | 81.2 | 10.8 |
| 2 | 95.4 | 4.2 | 87.7 | 8.6 | |
| 4 | 100.5 | 5.4 | 90.5 | 7.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chen, J.; Huang, B.; Kong, L.; Sun, F.; Li, L.; Peng, C.; Cai, H.; Hou, R. A Rapid Fluorescence Sensor for the Direct Quantification of Rongalite in Foodstuffs. Foods 2022, 11, 2650. https://doi.org/10.3390/foods11172650
Li H, Chen J, Huang B, Kong L, Sun F, Li L, Peng C, Cai H, Hou R. A Rapid Fluorescence Sensor for the Direct Quantification of Rongalite in Foodstuffs. Foods. 2022; 11(17):2650. https://doi.org/10.3390/foods11172650
Chicago/Turabian StyleLi, Hongfang, Jie Chen, Baowei Huang, Lingwei Kong, Feifei Sun, Lin Li, Chuanyi Peng, Huimei Cai, and Ruyan Hou. 2022. "A Rapid Fluorescence Sensor for the Direct Quantification of Rongalite in Foodstuffs" Foods 11, no. 17: 2650. https://doi.org/10.3390/foods11172650
APA StyleLi, H., Chen, J., Huang, B., Kong, L., Sun, F., Li, L., Peng, C., Cai, H., & Hou, R. (2022). A Rapid Fluorescence Sensor for the Direct Quantification of Rongalite in Foodstuffs. Foods, 11(17), 2650. https://doi.org/10.3390/foods11172650









