Flavor Differences of Edible Parts of Grass Carp between Jingpo Lake and Commercial Market
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Approval
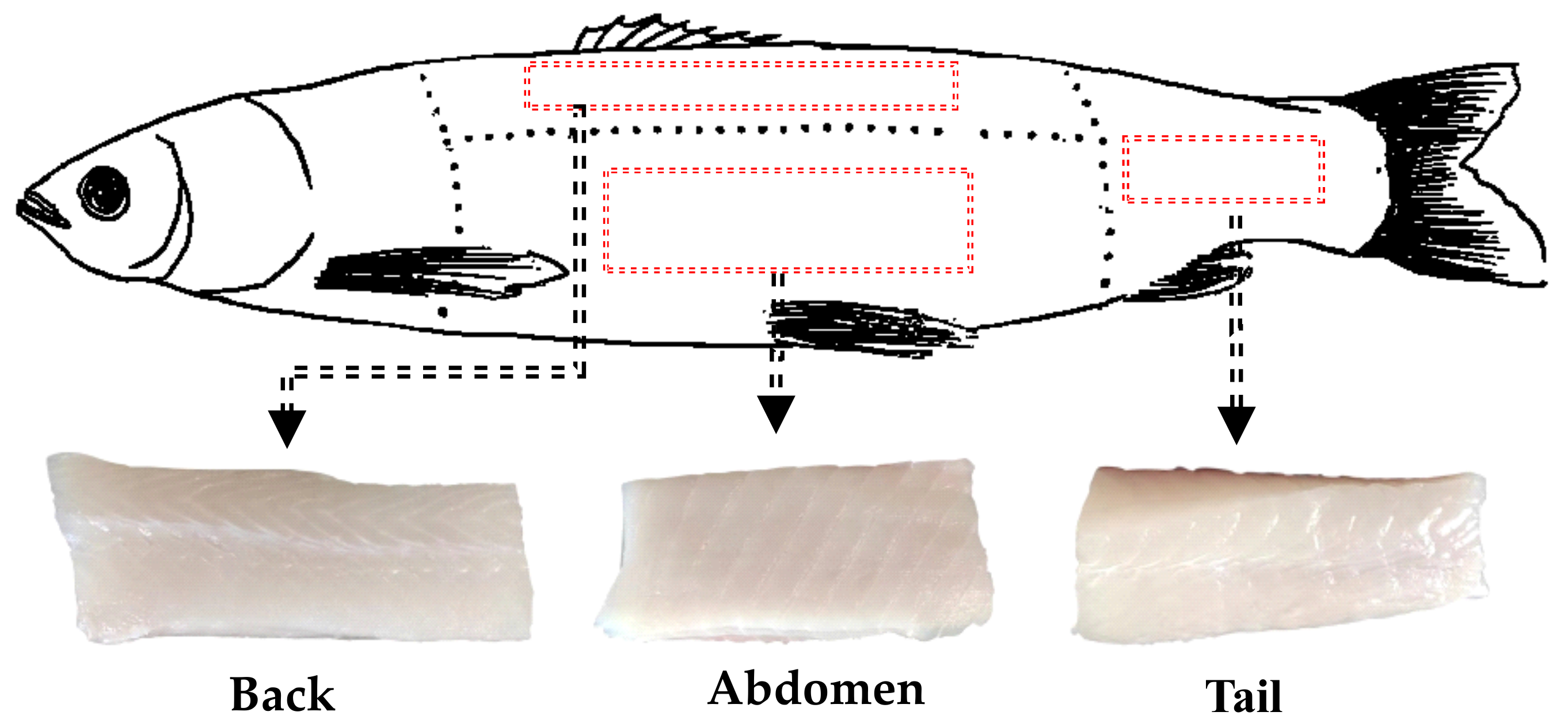
2.2. Materials
2.3. Sample Preparation
2.4. Physicochemical Analysis
2.5. Volatile Compound Analysis
2.6. E-Nose Analysis
2.7. E-Tongue Analysis
2.8. Determination of FAAs
2.9. ATP-Associated Compounds and K-Values
2.10. Sensory Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters
3.2. Volatile Compounds
3.3. E-Nose
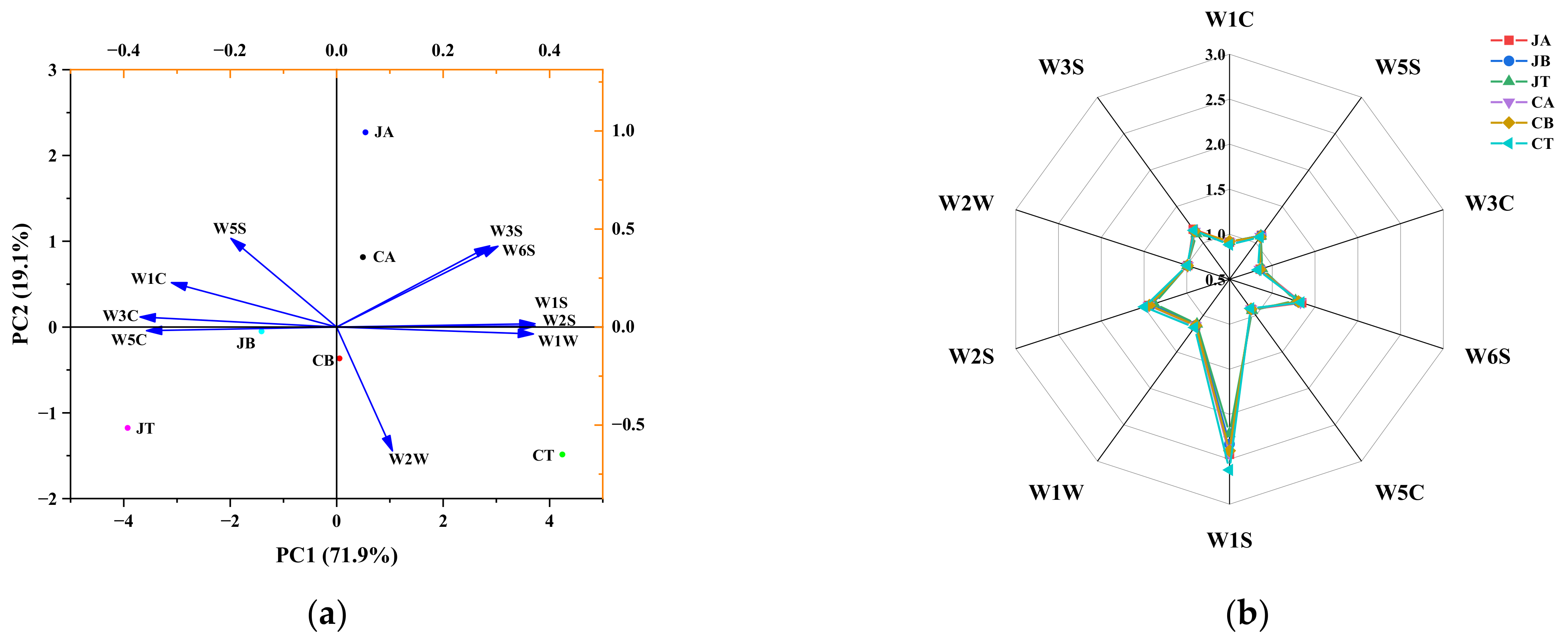
3.4. E-Tongue
3.5. FAAs
3.6. ATP-Associated Compounds and K-Values
3.7. Sensory Evaluation
3.8. Correlation Analysis between E-Nose, FAAs, and GC–MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisheries Department of Agriculture Ministry of China. China Fisheries Yearbook; Agriculture Press: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Du, H.; Lv, H.; Xu, Z.; Zhao, S.; Huang, T.; Manyande, A.; Xiong, S. The mechanism for improving the flesh quality of grass carp (Ctenopharyngodon idella) following the micro-flowing water treatment using a UPLC-QTOF/MS based metabolomics method. Food Chem. 2020, 327, 126777. [Google Scholar] [CrossRef]
- Mabuchi, R.; Ishimaru, A.; Tanaka, M.; Kawaguchi, O.; Tanimoto, S. Metabolic Profiling of Fish Meat by GC-MS Analysis, and Correlations with Taste Attributes Obtained Using an Electronic Tongue. Metabolites 2018, 9, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Van Houcke, J.; Medina, I.; Linssen, J.; Luten, J. Biochemical and volatile organic compound profile of European flat oyster (Ostrea edulis) and Pacific cupped oyster (Crassostrea gigas) cultivated in the Eastern Scheldt and Lake Grevelingen, the Netherlands. Food Control 2016, 68, 200–207. [Google Scholar] [CrossRef]
- Li, L.; Boyd, C.E.; Racine, P.; McNevin, A.A.; Somridhivej, B.; Minh, H.N.; Tinh, H.Q.; Godumala, R. Assessment of elemental profiling for distinguishing geographic origin of aquacultured shrimp from India, Thailand and Vietnam. Food Control 2017, 80, 162–169. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Wang, X.; Rogers, K.M.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC-MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, Y.; Jin, Y.; Deng, Y.; Zhao, Y. Impact of high hydrostatic pressure on non-volatile and volatile compounds of squid muscles. Food Chem. 2016, 194, 12–19. [Google Scholar] [CrossRef]
- Luo, X.; Xiao, S.; Ruan, Q.; Gao, Q.; An, Y.; Hu, Y.; Xiong, S. Differences in flavor characteristics of frozen surimi products reheated by microwave, water boiling, steaming, and frying. Food Chem. 2022, 372, 131260. [Google Scholar] [CrossRef]
- O’Neill, B.; Roux, A.L.; Hoffman, L.C. Comparative study of the nutritional composition of wild versus farmed yellowtail (Seriola lalandi). Aquaculture 2015, 448, 169–175. [Google Scholar] [CrossRef]
- Guy, J.; Nottingham, S. Fillet yield, biochemical composition, and consumer acceptance of farmed and wild mulloway. J. Aquat. Food Prod. Technol. 2014, 23, 608–620. [Google Scholar] [CrossRef]
- Rincón, L.; Castro, P.L.; Álvarez, B.; Hernández, M.D.; Álvarez, A.; Claret, A.; Guerrero, L.; Ginés, R. Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo). Aquaculture 2016, 451, 195–204. [Google Scholar] [CrossRef]
- Rodríguez-Barreto, D.; Jerez, S.; Cejas, J.R.; Martin, M.V.; Acosta, N.G.; Bolaños, A.; Lorenzo, A. Comparative study of lipid and fatty acid composition in different tissues of wild and cultured female broodstock of greater amberjack (Seriola dumerili). Aquaculture 2012, 360–361, 1–9. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, J.; Qiu, H.; Lai, X.; Li, J.; Wu, D.; Sheng, J.; Hong, Y. Comparison of nutritional quality and volatile flavor compounds among bighead carp from three aquaculture systems. Saudi J. Biol. Sci. 2021, 28, 4291–4299. [Google Scholar] [CrossRef]
- Jia, S.P.; Wang, L.; Zhang, J.M.; Zhang, L.; Ma, F.R.; Huang, M.L.; Liu, S.S.; Gong, J.H.; Zhang, M.; Yu, M.; et al. Comparative study on the morphological characteristics and nutritional quality of largemouth bass (Micropterus salmoides) cultured in an aquaculture system using land-based container with recycling water and a traditional pond system. Aquaculture 2022, 549, 737721. [Google Scholar] [CrossRef]
- Wang, L.; Jia, S.P.; Zhang, L.; Ma, F.R.; Zhang, M.; Yu, M.; Jiang, H.X.; Qiao, Z.G.; Li, X.J. Comparative study on nutritional quality and volatile flavor compounds of muscle in Cyprinus carpio haematopterus under wild, traditional pond and in-pond raceway system culture. Aquac. Rep. 2022, 25, 101194. [Google Scholar] [CrossRef]
- Xiao, N.Y.; Xu, H.Y.; Jiang, X.; Sun, T.T.; Luo, Y.X.; Shi, W.Z. Evaluation of aroma characteristics in grass carp mince as affected by different washing processes using an E-nose, HS-SPME-GC-MS, HS-GC-IMS, and sensory analysis. Food Res. Int. 2022, 158, 111584. [Google Scholar] [CrossRef]
- Du, H.; Chen, Q.; Liu, Q.; Wang, Y.; Kong, B. Evaluation of flavor characteristics of bacon smoked with different woodchips by HS-SPME-GC-MS combined with an electronic tongue and electronic nose. Meat Sci. 2021, 182, 108626. [Google Scholar] [CrossRef]
- Shi, J.; Nian, Y.; Da, D.; Xu, X.; Zhou, G.; Zhao, D.; Li, C. Characterization of flavor volatile compounds in sauce spareribs by gas chromatography–mass spectrometry and electronic nose. LWT 2020, 124, 109182. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Pei, Z.; Wei, P.; Xiang, D.; Cao, X.; Shen, X.; Li, C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Zhao, Y.; Zheng, X.; Lu, J.; Liang, Y. Simultaneous HPLC Determination of Amino Acids in Tea Infusion Coupled to Pre-column Derivatization with 2,4-Dinitrofluorobenzene. Food Anal. Methods. 2015, 9, 1307–1314. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, Y.; Jia, S.; Hong, H.; Liu, Y.; Luo, Y. Effects of pomegranate peel extract on quality and microbiota composition of bighead carp (Aristichthys nobilis) fillets during chilled storage. Food Microbiol. 2019, 82, 445–454. [Google Scholar] [CrossRef]
- Lazo, O.; Guerrero, L.; Alexi, N.; Grigorakis, K.; Claret, A.; Pérez, J.A.; Bou, R. Sensory characterization, physico-chemical properties and somatic yields of five emerging fish species. Food Res. Int. 2017, 100, 396–406. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Zhu, Y.; Wang, X.; Shi, W. Volatile components present in different parts of grass carp. J. Food Biochem. 2018, 42, e12668. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Escriche, I.; Serra, J.A. Comparison of physico-chemical parameters and composition of mussels (Mytilus galloprovincialis Lmk.) from different Spanish origins. Food Chem. 2009, 112, 295–302. [Google Scholar] [CrossRef]
- Cai, L.; Tong, F.; Tang, T.; Ao, Z.; Wei, Z.; Yang, F.; Shu, Y.; Liu, S.; Mai, K. Comparative evaluation of nutritional value and flavor quality of muscle in triploid and diploid common carp: Application of genetic improvement in fish quality. Aquaculture 2021, 541, 736780. [Google Scholar] [CrossRef]
- Song, S.; Tang, Q.; Fan, L.; Xu, X.; Song, Z.; Hayat, K.; Feng, T.; Wang, Y. Identification of pork flavour precursors from enzyme-treated lard using Maillard model system assessed by GC-MS and partial least squares regression. Meat Sci. 2017, 124, 15–24. [Google Scholar] [CrossRef]
- Ramírez, R.; Cava, R. Volatile profiles of dry-cured meat products from three different Iberian X Duroc genotypes. J. Agric. Food Chem. 2007, 55, 1923–1931. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Zhang, H.; Wang, H.; Kong, B. Influence of glycated nitrosohaemoglobin prepared from porcine blood cell on physicochemical properties, microbial growth and flavour formation of Harbin dry sausages. Meat Sci. 2019, 148, 96–104. [Google Scholar] [CrossRef]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, X.; Jin, M.; Jiao, L.; Sun, P.; Betancor, M.B.; Tocher, D.R.; Zhou, Q. Modification of nutritional values and flavor qualities of muscle of swimming crab (Portunus trituberculatus): Application of a dietary lipid nutrition strategy. Food Chem. 2020, 308, 125607. [Google Scholar] [CrossRef]
- Luo, J.; Nasiru, M.M.; Zhuang, H.; Zhou, G.; Zhang, J. Effects of partial NaCl substitution with high-temperature ripening on proteolysis and volatile compounds during process of Chinese dry-cured lamb ham. Food Res. Int. 2021, 140, 110001. [Google Scholar] [CrossRef]
- Moretti, V.M.; Vasconi, M.; Caprino, F.; Bellagamba, F. Fatty Acid Profiles and Volatile Compounds Formation During Processing and Ripening of a Traditional Salted Dry Fish Product. J. Food Process. Preserv. 2017, 41, e13133. [Google Scholar] [CrossRef]
- Mahmoud, M.A.A.; Buettner, A. Characterisation of aroma-active and off-odour compounds in German rainbow trout (Oncorhynchus mykiss). Part II: Case of fish meat and skin from earthen-ponds farming. Food Chem. 2017, 232, 841–849. [Google Scholar] [CrossRef]
- Montanari, C.; Bargossi, E.; Gardini, A.; Lanciotti, R.; Magnani, R.; Gardini, F.; Tabanelli, G. Correlation between volatile profiles of Italian fermented sausages and their size and starter culture. Food Chem. 2016, 192, 736–744. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef]
- Wu, W.H.; Zhou, Y.; Wang, G.Y.; Zhu, R.J.; Ge, C.R.; Liao, G.Z. Changes in the physicochemical properties and volatile flavor compounds of dry-cured Chinese Laowo ham during processing. J. Food Process. Preserv. 2020, 44, e14593. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Apetrei, C.; Lozano, J.; Anyogu, A. Potential use of electronic noses, electronic tongues and biosensors as multisensor systems for spoilage examination in foods. Trends Food Sci. Technol. 2018, 80, 71–92. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Wang, W.; Liu, J. Comparison of proximate composition, amino acid and fatty acid profiles in wild, pond- and cage-cultured longsnout catfish (Leiocassis longirostris). Int. J. Food Sci. Technol. 2012, 47, 1772–1776. [Google Scholar] [CrossRef]
- Wu, T.; Mao, L. Influences of hot air drying and microwave drying on nutritional and odorous properties of grass carp (Ctenopharyngodon idellus) fillets. Food Chem. 2008, 110, 647–653. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Chen, S.J.F.S.; Research, T. Comparison of active non-volatile taste components in the viscera and adductor muscles of oyster (Ostrea rivularis Gould). Food Sci. Technol. Res. 2013, 19, 417–424. [Google Scholar] [CrossRef]
- Jiao, S.; Nie, M.; Song, H.; Xu, D.; You, F. Physiological responses to cold and starvation stresses in the liver of yellow drum (Nibea albiflora) revealed by LC-MS metabolomics. Sci. Total Environ. 2020, 715, 136940. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Song, S.; Wang, Z.; Kong, C.; Luo, Y. The role of microorganisms in the degradation of adenosine triphosphate (ATP) in chill-stored common carp (Cyprinus carpio) fillets. Food Chem. 2017, 224, 347–352. [Google Scholar] [CrossRef]
- Drengstig, A.; Bergheim, A. Commercial land-based farming of European lobster (Homarus gammarus L.) in recirculating aquaculture system (RAS) using a single cage approach. Aquac. Eng. 2013, 53, 14–18. [Google Scholar] [CrossRef]
- Johnston, I.A.; Li, X.J.; Vieira, V.L.A.; Nickell, D.; Dingwall, A.; Alderson, R.; Campbell, P.; Bickerdike, R. Muscle and flesh quality traits in wild and farmed Atlantic salmon. Aquaculture 2006, 256, 323–336. [Google Scholar] [CrossRef]
- Farmer, L.J.; McConnell, J.M.; Kilpatrick, D.J. Sensory characteristics of farmed and wild Atlantic salmon. Aquaculture 2000, 187, 105–125. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Merlo, T.C.; Lorenzo, J.M.; Saldaña, E.; Patinho, I.; Oliveira, A.C.; Menegali, B.S.; Selani, M.M.; Domínguez, R.; Contreras-Castillo, C.J. Relationship between volatile organic compounds, free amino acids, and sensory profile of smoked bacon. Meat Sci. 2021, 181, 108596. [Google Scholar] [CrossRef]



| Samples | Content (g/100g) | |||||
|---|---|---|---|---|---|---|
| JA | CA | JB | CB | JT | CT | |
| Crude protein | 20.14 ± 0.38 b | 17.59 ± 0.23 d | 21.55 ± 0.57 a | 18.50 ± 0.46 c | 20.04 ± 0.33 b | 16.86 ± 0.28 d |
| Crude fat | 4.84 ± 0.13 b | 6.13 ± 0.12 a | 4.70 ± 0.19 b | 5.94 ± 0.21 a | 4.46 ± 0.15 b | 5.98 ± 0.12 a |
| Moisture | 73.92 ± 4.24 a | 75.79 ± 4.78 a | 72.95 ± 4.89 a | 75.29 ± 1.99 a | 74.98 ± 2.32 a | 76.36 ± 5.92 a |
| Ash | 1.18 ± 0.01 ab | 1.05 ± 0.02 d | 1.22 ± 0.01 a | 1.09 ± 0.01 c | 1.16 ± 0.02 b | 1.05 ± 0.01 d |
| No | Volatile Compounds | Content (μg kg−1) | |||||
|---|---|---|---|---|---|---|---|
| JA | CA | JB | CB | JT | CT | ||
| Alcohols | |||||||
| 1 | 1-Octanol | – | – | 1.87 ± 0.12 a | 1.20 ± 0.02 b | – | – |
| 2 | 1,3,5-Benzetriol | 1.05 ± 0.03 a | – | 0.87 ± 0.01 a | – | – | – |
| 3 | 11-Bromo-1-undecanol | – | – | 1.78 ± 0.01 a | – | – | – |
| 4 | Palmidrol | – | – | 2.66 ± 0.23 a | – | – | – |
| 5 | Sorbitol | – | – | 1.49 ± 0.01 a | – | – | – |
| 6 | 1-Tetradecanol | – | – | 2.64 ± 0.21 b | – | 4.99 ± 0.13 a | – |
| 7 | Hexaethylene glycol | – | – | 2.26 ± 0.19 a | – | – | – |
| 8 | Isoamyl alcohol | – | – | 1.06 ± 0.01 a | – | – | – |
| 9 | 2,5,8,11,14-Pentaoxahexadecan-16-ol | – | – | 1.38 ± 0.08 a | – | – | – |
| 10 | Glycerol, 3TBDMS derivative | – | – | 1.52 ± 0.12 a | – | – | – |
| 11 | 3,4-Dihydroxyphenylglycol, 4TMS derivative | – | – | 1.53 ± 0.18 a | – | – | – |
| 12 | Tetraethylene glycol | – | – | 1.50 ± 0.10 a | – | – | – |
| 13 | 2-[2-[2-[2-[2-[2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol | – | – | 0.92 ± 0.02 a | – | – | – |
| 14 | 4-Octanol, 4,7-dimethyl- | – | – | 2.64 ± 0.20 a | – | – | – |
| 15 | 1,5-Anhydro-2-O-acetyl-3,4,6-tri-O-methyl-D-glucitol | – | – | 0.94 ± 0.01 a | – | – | – |
| 16 | (R)-2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide | – | – | 1.07 ± 0.01 a | – | – | – |
| 17 | 1-nonanol | 0.81 ± 0.02 b | 1.45 ± 0.02 a | – | 1.39 ± 0.02 a | – | – |
| 18 | 1-Hexanol | 19.88 ± 0.69 a | 21.11 ± 0.87 a | 20.71 ± 0.59 a | 22.93 ± 0.54 a | 17.59 ± 0.60 b | 17.64 ± 0.61 b |
| 19 | 4-Methylmannitol | – | – | – | – | 2.68 ± 0.02 a | – |
| 20 | 1-Butanol,3-(1-ethoxyethoxy)-4,4,4-trifluoro- | – | – | – | – | 2.34 ± 0.01 a | – |
| 21 | Triethylene glycol monododecyl ether | – | – | – | 1.45 ± 0.02 b | 2.68 ± 0.02 a | – |
| 22 | Glycerol, 1,2-di(TMS)- | – | – | – | 4.00 ± 0.03 a | – | – |
| 23 | 3-Methyl-5-methoxy-1-pentanol | – | – | – | 1.34 ± 0.01 a | – | – |
| 24 | 3-Methoxy-hexane-1,6-diol | – | – | – | 1.27 ± 0.02 a | – | – |
| 25 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | – | 5.62 ± 0.26 b | 6.17 ± 0.04 a | – | – | – |
| 26 | 1,2,4-Cyclopentanetriol | – | 1.32 ± 0.02 a | – | – | – | – |
| 27 | 2-Octyldecanol | – | – | – | – | – | 0.44 ± 0.01 a |
| 28 | Nonaethylene glycol | – | – | – | – | – | 23.67 ± 0.18 a |
| 29 | Fucoxanthin | – | – | – | – | 2.95 ± 0.12 a | – |
| Total alcohols | 21.74 ± 0.74 f | 29.50 ± 1.17 e | 52.47 ± 2.14 a | 33.58 ± 0.66 c | 33.23 ± 0.90 c | 41.75 ± 0.80 b | |
| Aldehydes | |||||||
| 30 | 2,5-Dihydroxybenzaldehyde | 3.77 ± 0.02 c | 4.34 ± 0.08 b | 3.00 ± 0.10 e | 4.16 ± 0.02 b | 6.61 ± 0.07 a | 4.30 ± 0.06 b |
| 31 | 1-nonanal | 3.52 ± 0.10 a | 1.07 ± 0.03 d | 3.59 ± 0.03 a | 1.13 ± 0.01 d | 3.06 ± 0.11 b | 2.58 ± 0.05 c |
| 32 | 3-Methylbutanal | 6.97 ± 0.05 a | 4.16 ± 0.24 d | 5.81 ± 0.34 c | 3.91 ± 0.32 e | 6.42 ± 0.56 b | 3.93 ± 0.16 e |
| 33 | Hexanal | – | – | – | – | 2.37 ± 0.02 a | – |
| 34 | Vanillin | 4.38 ± 0.10 a | – | 3.43 ± 0.12 b | – | – | – |
| 35 | 3-Hydroxy-4-methoxybenzaldehyde | – | – | – | 2.72 ± 0.05 b | 4.25 ± 0.16 a | – |
| 36 | 2-dodecenal | – | – | – | – | 1.91 ± 0.03 b | 3.98 ± 0.09 a |
| Total aldehydes | 18.64 ± 0.29 b | 9.57 ± 0.35 e | 15.83 ± 0.59 c | 11.92 ± 0.40 d | 24.62 ± 0.95 a | 14.79 ± 0.36 c | |
| Ketones | |||||||
| 37 | Xanthoxylin | – | – | – | 8.65 ± 0.17 a | – | – |
| 38 | 5-Decanone | – | – | – | – | – | 1.34 ± 0.02 a |
| 39 | 5,5-dichloro-4-Spirohexanone | – | 1.82 ± 0.08 a | – | – | – | – |
| Total ketones | 0 | 1.82 ± 0.08 b | 0 | 8.65 ± 0.17 a | 0 | 1.34 ± 0.02 c | |
| Esters | |||||||
| 40 | Butyric acid pentadecyl ester | – | – | – | 2.62 ± 0.11 a | – | – |
| 41 | Propanoic acid, dimethyl (ethenyl)silyl ester | – | – | – | – | – | 1.95 ± 0.04 a |
| 42 | Diglycolic acid, 2-chloro-6-fluorophenyl nonyl ester | – | – | – | – | – | 1.86 ± 0.02 a |
| 43 | Diethyl sulfate | – | – | 0.86 ± 0.02 a | – | – | – |
| 44 | Methyl myristoleate | – | – | 1.30 ± 0.03 a | – | – | – |
| 45 | Isopropyl palmitate | 0.89 ± 0.02 b | – | 2.29 ± 0.14 a | – | – | – |
| 46 | Triethyl borate | – | – | 1.11 ± 0.01 a | – | – | – |
| 47 | Glycerol monostearate | – | – | 1.58 ± 0.08 a | – | – | – |
| 48 | di(Butoxyethyl)adipate | – | – | 2.04 ± 0.10 a | – | – | – |
| 49 | Methyl 2-hydroxystearate | – | – | 0.89 ± 0.02 a | – | – | – |
| 50 | Dimethyl dl-malate | – | – | 0.84 ± 0.01 a | – | – | – |
| 51 | Methyl acetylglycinate | – | – | 0.80 ± 0.03 a | – | – | – |
| 52 | Isobutyl 3-hydroxy-2-methylenebutanoate | – | – | 0.85 ± 0.01 a | – | – | – |
| 53 | Hexanoic acid, cyclohexyl ester | – | – | 0.86 ± 0.02 a | – | – | – |
| 54 | Propanoic acid, 2-methyl-, octyl ester | – | – | 1.06 ± 0.01 a | – | – | – |
| 55 | Hexacosanoic acid, methyl ester | – | – | 1.60 ± 0.09 a | – | – | – |
| 56 | Heptanoic acid, octyl ester | – | – | 0.94 ± 0.03 a | – | – | – |
| 57 | Methyl benzoate | 3.19 ± 0.13 e | – | 6.24 ± 0.29 b | 5.21 ± 0.10 c | 7.66 ± 0.32 a | 6.12 ± 0.21 b |
| 58 | Propanoic acid, 3-ethoxy-, ethyl ester | – | – | 1.86 ± 0.08 a | – | – | – |
| 59 | Heptanoic acid, propyl ester | – | – | 0.87 ± 0.05 a | – | – | – |
| 60 | ethyl-2-methylbutanoate | – | – | 6.01 ± 0.62 a | – | – | – |
| 61 | ENT-337 | – | – | 0.89 ± 0.02 a | – | – | – |
| 62 | L-Citrulline, N,N’-bis(dimethylaminomethylene)-, methyl ester | – | – | 1.88 ± 0.07 a | – | – | – |
| 63 | 2-Isoxazolidinecarboxylic acid, ethyl ester | – | – | 0.79 ± 0.02 a | – | – | – |
| 64 | Silicic acid, diethyl bis(trimethylsilyl) ester | 3.62 ± 0.22 b | – | – | 4.18 ± 0.31 a | – | – |
| 65 | Glycylglycine ethyl ester | 1.11 ± 0.02 a | – | – | – | – | – |
| 66 | Carbamodithioic acid, diethyl-, methyl ester | 3.67 ± 0.31 b | – | – | 9.42 ± 0.49 a | – | – |
| 67 | Imidodicarbonic acid, diethyl ester | 0.95 ± 0.05 a | – | – | – | – | – |
| 68 | (2-Bromomethyl-[1,3]dioxolan-2-yl)-acetic acid, methyl ester | – | – | – | – | 2.42 ± 0.14 a | – |
| 69 | Pentyl (3S)-3-hydroxy-5-methoxypentanoate | – | – | – | 0.98 ± 0.01 c | 3.16 ± 0.16 a | 2.45 ± 0.08 b |
| 70 | Butanoic acid, 4-ethoxy-, methyl ester | – | – | – | – | 3.93 ± 0.28 a | – |
| 71 | (E)-3,7,11-Trimethyldodec-2-enoic acid, methyl ester | – | – | – | – | 1.80 ± 0.09 a | – |
| 72 | Heptadecanoic acid, 9-methyl-, methyl ester | – | – | – | – | 4.28 ± 0.44 a | – |
| 73 | Pentanoic acid, 2-hydroxy-3-methyl-, methyl ester | – | – | – | – | 2.70 ± 0.10 a | – |
| 74 | Boric acid (H3BO3), tris(1-methylethyl) ester | – | – | – | – | 1.52 ± 0.09 a | – |
| 75 | 4-Methylmannonic.delta.- lactone | – | – | – | – | 1.99 ± 0.11 a | – |
| 76 | 3-(1-Ethoxy-ethoxy)-2-ethyl-butyric acid, ethyl ester | – | – | – | – | 3.31 ± 0.28 a | – |
| 77 | Isobutyl 2,5,8,11-tetraoxatridecan-13-yl carbonate | – | – | – | – | 2.10 ± 0.12 a | – |
| 78 | 3-Deoxy-d-mannoic lactone | – | – | – | 0.98 ± 0.02 a | – | – |
| 79 | Propanoic acid, 2-methyl-, decyl ester | – | – | – | 1.76 ± 0.15 a | – | – |
| 80 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | – | – | – | 1.28 ± 0.04 a | – | – |
| 81 | Trimethylene borate | – | – | – | 3.17 ± 0.36 a | – | – |
| 82 | Methyl octa-O-methyllaminaribionate | – | – | – | 1.10 ± 0.09 a | – | – |
| 83 | Methyl-(aminosulfanyl)formate | – | – | – | 1.14 ± 0.04 a | – | – |
| 84 | Propyl (3S)-3-hydroxy-5-methoxypentanoate | – | – | – | 2.21 ± 0.20 a | – | – |
| 85 | Ethanol, 2-[2-(2-methoxyethoxy)ethoxy]-, acetate | – | – | – | 1.30 ± 0.07 a | – | – |
| 86 | Tetraethyleneglycol monomethylether | – | – | – | 0.99 ± 0.03 a | – | – |
| 87 | Butanoic acid, nonyl ester | – | 1.83 ± 0.24 a | – | – | – | – |
| 88 | Methyl 3-hydroxytetradecanoate | – | – | – | – | – | 1.37 ± 0.11 a |
| 89 | Octaethylene glycol monododecyl ether | – | – | – | – | – | 2.07 ± 0.09 a |
| 90 | Nonanoic acid, 9-oxo-, ethyl ester | – | – | – | – | – | 1.60 ± 0.13 a |
| 91 | Methyl 5-methoxy-3-oxovalerate | – | – | – | – | – | 1.28 ± 0.07 a |
| 92 | 7-Hexadecenoic acid, methyl ester, (Z)- | – | – | – | – | – | 9.15 ± 0.83 a |
| Total esters | 13.43 ± 0.75 d | 1.83 ± 0.24 e | 35.56 ± 1.82 b | 36.34 ± 2.02 b | 42.27 ± 2.13 a | 27.85 ± 1.61 c | |
| Hydrocarbons | |||||||
| 93 | Hexane, 3-methyl- | – | – | 1.44 ± 0.08 a | – | – | – |
| 94 | Trichloromethane | – | – | 1.20 ± 0.03 a | 1.23 ± 0.04 a | – | – |
| 95 | Heneicosane | 7.34 ± 0.52 d | 2.76 ± 0.14 e | 2.32 ± 0.12 f | 9.91 ± 0.43 c | 28.41 ± 0.98 b | 33.37 ± 2.09 a |
| 96 | Pentatriacontane | – | – | 3.05 ± 0.24 a | – | – | – |
| 97 | Dodecane, 2-methyl- | – | – | 1.64 ± 0.10 a | – | – | – |
| 98 | Propane, 1,1’-[ethylidenebis(oxy)]bis- | – | – | 1.05 ± 0.06 a | – | – | – |
| 99 | trans-Calamenene | – | – | 0.93 ± 0.06 a | – | – | – |
| 100 | Heptadecane | 1.92 ± 0.10 b | – | – | – | 5.6 ± 0.30 a | – |
| 101 | cis-Calamenene | 1.55 ± 0.19 b | – | – | – | 3.26 ± 0.24 a | – |
| 102 | Oxetane, 2-propyl- | – | – | – | – | – | 1.31 ± 0.09 a |
| 103 | Heptadecane, 7-methyl- | – | – | – | – | – | 18.73 ± 2.05 a |
| 104 | 1,4,7-Triazacyclononane, 1-benzoyl- | – | – | – | – | – | 2.69 ± 0.08 a |
| 105 | 1-Tetracosene | – | – | – | – | – | 3.99 ± 0.23 a |
| 106 | 1-Eicosyne | – | – | – | – | 2.00 ± 0.10 a | |
| Total hydrocarbons | 10.81 ± 0.81 e | 2.76 ± 0.14 f | 11.63 ± 0.79 c | 11.14 ± 0.47 d | 37.27 ± 1.52 b | 62.09 ± 4.64 a | |
| Ethers | |||||||
| 107 | Estragole | – | – | 4.93 ± 0.65 a | – | – | – |
| 108 | 2-(2-Methoxyethoxy)ethanol | – | – | 1.07 ± 0.02 a | – | – | – |
| 109 | 2-[2-(hexyloxy)ethoxy]-ethanol | – | – | 0.93 ± 0.05 a | – | – | – |
| 110 | Tetraethylene glycol diethyl ether | – | – | 1.04 ± 0.10 a | – | – | – |
| 111 | Ethanol, 2-(2-ethoxyethoxy)- | – | – | 0.78 ± 0.02 a | – | – | – |
| 112 | Undecaethylene glycol monomethyl ether | 1.16 ± 0.10 a | – | – | – | – | – |
| 113 | Eicosyl methyl ether | – | 2.25 ± 0.16 a | – | – | – | – |
| 114 | Anethole | 3.12 ± 0.16 c | 2.90 ± 0.10 c | 4.28 ± 0.29 b | 2.63 ± 0.11 e | 6.43 ± 0.46 a | 2.39 ± 0.08 d |
| Total ethesrs | 4.28 ± 0.26 d | 5.15 ± 0.26 c | 13.03 ± 1.13 a | 2.63 ± 0.11 e | 6.43 ± 0.46 b | 2.39 ± 0.08 f | |
| Others | |||||||
| 115 | 3-Phenylindole | – | – | 1.76 ± 0.08 a | – | – | – |
| 116 | 1,2-Bis(trimethylsilyl)benzene | – | – | 0.91 ± 0.03 a | – | – | – |
| 117 | Butylated Hydroxytoluene | 2.61 ± 0.26 b | – | 3.59 ± 0.15 a | – | – | – |
| 118 | Azulene | – | 1.38 ± 0.08 a | – | – | – | – |
| 119 | Naphthalene | – | – | – | 1.45 ± 0.08 a | – | 1.36 ± 0.06 a |
| Total others | 5.73 ± 0.48 c | 4.28 ± 0.38 d | 10.54 ± 0.55 a | 4.08 ± 0.19 e | 6.43 ± 0.46 b | 3.75 ± 0.16 f | |
| Amino Acid Species | Taste Attribute | Content (g Amino Acid/100 g Protein) | |||||
|---|---|---|---|---|---|---|---|
| 2222 | JA | CA | JB | CB | JT | CT | |
| Aspartic acid ▲ (Asp) | Umami/sour (+) | 5.96 ± 0.17 a | 5.46 ± 0.27 c | 5.69 ± 0.13 b | 5.15 ± 0.21 d | 5.70 ± 0.16 b | 5.18 ± 0.23 d |
| Threonine ▲ (Thr) | Sweet (+) | 2.01 ± 0.08 a | 1.92 ± 0.03 ab | 1.96 ± 0.09 ab | 1.80 ± 0.07 b | 2.00 ± 0.04 a | 1.83 ± 0.17 b |
| Serine ▲ (Ser) | Sweet (+) | 2.11 ± 0.02 a | 2.03 ± 0.04 ab | 2.04 ± 0.04 ab | 1.91 ± 0.06 b | 2.05 ± 0.03 ab | 1.92 ± 0.02 b |
| Glutamic acid ▲ (Glu) | Umami/sour (+) | 7.94 ± 0.06 a | 7.32 ± 0.11 d | 7.53 ± 0.12 c | 6.77 ± 0.19 f | 7.78 ± 0.08 b | 6.94 ± 0.18 e |
| Glycine ▲ (Gly) | Sweet (+) | 3.20 ± 0.01 a | 2.95 ± 0.08 b | 2.84 ± 0.04 bc | 2.62 ± 0.05 d | 2.99 ± 0.01 b | 2.73 ± 0.01 cd |
| Alanine ▲ (Ala) | Sweet (+) | 3.66 ± 0.16 a | 3.37 ± 0.16 b | 3.53 ± 0.13 ab | 3.17 ± 0.13 c | 3.47 ± 0.15 b | 3.18 ± 0.12 c |
| Cysteine (Cys) | Bitter/sweet/sulfur (-) | 0.15 ± 0.02 a | 0.10 ± 0.01 a | 0.18 ± 0.02 a | 0.17 ± 0.03 a | 0.23 ± 0.01 a | 0.15 ± 0.02 a |
| Valine ▼ (Val) | Sweet/bitter (-) | 1.57 ± 0.13 a | 1.48 ± 0.10 a | 1.53 ± 0.07 a | 1.42 ± 0.13 a | 1.57 ± 0.13 a | 1.47 ± 0.06 a |
| Methionine ▼ (Met) | Bitter/sweet/sulfur (-) | 1.48 ± 0.03 b | 1.46 ± 0.09 b | 1.55 ± 0.07 b | 1.47 ± 0.16 b | 1.74 ± 0.10 a | 1.44 ± 0.14 b |
| Isoleucine ▼ (Ile) | Bitter (-) | 1.32 ± 0.04 a | 1.27 ± 0.12 a | 1.29 ± 0.07 a | 1.27 ± 0.11 a | 1.33 ± 0.15 a | 1.26 ± 0.14 a |
| Leucine ▼ (Leu) | Bitter (-) | 4.11 ± 0.15 a | 3.76 ± 0.17 b | 4.01 ± 0.17 a | 3.62 ± 017 b | 4.04 ± 0.14 a | 3.63 ± 0.19 b |
| Tyrosine ▼ (Tyr) | Bitter (-) | 1.96 ± 0.08 a | 1.93 ± 0.04 a | 2.00 ± 0.02 a | 1.88 ± 0.04 a | 2.04 ± 0.05 a | 1.89 ± 0.10 a |
| Phenylalanine ▼ (Phe) | Bitter (-) | 2.13 ± 0.18 a | 1.93 ± 0.17 bc | 2.07 ± 0.13 ab | 1.84 ± 0.12 c | 2.03 ± 0.12 ab | 1.83 ± 0.11 c |
| Lysine ▼ (Lys) | Sweet/bitter (-) | 4.87 ± 0.15 a | 4.38 ± 0.13 c | 4.63 ± 0.16 b | 4.18 ± 0.18 d | 4.68 ± 0.14 b | 4.23 ± 0.16 cd |
| Histidine ▼ (His) | Bitter (-) | 1.57 ± 0.02 a | 1.33 ± 0.04 bc | 1.63 ± 0.08 a | 1.31 ± 0.03 bc | 1.47 ± 0.09 ab | 1.29 ± 0.07 c |
| Arginine ▼ (Arg) | Sweet/bitter (-) | 2.96 ± 0.01 a | 2.75 ± 0.08 b | 2.82 ± 0.06 ab | 2.66 ± 0.08 b | 2.90 ± 0.11 a | 2.67 ± 0.05 b |
| Proline ▲ (Pro) | Sweet/bitter (+) | 2.09 ± 0.03 a | 1.97 ± 0.03 ab | 1.95 ± 0.03 ab | 1.73 ± 0.04 c | 2.03 ± 0.05 a | 1.85 ± 0.03 bc |
| Total | 49.09 ± 2.54 a | 45.42 ± 1.78 bc | 47.24 ± 1.20 b | 42.96 ± 0.94 c | 48.05 ± 2.61 ab | 43.46 ± 1.65 c | |
| ATP-Associated Compounds | Content (mg/kg) | |||||
|---|---|---|---|---|---|---|
| JA | CA | JB | CB | JT | CT | |
| ATP | 156.39 ± 6.86 b | 151.62 ± 5.12 c | 171.55 ± 6.51 a | 165.04 ± 6.43 a | 151.61 ± 5.27 c | 148.07 ± 6.11 d |
| ADP | 272.65 ± 10.86 b | 231.94 ± 11.23 d | 282.26 ± 10.22 a | 248.25 ± 11.36 c | 218.52 ± 10.13 e | 218.21 ± 11.38 e |
| AMP | 84.39 ± 5.92 b | 68.66 ± 5.90 d | 87.91 ± 5.91 a | 60.78 ± 5.90 e | 81.19 ± 4.63 c | 66.75 ± 6.58 d |
| IMP | 1951.19 ± 36.34 d | 2009.11 ± 43.91 c | 2258.22 ± 40.46 a | 2243.75 ± 43.26 a | 2113.24 ± 37.20 b | 2101.44 ± 42.98 b |
| HxR | 202.29 ± 12.03 e | 212.88 ± 11.45 d | 264.10 ± 11.49 a | 200.21 ± 11.76 e | 258.69 ± 11.95 b | 231.49 ± 10.35 c |
| Hx | 72.98 ± 4.52 a | 62.23 ± 2.54 b | 29.82 ± 2.56 e | 41.64 ± 3.43 d | 39.00 ± 3.81 d | 55.69 ± 2.26 c |
| k | 10.05 ± 0.43 a | 10.05 ± 0.41 a | 9.50 ± 0.52 a | 8.17 ± 0.99 a | 10.40 ± 0.38 a | 10.18 ± 0.87 a |
| Samples | JA | CA | JB | CB | JT | CT |
|---|---|---|---|---|---|---|
| Texture | ||||||
| Tenderness | 4.04 ± 0.26 a | 2.82 ± 0.33 b | 3.94 ± 0.22 a | 2.69 ± 0.36 b | 3.36 ± 0.43 ab | 3.12 ± 0.38 ab |
| Springiness | 3.91 ± 0.22 a | 2.51 ± 0.42 b | 3.87 ± 0.41 a | 2.59 ± 0.36 b | 3.70 ± 0.52 a | 2.60 ± 0.43 b |
| Chewiness | 4.23 ± 0.34 a | 3.13 ± 0.56 ab | 3.92 ± 0.46 ab | 3.85 ± 0.26 ab | 2.83 ± 0.20 ab | 2.71 ± 0.55 b |
| Odor | ||||||
| Fishy | 2.38 ± 0.31 b | 3.47 ± 0.29 a | 2.17 ± 0.51 b | 3.44 ± 0.49 a | 2.18 ± 0.21 b | 3.60 ± 0.28 a |
| Earthy | 1.20 ± 0.32 b | 3.11 ± 0.34 a | 1.18 ± 0.18 b | 3.88 ± 0.41 a | 1.54 ± 0.40 b | 2.99 ± 0.30 a |
| Taste | ||||||
| Fishy | 2.87 ± 0.28 ab | 3.61 ± 0.37 a | 2.75 ± 0.25 b | 3.65 ± 0.18 a | 2.80 ± 0.29 b | 3.18 ± 0.33 ab |
| Earthy | 1.10 ± 0.37 b | 3.24 ± 0.40 a | 1.45 ± 0.49 b | 3.26 ± 0.54 a | 1.68 ± 0.12 b | 3.29 ± 0.29 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Pan, D.; Du, H.; Ma, J.; Kong, B.; Diao, J. Flavor Differences of Edible Parts of Grass Carp between Jingpo Lake and Commercial Market. Foods 2022, 11, 2594. https://doi.org/10.3390/foods11172594
Chen H, Pan D, Du H, Ma J, Kong B, Diao J. Flavor Differences of Edible Parts of Grass Carp between Jingpo Lake and Commercial Market. Foods. 2022; 11(17):2594. https://doi.org/10.3390/foods11172594
Chicago/Turabian StyleChen, Hongsheng, Deyin Pan, Hongzhen Du, Jinming Ma, Baohua Kong, and Jingjing Diao. 2022. "Flavor Differences of Edible Parts of Grass Carp between Jingpo Lake and Commercial Market" Foods 11, no. 17: 2594. https://doi.org/10.3390/foods11172594
APA StyleChen, H., Pan, D., Du, H., Ma, J., Kong, B., & Diao, J. (2022). Flavor Differences of Edible Parts of Grass Carp between Jingpo Lake and Commercial Market. Foods, 11(17), 2594. https://doi.org/10.3390/foods11172594







