Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging
Abstract
1. Introduction
2. Materials and Methods
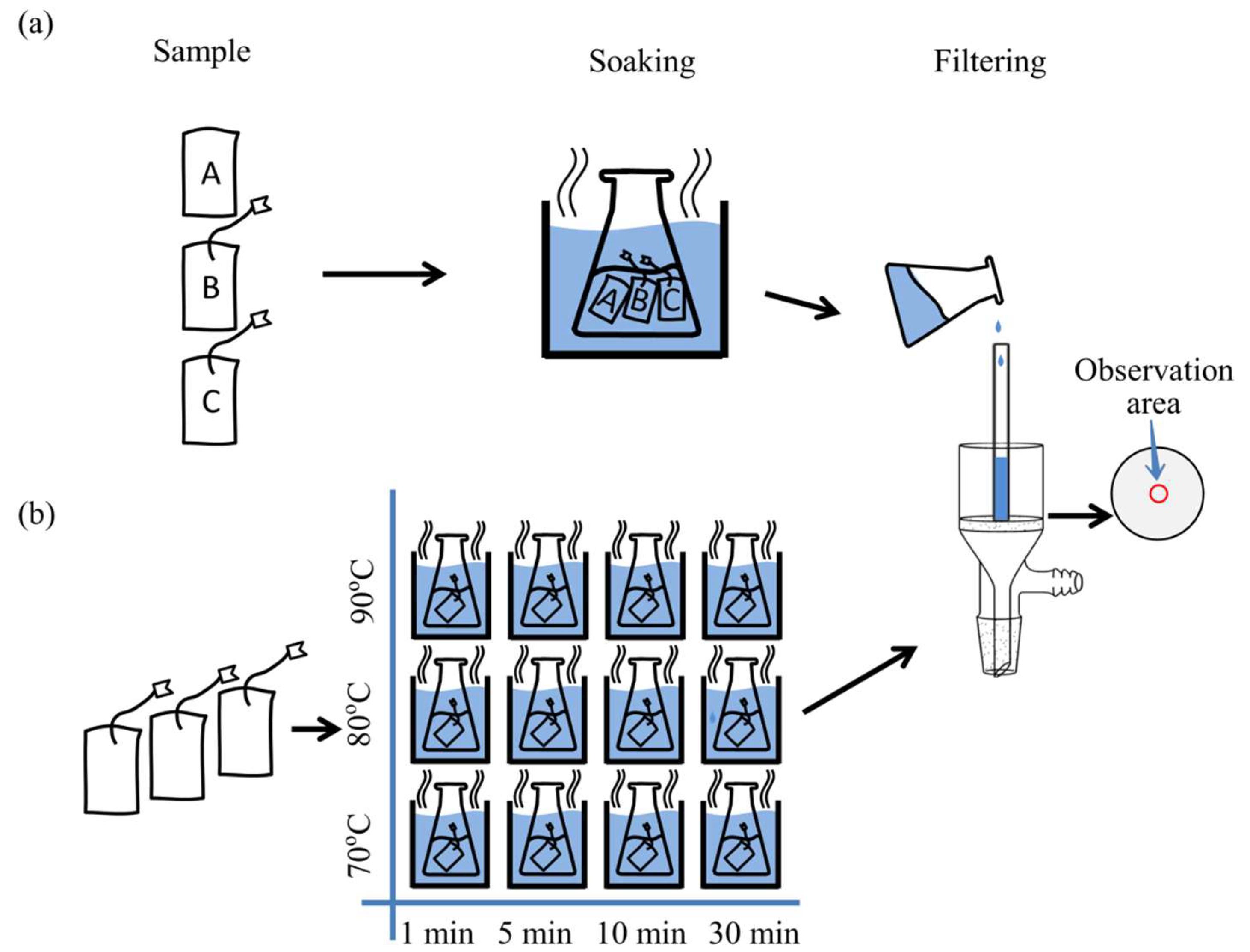
2.1. Samples and Pretreatment
2.2. MP Separation
2.3. Raman Spectra and Images Acquisitions
2.4. Date and Image Processing
3. Results and Discussion
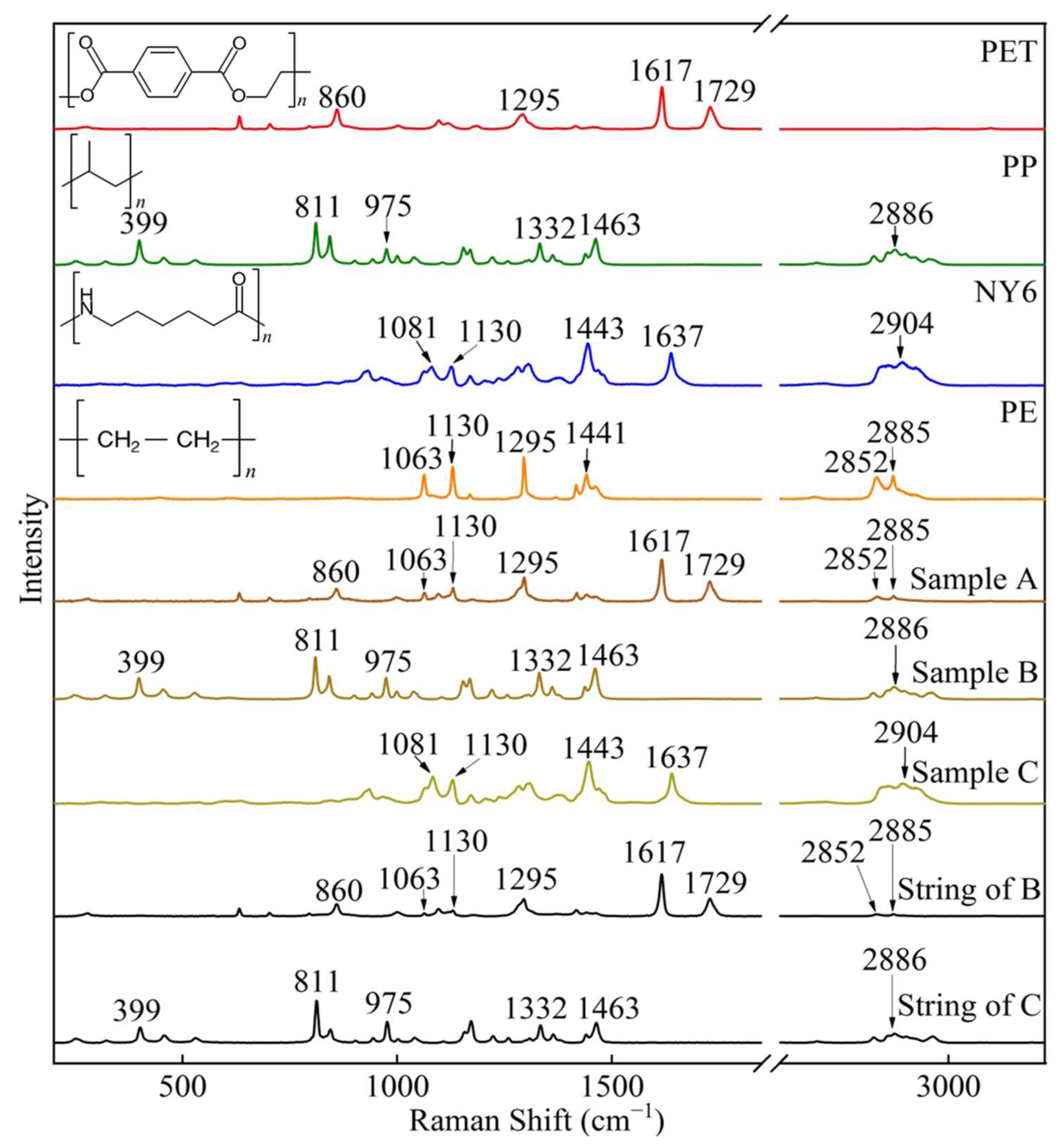
3.1. Raman Spectral Properties of Different Filter Bags
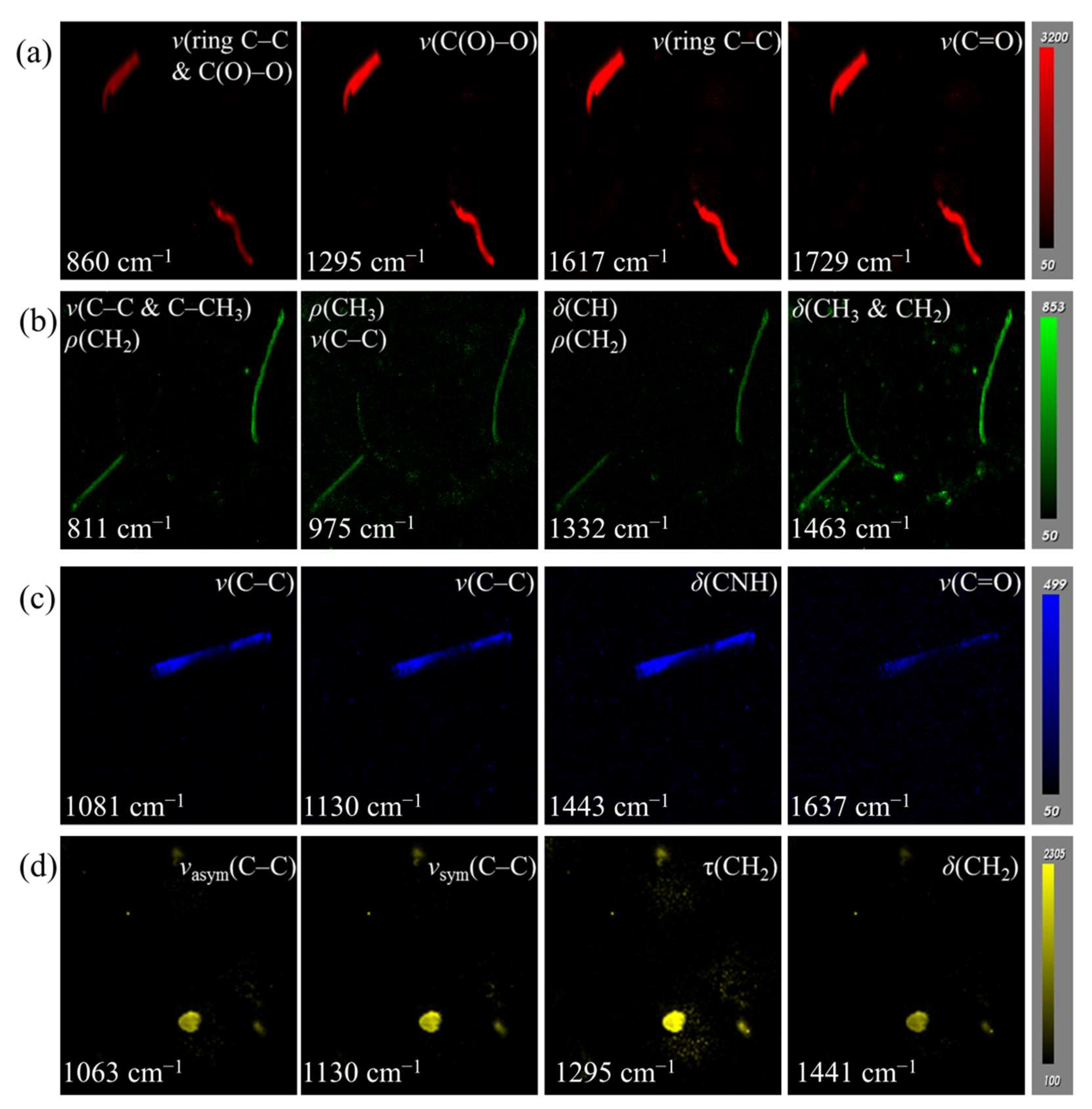
3.2. Optimization of Peak Area Imaging for Various MPs
3.3. MP Mapping Using Raman Images Combined with Chemometrics
3.3.1. MP Mapping by Peak Area Imaging at Single Characteristic Peak
3.3.2. MP Mapping by Raman Imaging Combined with DCLS
3.4. Simultaneous Identification of Various MPs Released from Filter Bags
3.5. Exploration of the Origin of MPs from Filter Bags
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rainieri, S.; Barranco, A. Microplastics, a food safety issue? Trends Food Sci. Technol. 2019, 84, 55–57. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of Polyethylene Terephthalate and Polycarbonate Microplastics in Infant and Adult Feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef]
- Lee, M.-R.; Lai, F.-Y.; Dou, J.; Lin, K.-L.; Chung, L.-W. Determination of Trace Leaching Phthalate Esters in Water and Urine from Plastic Containers by Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Anal. Lett. 2011, 44, 676–686. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Sripada, K.; Wierzbicka, A.; Abass, K.; Grimalt, J.O.; Erbe, A.; Rollin, H.B.; Weihe, P.; Diaz, G.J.; Singh, R.R.; Visnes, T.; et al. A Children’s Health Perspective on Nano- and Microplastics. Environ. Health Perspect. 2022, 130, 015001. [Google Scholar] [CrossRef]
- Wang, W.; Do, A.T.N.; Kwon, J.H. Ecotoxicological effects of micro- and nanoplastics on terrestrial food web from plants to human beings. Sci. Total Environ. 2022, 834, 155333. [Google Scholar] [CrossRef]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernandez, A. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Oceans. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Süssmann, J.; Krause, T.; Martin, D.; Walz, E.; Greiner, R.; Rohn, S.; Fischer, E.K.; Fritsche, J. Evaluation and optimisation of sample preparation protocols suitable for the analysis of plastic particles present in seafood. Food Control 2021, 125, 107969. [Google Scholar] [CrossRef]
- Praveena, S.M.; Laohaprapanon, S. Quality assessment for methodological aspects of microplastics analysis in bottled water—A critical review. Food Control 2021, 130, 108285. [Google Scholar] [CrossRef]
- Nalbone, L.; Cincotta, F.; Giarratana, F.; Ziino, G.; Panebianco, A. Microplastics in fresh and processed mussels sampled from fish shops and large retail chains in Italy. Food Control 2021, 125, 108003. [Google Scholar] [CrossRef]
- Panebianco, A.; Nalbone, L.; Giarratana, F.; Ziino, G. First discoveries of microplastics in terrestrial snails. Food Control 2019, 106, 106722. [Google Scholar] [CrossRef]
- Mercogliano, R.; Avio, C.G.; Regoli, F.; Anastasio, A.; Colavita, G.; Santonicola, S. Occurrence of Microplastics in Commercial Seafood under the Perspective of the Human Food Chain. A Review. J. Agric. Food Chem. 2020, 68, 5296–5301. [Google Scholar] [CrossRef]
- Li, Q.; Ma, C.; Zhang, Q.; Shi, H. Microplastics in shellfish and implications for food safety. Curr. Opin. Food Sci. 2021, 40, 192–197. [Google Scholar] [CrossRef]
- Guo, X.; Lin, H.; Xu, S.; He, L. Recent Advances in Spectroscopic Techniques for the Analysis of Microplastics in Food. J. Agric. Food Chem. 2022, 70, 1410–1422. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics and Nanoplastics: Emerging Contaminants in Food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wang, X.; Ren, T.; Wang, J.; Shan, J. Microplastics contamination in food and beverages: Direct exposure to humans. J. Food Sci. 2021, 86, 2816–2837. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Mei, T.; Xu, M.; Lu, Z.; Dai, H.; Pi, F.; Wang, J. Estimation of contamination level in microplastic-exposed crayfish by laser confocal micro-Raman imaging. Food Chem. 2022, 397, 133844. [Google Scholar] [CrossRef]
- Fadare, O.O.; Wan, B.; Guo, L.H.; Zhao, L. Microplastics from consumer plastic food containers: Are we consuming it? Chemosphere 2020, 253, 126787. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in take-out food containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef] [PubMed]
- Hee, Y.Y.; Weston, K.; Suratman, S. The effect of storage conditions and washing on microplastic release from food and drink containers. Food Packag. Shelf Life 2022, 32, 100826. [Google Scholar] [CrossRef]
- Weisser, J.; Beer, I.; Hufnagl, B.; Hofmann, T.; Lohninger, H.; Ivleva, N.P.; Glas, K. From the Well to the Bottle: Identifying Sources of Microplastics in Mineral Water. Water 2021, 13, 841. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef]
- Prata, J.C.; Paco, A.; Reis, V.; Da Costa, J.P.; Fernandes, A.J.S.; Da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of microplastics in white wines capped with polyethylene stoppers using micro-Raman spectroscopy. Food Chem. 2020, 331, 127323. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; King, A.E.; Yu, L.H. Analytical Chemistry of Plastic Debris: Sampling, Methods, and Instrumentation. In Microplastic in the Environment: Pattern and Process; Bank, M.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 17–67. [Google Scholar]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An overview on separation, identification and characterization of microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Dong, M.T.; She, Z.B.; Xiong, X.; Ouyang, G.; Luo, Z.J. Automated analysis of microplastics based on vibrational spectroscopy: Are we measuring the same metrics? Anal. Bioanal. Chem. 2022, 414, 3359–3372. [Google Scholar] [CrossRef]
- Sobhani, Z.; Al Amin, M.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics by Raman mapping. Anal. Chim. Acta 2019, 1077, 191–199. [Google Scholar] [CrossRef]
- Ilia, M.P.; Kirill, K.; Junyeol, K.; Kyle, D.; Masaru, K. Photothermal infrared imaging: Identification and visualization of micro- and nanoplastics in environmental matrices. In Advanced Chemical Microscopy for Life Science and Translational Medicine; SPIE: Bellingham, WA, USA, 2021; Volume 2, pp. 28–34. [Google Scholar]
- Olson, N.E.; Xiao, Y.; Lei, Z.Y.; Ault, A.P. Simultaneous Optical Photothermal Infrared (O-PTIR) and Raman Spectroscopy of Submicrometer Atmospheric Particles. Anal. Chem. 2020, 92, 9932–9939. [Google Scholar] [CrossRef] [PubMed]
- Jha, D.K.; Dhekne, P.P.; Patwardhan, A.W. Characterization and evaluation of tea bag papers. J. Food Sci. Technol. 2020, 57, 3060–3070. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Schleusener, J.; Carrer, V.; Patzelt, A.; Guo, S.; Bocklitz, T.; Coderch, L.; Lademann, J.; Darvin, M.E. Confocal Raman imaging of skin sections containing hair follicles using classical least squares regression and multivariate curve resolution–alternating least squares. Quantum Electron. 2019, 49, 6–12. [Google Scholar] [CrossRef]
- Rebiere, H.; Martin, M.; Ghyselinck, C.; Bonnet, P.A.; Brenier, C. Raman chemical imaging for spectroscopic screening and direct quantification of falsified drugs. J. Pharm. Biomed. Anal. 2018, 148, 316–323. [Google Scholar] [CrossRef]
- Xu, J.L.; Lin, X.; Hugelier, S.; Herrero-Langreo, A.; Gowen, A.A. Spectral imaging for characterization and detection of plastic substances in branded teabags. J. Hazard. Mater. 2021, 418, 126328. [Google Scholar] [CrossRef]
- Nava, V.; Frezzotti, M.L.; Leoni, B. Raman Spectroscopy for the Analysis of Microplastics in Aquatic Systems. Appl. Spectrosc. 2021, 75, 1341–1357. [Google Scholar] [CrossRef]
- Boerio, F.J.; Bahl, S.K.; Mcgraw, G.E. Vibrational analysis of polyethylene terephthalate and its deuterated derivatives. J. Polym. Sci. Polym. Phys. Ed. 1976, 14, 1029–1046. [Google Scholar] [CrossRef]
- Sagitova, E.A.; Donfack, P.; Nikolaeva, G.Y.; Prokhorov, K.A.; Zavgorodnev, Y.V.; Pashinin, P.P.; Ushakova, T.M.; Novokshonova, L.A.; Starchak, E.E.; Guseva, M.A.; et al. Regularity modes in Raman spectra of polyolefins: Part II. Polyethylene and ethylene copolymers. Vib. Spectrosc. 2016, 84, 139–145. [Google Scholar] [CrossRef]
- Andreassen, E. Infrared and Raman spectroscopy of polypropylene. In Polypropylene; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Guo, X.; Lin, Z.; Wang, Y.; He, Z.; Wang, M.; Jin, G. In-Line Monitoring the Degradation of Polypropylene under Multiple Extrusions Based on Raman Spectroscopy. Polymers 2019, 11, 1698. [Google Scholar] [CrossRef]
- Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Effect of the Electrospinning Process on Polymer Crystallization Chain Conformation in Nylon-6 and Nylon-12. Macromolecules 2004, 37, 877–881. [Google Scholar] [CrossRef]
- Zhang, L.; Henson, M.J.; Sekulic, S.S. Multivariate data analysis for Raman imaging of a model pharmaceutical tablet. Anal. Chim. Acta 2005, 545, 262–278. [Google Scholar] [CrossRef]
- Ranjan, V.P.; Joseph, A.; Goel, S. Microplastics and other harmful substances released from disposable paper cups into hot water. J. Hazard. Mater. 2021, 404, 124118. [Google Scholar] [CrossRef] [PubMed]


| Soaking Sample Type | Soaking Temperature (°C) | Soaking Time (min) | |||
|---|---|---|---|---|---|
| 1 | 5 | 10 | 30 | ||
| A | 70 |  |  |  |  |
| 80 |  |  |  |  | |
| 90 |  |  |  |  | |
| B | 70 |  |  |  |  |
| 80 |  |  |  |  | |
| 90 |  |  |  |  | |
| C | 70 |  |  |  |  |
| 80 |  |  |  |  | |
| 90 |  |  |  |  | |
 : Red: PET, Green: PP, Blue: NY6, Yellow: PE.
: Red: PET, Green: PP, Blue: NY6, Yellow: PE.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, T.; Wang, J.; Xiao, X.; Lv, J.; Li, Q.; Dai, H.; Liu, X.; Pi, F. Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging. Foods 2022, 11, 2871. https://doi.org/10.3390/foods11182871
Mei T, Wang J, Xiao X, Lv J, Li Q, Dai H, Liu X, Pi F. Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging. Foods. 2022; 11(18):2871. https://doi.org/10.3390/foods11182871
Chicago/Turabian StyleMei, Tingna, Jiahua Wang, Xiaofeng Xiao, Jingwen Lv, Qiaocong Li, Huang Dai, Xiaodan Liu, and Fuwei Pi. 2022. "Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging" Foods 11, no. 18: 2871. https://doi.org/10.3390/foods11182871
APA StyleMei, T., Wang, J., Xiao, X., Lv, J., Li, Q., Dai, H., Liu, X., & Pi, F. (2022). Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging. Foods, 11(18), 2871. https://doi.org/10.3390/foods11182871








