Phytochemical Profile and Antioxidant, Antiproliferative, and Antimicrobial Properties of Rubus idaeus Seed Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Preparation of Extracts
2.2. Phytochemical Characterization
2.3. Redox Active Proprieties
2.4. Cell Culture
2.5. Cellular Antioxidant Activity
2.6. Antiproliferative Activity
2.7. Microbiological Characterization of WRSP
2.8. Determination of Antibacterial Activity of WRSP
2.9. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Characterization
3.2. Antioxidant Proprieties
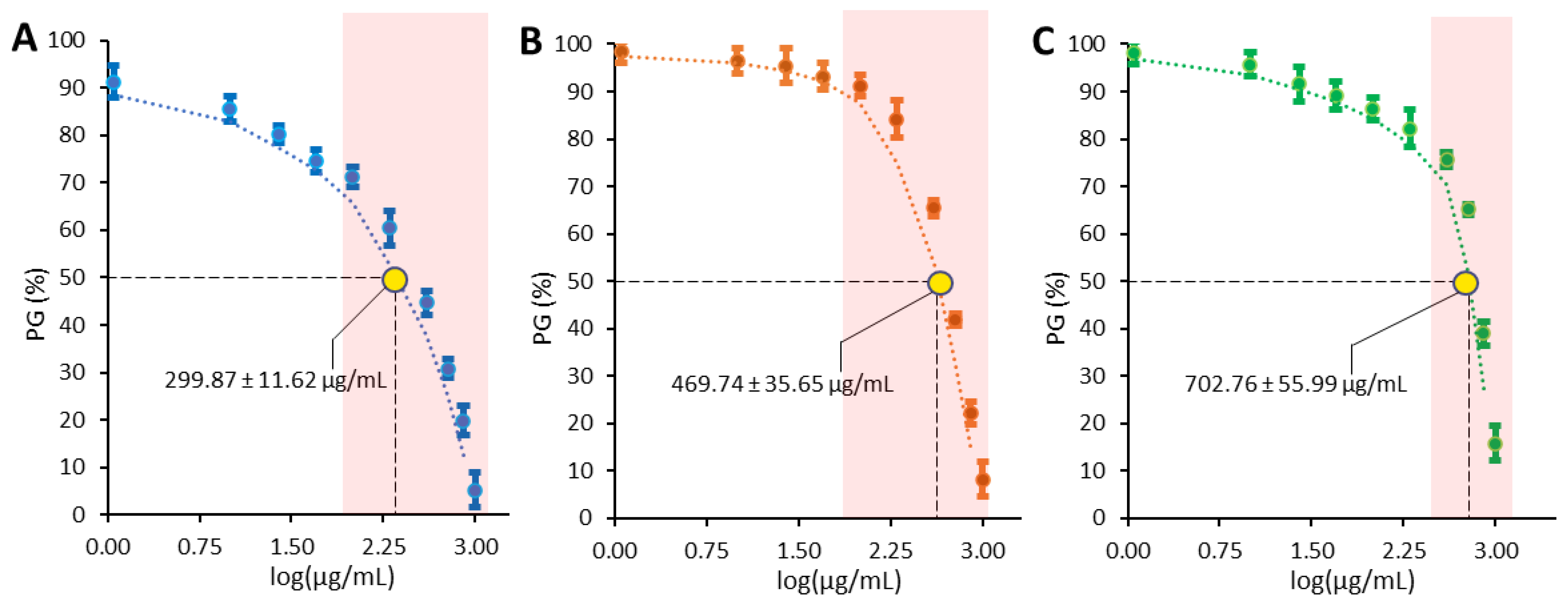
3.3. Antiproliferative Activity
3.4. Microbiological Characterization and Inhibitory Properties of WRSP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste-Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Bertocci, F.; Mannino, G. Can Agri-Food Waste Be a Sustainable Alternative in Aquaculture? A Bibliometric and Meta-Analytic Study on Growth Performance, Innate Immune System, and Antioxidant Defenses. Foods 2022, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Socaci, S.A.; Farcas, A.C.; Vodnar, D.C.; Tofana, M. Food Wastes as Valuable Sources of Bioactive Molecules; IntechOpen: London, UK, 2017; pp. 75–93. [Google Scholar]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Campobenedetto, C.; Mannino, G.; Agliassa, C.; Acquadro, A.; Contartese, V.; Garabello, C.; Bertea, C.M. Transcriptome Analyses and Antioxidant Activity Profiling Reveal the Role of a Lignin-Derived Biostimulant Seed Treatment in Enhancing Heat Stress Tolerance in Soybean. Plants 2020, 9, 1308. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P. Red raspberry (Rubus idaeus L.) seed oil: A review. Plants 2021, 10, 944. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: www.fao.org/faostat (accessed on 24 August 2022).
- George, B.P.; Abrahamse, H. Traditional Uses and Bioactivities of Common Rubus Species With Reference to Cancer: A Mini-Review. In Phytopharmaceuticals; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 259–270. [Google Scholar]
- Mihailović, N.R.; Mihailović, V.B.; Ćirić, A.R.; Srećković, N.Z.; Cvijović, M.R.; Joksović, L.G. Analysis of Wild Raspberries (Rubus idaeus L.): Optimization of the Ultrasonic-Assisted Extraction of Phenolics and a New Insight in Phenolics Bioaccessibility. Plant Foods Hum. Nutr. 2019, 74, 399–404. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Derrick, S.A.; Kristo, A.S.; Reaves, S.K.; Sikalidis, A.K. Effects of Dietary Red Raspberry Consumption on Pre-Diabetes and Type 2 Diabetes Mellitus Parameters. Int. J. Environ. Res. Public Health 2021, 18, 9364. [Google Scholar] [CrossRef]
- Veljkovic, B.; Djordjevic, N.; Dolicanin, Z.; Licina, B.; Topuzovic, M.; Stankovic, M.; Zlatic, N.; Dajic-Stevanovic, Z. Antioxidant and anticancer properties of leaf and fruit extracts of the wild raspberry (Rubus idaeus L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 359–367. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Naji-Tabasi, S.; Emadzadeh, B.; Shahidi-Noghabi, M.; Abbaspour, M.; Akbari, E. Physico-chemical and antioxidant properties of barberry juice powder and its effervescent tablets. Chem. Biol. Technol. Agric. 2021, 8, 23. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Schieber, A.; Kolodziejczyk, P. Characterization of Canadian black currant (Ribes nigrum L.) seed oils and residues. J. Agric. Food Chem. 2009, 57, 11528–11536. [Google Scholar] [CrossRef] [PubMed]
- Helbig, D.; Böhm, V.; Wagner, A.; Schubert, R.; Jahreis, G. Berry seed press residues and their valuable ingredients with special regard to black currant seed press residues. Food Chem. 2008, 111, 1043–1049. [Google Scholar] [CrossRef]
- Kosmala, M.; Zdunczyk, Z.; Juskiewicz, J.; Jurgonski, A.; Karlinska, E.; Macierzynski, J.; Jańczak, R.; Roj, E. Chemical composition of defatted strawberry and raspberry seeds and the effect of these dietary ingredients on polyphenol metabolites, intestinal function, and selected serum parameters in rats. J. Agric. Food Chem. 2015, 63, 2989–2996. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Moore, J.; Cheng, Z.; Luther, M.; Rao, J.N.; Wang, J.Y.; Yu, L.L. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. J. Agric. Food Chem. 2006, 54, 3773–3778. [Google Scholar] [CrossRef]
- Kang, I.; Espín, J.C.; Carr, T.P.; Tomás-Barberán, F.A.; Chung, S. Raspberry seed flour attenuates high-sucrose diet-mediated hepatic stress and adipose tissue inflammation. J. Nutr. Biochem. 2016, 32, 64–72. [Google Scholar] [CrossRef]
- Mannino, G.; Serio, G.; Bertea, C.M.; Chiarelli, R.; Lauria, A.; Gentile, C. Phytochemical profile and antioxidant properties of the edible and non-edible portions of black sapote (Diospyros digyna Jacq.). Food Chem. 2022, 380, 132137. [Google Scholar] [CrossRef]
- Vigliante, I.; Mannino, G.; Maffei, M.E. Chemical Characterization and DNA Fingerprinting of Griffonia simplicifolia Baill. Molecules 2019, 24, 1032. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Lauria, A.; Mannino, S.; Gentile, C.; Mannino, G.; Martorana, A.; Peri, D. DRUDIT: Web-based DRUgs DIscovery Tools to design small molecules as modulators of biological targets. Bioinformatics 2020, 36, 1562–1569. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Vigliante, I.; Mannino, G.; Maffei, M.E. OxiCyan®, a phytocomplex of bilberry (Vaccinium myrtillus) and spirulina (Spirulina platensis), exerts both direct antioxidant activity and modulation of ARE/Nrf2 pathway in HepG2 cells. J. Funct. Foods 2019, 61, 103508. [Google Scholar] [CrossRef]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological profile and bioactive properties of insect powders used in food and feed formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A.; Settanni, L.; Braga, T.M.; de Fatima Silva Lopes, M.; Suzzi, G. An investigation of the bacteriocinogenic potential of lactic acid bacteria associated with wheat (Triticum durum) kernels and non-conventional flours. LWT-Food Sci. Technol. 2008, 41, 1173–1182. [Google Scholar] [CrossRef]
- Gaglio, R.; Guarcello, R.; Venturella, G.; Palazzolo, E.; Francesca, N.; Moschetti, G.; Settanni, L.; Saporita, P.; Gargano, M.L. Microbiological, chemical and sensory aspects of bread supplemented with different percentages of the culinary mushroom Pleurotus eryngii in powder form. Int. J. Food Sci. Technol. 2019, 54, 1197–1205. [Google Scholar] [CrossRef]
- Barbaccia, P.; Busetta, G.; Barbera, M.; Alfonzo, A.; Garofalo, G.; Francesca, N.; Moscarelli, A.; Moschetti, G.; Settanni, L.; Gaglio, R. Effect of grape pomace from red cultivar’Nero d’Avola’on the microbiological, physicochemical, phenolic profile and sensory aspects of ovine Vastedda-like stretched cheese. J. Appl. Microbiol. 2021, 133, 130–144. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Gentile, C.; Mannino, G.; Palazzolo, E.; Gianguzzi, G.; Perrone, A.; Serio, G.; Farina, V. Pomological, Sensorial, Nutritional and Nutraceutical Profile of Seven Cultivars of Cherimoya (Annona cherimola Mill). Foods 2021, 10, 35. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Lee, Y.A.; Cho, E.J.; Yokozawa, T. Effects of proanthocyanidin preparations on hyperlipidemia and other biomarkers in mouse model of type 2 diabetes. J. Agric. Food Chem. 2008, 56, 7781–7789. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods—A Review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Kaur, K.; Asthir, B.; Verma, D.K. Biosynthesis, bioavailability, and metabolism of plant polyphenols: Biological activities and their potential benefits in human health. In Phytochemicals in Food and Health; Apple Academic Press: Cambridge, MA, USA, 2021; pp. 231–255. ISBN 1003082122. [Google Scholar]
- Aneklaphakij, C.; Saigo, T.; Watanabe, M.; Naake, T.; Fernie, A.R.; Bunsupa, S.; Satitpatipan, V.; Tohge, T. Diversity of chemical structures and biosynthesis of polyphenols in Nut-bearing species. Front. Plant Sci. 2021, 12, 642581. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W. Oxidative Stress—Antioxidants in Foods. Ernahrungs Umschau 2011, 58, 536–541. [Google Scholar]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Caradonna, F.; Consiglio, O.; Luparello, C.; Gentile, C. Science and healthy meals in the world: Nutritional epigenomics and nutrigenetics of the mediterranean diet. Nutrients 2020, 12, 1748. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Allegra, M.; Gentile, C.; Tesoriere, L.; Livrea, M.A. Protective effect of melatonin against cytotoxic actions of malondialdehyde: An in vitro study on human erythrocytes. J. Pineal Res. 2002, 32, 187–193. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals—An overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef]
- Almanza-Aguilera, E.; Ceballos-Sánchez, D.; Achaintre, D.; Rothwell, J.A.; Laouali, N.; Severi, G.; Katzke, V.; Johnson, T.; Schulze, M.B.; Palli, D. Urinary Concentrations of (+)-Catechin and (-)-Epicatechin as Biomarkers of Dietary Intake of Flavan-3-ols in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Nutrients 2021, 13, 4157. [Google Scholar] [CrossRef]
- Cossarizza, A.; Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L. Quercetin and cancer chemoprevention. Evid. Based Complement. Altern. Med. 2011, 2011, 591356. [Google Scholar]
- Ekström, A.M.; Serafini, M.; Nyren, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary quercetin intake and risk of gastric cancer: Results from a population-based study in Sweden. Ann. Oncol. 2011, 22, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Wang, P.; Vadgama, J.V.; Said, J.W.; Magyar, C.E.; Doan, N.; Heber, D.; Henning, S.M. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J. Nutr. Biochem. 2014, 25, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Iglesias, M.J.; Novio, S.; García, C.; Pérez-Muñuzuri, M.E.; Martínez, M.-C.; Santiago, J.-L.; Boso, S.; Gago, P.; Freire-Garabal, M. Co-Adjuvant Therapy Efficacy of Catechin and Procyanidin B2 with Docetaxel on Hormone-Related Cancers In Vitro. Int. J. Mol. Sci. 2021, 22, 7178. [Google Scholar] [CrossRef]
- Dlugaszewska, J.; Ratajczak, M.; Kamińska, D.; Gajecka, M. Are dietary supplements containing plant-derived ingredients safe microbiologically? Saudi Pharm. J. 2019, 27, 240–245. [Google Scholar] [CrossRef]
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Can-Cauich, C.A.; Sauri-Duch, E.; Betancur-Ancona, D.; Chel-Guerrero, L.; González-Aguilar, G.A.; Cuevas-Glory, L.F.; Pérez-Pacheco, E.; Moo-Huchin, V.M. Tropical fruit peel powders as functional ingredients: Evaluation of their bioactive compounds and antioxidant activity. J. Funct. Foods 2017, 37, 501–506. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New insights on the use of polyphenols as natural preservatives and their emerging safety concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Phyo, H.M.; Galeboe, O.; Yao, W. Application of essential oils as preservatives in food systems: Challenges and future prospectives—A review. Phytochem. Rev. 2021, 21, 1209–1246. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Microbiologia Degli Alimenti; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 8847007860. [Google Scholar]
- Gribble, A.; Mills, J.; Brightwell, G. The spoilage characteristics of Brochothrix thermosphacta and two psychrotolerant Enterobacteriacae in vacuum packed lamb and the comparison between high and low pH cuts. Meat Sci. 2014, 97, 83–92. [Google Scholar] [CrossRef]
- Caldera, L.; Franzetti, L.; Van Coillie, E.; De Vos, P.; Stragier, P.; De Block, J.; Heyndrickx, M. Identification, enzymatic spoilage characterization and proteolytic activity quantification of Pseudomonas spp. isolated from different foods. Food Microbiol. 2016, 54, 142–153. [Google Scholar] [CrossRef]
- Miceli, A.; Gaglio, R.; Francesca, N.; Ciminata, A.; Moschetti, G.; Settanni, L. Evolution of shelf life parameters of ready-to-eat escarole (Cichorium endivia var. latifolium) subjected to different cutting operations. Sci. Hortic. 2019, 247, 175–183. [Google Scholar] [CrossRef]
- Settanni, L.; Gaglio, R.; Stucchi, C.; De Martino, S.; Francesca, N.; Moschetti, G. Presence of pathogenic bacteria in ice cubes and evaluation of their survival in different systems. Ann. Microbiol. 2017, 67, 827–835. [Google Scholar] [CrossRef]
- Podkowik, M.; Seo, K.S.; Schubert, J.; Tolo, I.; Robinson, D.A.; Bania, J.; Bystroń, J. Genotype and enterotoxigenicity of Staphylococcus epidermidis isolate from ready to eat meat products. Int. J. Food Microbiol. 2016, 229, 52–59. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665. [Google Scholar] [CrossRef]

| # | RT [Min] | m/z | MS/MS | CAS-ID | Chemical Formula | Compound(s) | mg/100 g | ||
|---|---|---|---|---|---|---|---|---|---|
| Flavanonols | |||||||||
| 1 | 10.7 | 287 | 480-20-6 | C15H12O6 | Dihydrokaempferol | 12.74 ± 0.73 | |||
| 2 | 13.6 | 481 | 319 | n.a. | C21H22O13 | Dihydromyricetin-3-O-glucoside | 4.38 ± 0.11 | ||
| 19 | 28.2 | 319 | 27,200-12-0 | C15H12O8 | Dihydromyricetin | 64.15 ± 3.96 | |||
| Flavonols | |||||||||
| 6 | 15.7 | 625 | 479 | 463 | 317 | 41,093-68-9 | C27H30O17 | Myricetin-rutinoside | 0.95 ± 0.06 |
| 7 | 22.2 | 433 | 301 | 572-30-5 | C20H18O11 | Quercetin-3-O-arabinoside | 5.25 ± 0.07 | ||
| 9 | 23.2 | 433 | 549-32-6 | C20H18O11 | Quercetin-3-O-xyloside | 3.83 ± 0.23 | |||
| 10 | 24.2 | 625 | 463 | 301 | 6892-74-6 | C27H30O17 | Quercetin-3,7-O-diglucoside | 3.11 ± 0.09 | |
| 11 | 24.4 | 463 | 301 | 482-36-0 | C21H20O12 | Quercetin-3-O-galactoside | 0.44 ± 0.01 | ||
| 12 | 24.8 | 301 | 117-39-5 | C15H10O7 | Quercetin | 697.84 ± 16.81 | |||
| 14 | 25 | 447 | 285 | 482-35-9 | C21H20O12 | Kaempferol-3-O-glucoside | 0.46 ± 0.02 | ||
| 15 | 25 | 463 | 301 | 482-35-9 | C21H20O12 | Quercetin-3-O-glucoside | 0.71 ± 0.02 | ||
| 16 | 26.4 | 477 | 176 | 22,688-79-5 | C21H18O13 | Quercetin-3-O-glucoronide | 2.44 ± 0.13 | ||
| 22 | 31.1 | 317 | 529-44-2 | C15H10O8 | Myricetin | 18.23 ± 0.75 | |||
| 24 | 32.7 | 285 | 520-18-3 | C15H10O6 | Kaempferol | 5.05 ± 0.27 | |||
| 25 | 33.9 | 609 | 463 | 447 | 301 | 153-18-4 | C27H30O16 | Quercetin-3-O-rutinoside | 0.48 ± 0.03 |
| 34 | 45.6 | 593 | 447 | 431 | 285 | 17,650-84-9 | C27H30O15 | Kaempherol-3-O-rutinoside | 4.82 ± 0.28 |
| 35 | 45.6 | 595 | 294 | 83,048-35-5 | C26H28O16 | Quercetin-3-O-sambunioside | 2.42 ± 0.05 | ||
| Flavonones | |||||||||
| 18 | 27.7 | 447 | 285 | 20,344-46-1 | C21H20O11 | Luteolin-3-O-glucoside | 6.32 ± 0.31 | ||
| 26 | 35.2 | 285 | 491-70-3 | C15H10O6 | Luteolin | 2.32 ± 0.14 | |||
| Flavanones | |||||||||
| 20 | 29.4 | 451 | 289 | 20,344-46-1 | C21H22O10 | Naringenin-3-O-galactoside | 2.17 ± 0.11 | ||
| 27 | 36.4 | 287 | 552-58-9 | C21H22O11 | Eriodictyol | 5.14 ± 0.26 | |||
| 37 | 57.8 | 595 | 433 | 271 | n.a. | C27H32O15 | Naringenin-3,7-O-diglucoside | 5.66 ± 0.12 | |
| O-methylated flavonol | |||||||||
| 33 | 44.6 | 639 | 477 | 315 | n.a. | C28H32O17 | Isohermentin-3,7-O-diglucoside | 1.33 ± 0.07 | |
| # | RT [Min] | M-H | MS/MS | CAS-ID | Chemical Formula | Compound(s) | mg/100 g | ||
|---|---|---|---|---|---|---|---|---|---|
| Flavan-3-ols | |||||||||
| 3 | 14.2 | 451 | 289 | n.a. | C21H24O11 | Catechin-3-O-galactoside | 0.75 ± 0.03 | ||
| 4 | 14.9 | 451 | 289 | n.a. | C21H24O11 | Catechin-3-O-glucoside | 1.32 ± 0.03 | ||
| 5 | 15.6 | 597 | 451 | 435 | 289 | n.a. | C27H34O15 | Catechin-3-O-rutinoside | 7.34 ± 0.18 |
| 21 | 29.7 | 289 | 154-23-4 | C15H14O6 | Catechin | 122 ± 3.48 | |||
| 36 | 51.2 | 613 | 451 | 289 | n.a. | C27H34O16 | Catechin-3,7-O-diglucoside | 2.46 ± 0.14 | |
| Proanthocyanidins | |||||||||
| 8 | 22.8 | 577 | 289 | 29,106-49-8 | C30H26O12 | Dimer B-Type PAC | 399.61 ± 5.95 | ||
| 13 | 24.9 | 865 | 575 | 289 | 65,085-09-8 | C45H38O18 | Trimer A-Type PAC | 14.26 ± 0.17 | |
| 17 | 27.5 | 1153 | 867 | 577 | n.a. | C60H52O24 | Tetramer B-Type PAC | 12.63 ± 0.41 | |
| 23 | 31.8 | 1439 | 1437 | 1151 | 575 | n.a. | C75H60O30 | Pentamer A-Type PAC | 56.16 ± 3.83 |
| 28 | 37.2 | 2012 | 1151 | 863 | 289 | n.a. | C90H72O36 | Esamer A-Type PAC | 12.05 ± 0.5 |
| 29 | 37.2 | 2014 | 1153 | 861 | 577 | n.a. | C90H78O36 | Esamer B-Type PAC | 5.16 ± 0.17 |
| 30 | 37.5 | 1437 | 1151 | 863 | 575 | n.a. | C75H60O30 | Pentamer A-Type PAC | 30.88 ± 1.58 |
| 31 | 38.8 | 1151 | 863 | 575 | 289 | n.a. | C60H48O24 | Tetramer A-Type PAC | 13.35 ± 0.64 |
| 32 | 42.5 | 1441 | 1155 | 865 | 289 | n.a. | C75H65O30 | Pentamer B-Type PAC | 77.33 ± 3.16 |
| Species | Strains | Source of Isolation | Inhibition (mm) | MIC (mg/mL) |
|---|---|---|---|---|
| Pro-technological | ||||
| En. mundtii | WFE3 | Wheat flours | - | n.d. |
| F. sanfranciscensis | SD22 | Sourdough | - | n.d. |
| Lt. sakei | SP255 | Salami | - | n.d. |
| Lv. brevis | SD70 | Sourdough | - | n.d. |
| Lc. lactis | CAG4 | Curd | - | n.d. |
| Ln. mesenteroides | MISE643 | Raw ewe’s milk | - | n.d. |
| Spoilage | ||||
| Br. thermosphacta | SP10 | Pork meat | 23.0 ± 0.4 | 25 |
| P. fluorescens | 4G628 | Ready to eat salad | 19.3 ± 0.2 | 25 |
| P. lactis | SP198 | Salami | 17.8 ± 0.2 | 25 |
| P. poae | 4G558 | Ready to eat salad | 19.0 ± 0.4 | 25 |
| Pathogenic | ||||
| A. guillouiae | ICE24 | Food ice cubes | 18.2 ± 0.3 | 25 |
| B. cereus | ICE70 | Food ice cubes | 13.5 ± 0.2 | 100 |
| E. coli | PSL52 | PDO Pecorino Siciliano cheese | - | n.d. |
| L. monocytogenes | 13BO | Gorgonzola cheese | - | n.d. |
| P. aeruginosa | PSA68 | Animal tissue | - | n.d. |
| S. Enteritidis | ATCC13076 | Unknown | - | n.d. |
| S. Typhimurium | 50432 | Molluscs | - | n.d. |
| St. aureus | ATCC33862 | Unknown | 17.8 ± 0.2 | 25 |
| St. epidermidis | ICE244 | Food ice cubes | 18.6 ± 0.1 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannino, G.; Serio, G.; Gaglio, R.; Busetta, G.; La Rosa, L.; Lauria, A.; Settanni, L.; Gentile, C. Phytochemical Profile and Antioxidant, Antiproliferative, and Antimicrobial Properties of Rubus idaeus Seed Powder. Foods 2022, 11, 2605. https://doi.org/10.3390/foods11172605
Mannino G, Serio G, Gaglio R, Busetta G, La Rosa L, Lauria A, Settanni L, Gentile C. Phytochemical Profile and Antioxidant, Antiproliferative, and Antimicrobial Properties of Rubus idaeus Seed Powder. Foods. 2022; 11(17):2605. https://doi.org/10.3390/foods11172605
Chicago/Turabian StyleMannino, Giuseppe, Graziella Serio, Raimondo Gaglio, Gabriele Busetta, Lorenza La Rosa, Antonino Lauria, Luca Settanni, and Carla Gentile. 2022. "Phytochemical Profile and Antioxidant, Antiproliferative, and Antimicrobial Properties of Rubus idaeus Seed Powder" Foods 11, no. 17: 2605. https://doi.org/10.3390/foods11172605
APA StyleMannino, G., Serio, G., Gaglio, R., Busetta, G., La Rosa, L., Lauria, A., Settanni, L., & Gentile, C. (2022). Phytochemical Profile and Antioxidant, Antiproliferative, and Antimicrobial Properties of Rubus idaeus Seed Powder. Foods, 11(17), 2605. https://doi.org/10.3390/foods11172605









