Pomegranate (Punica granatum L.) Peel Extracts as Antimicrobial and Antioxidant Additives Used in Alfalfa Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pomegranate Peel Extracts
2.2. Identification and Quantification of Individual Phenolic Compounds
2.3. Total Phenolic and Flavonoid Content
2.4. Antioxidant Capacity
2.5. In Vitro Antimicrobial Activity
2.6. Anti-Quorum Sensing Potential
2.7. Application of PPE to Alfalfa Sprouts
2.8. Microbiological Analysis of Treated Alfalfa Sprouts
2.9. Antioxidant Capacity of Treated Alfalfa Sprouts
2.10. Statistical Analysis
3. Results and Discussion
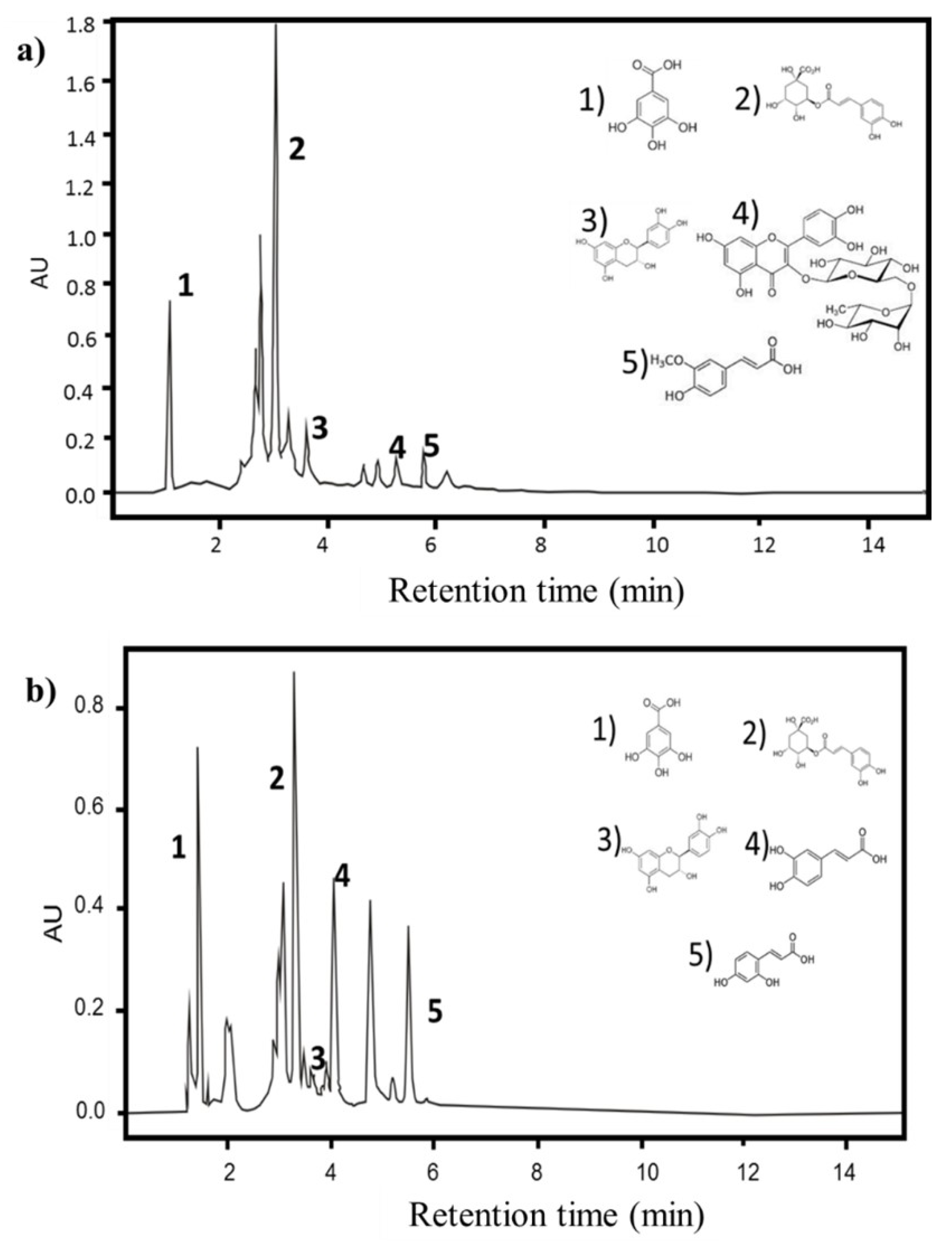
3.1. Identification and Quantification of Phenolic and Flavonoid Content and the Antioxidant Capacity of PPE
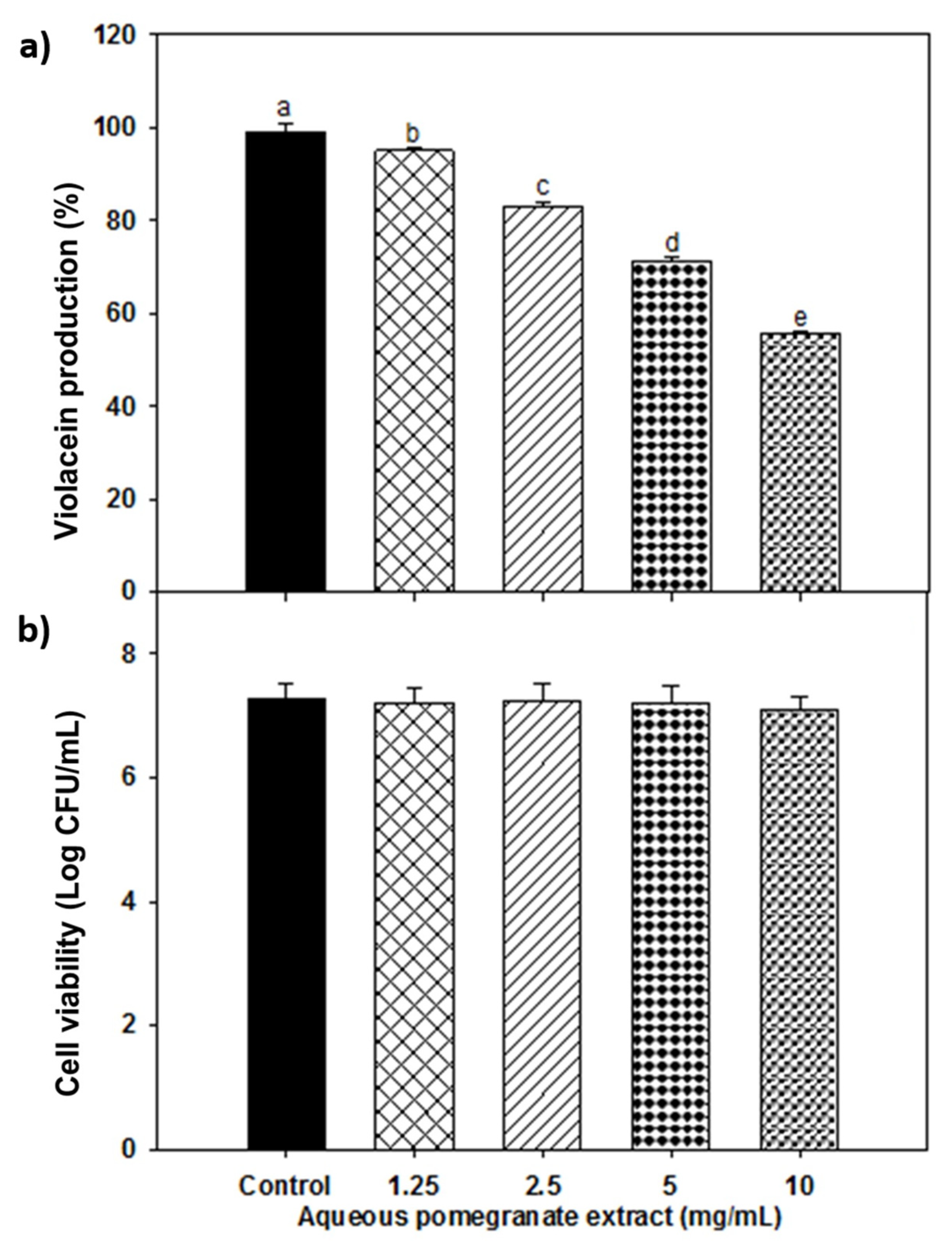
3.2. Antimicrobial Activity against Pathogenic Bacteria and Anti-Quorum Sensing Properties of PPE
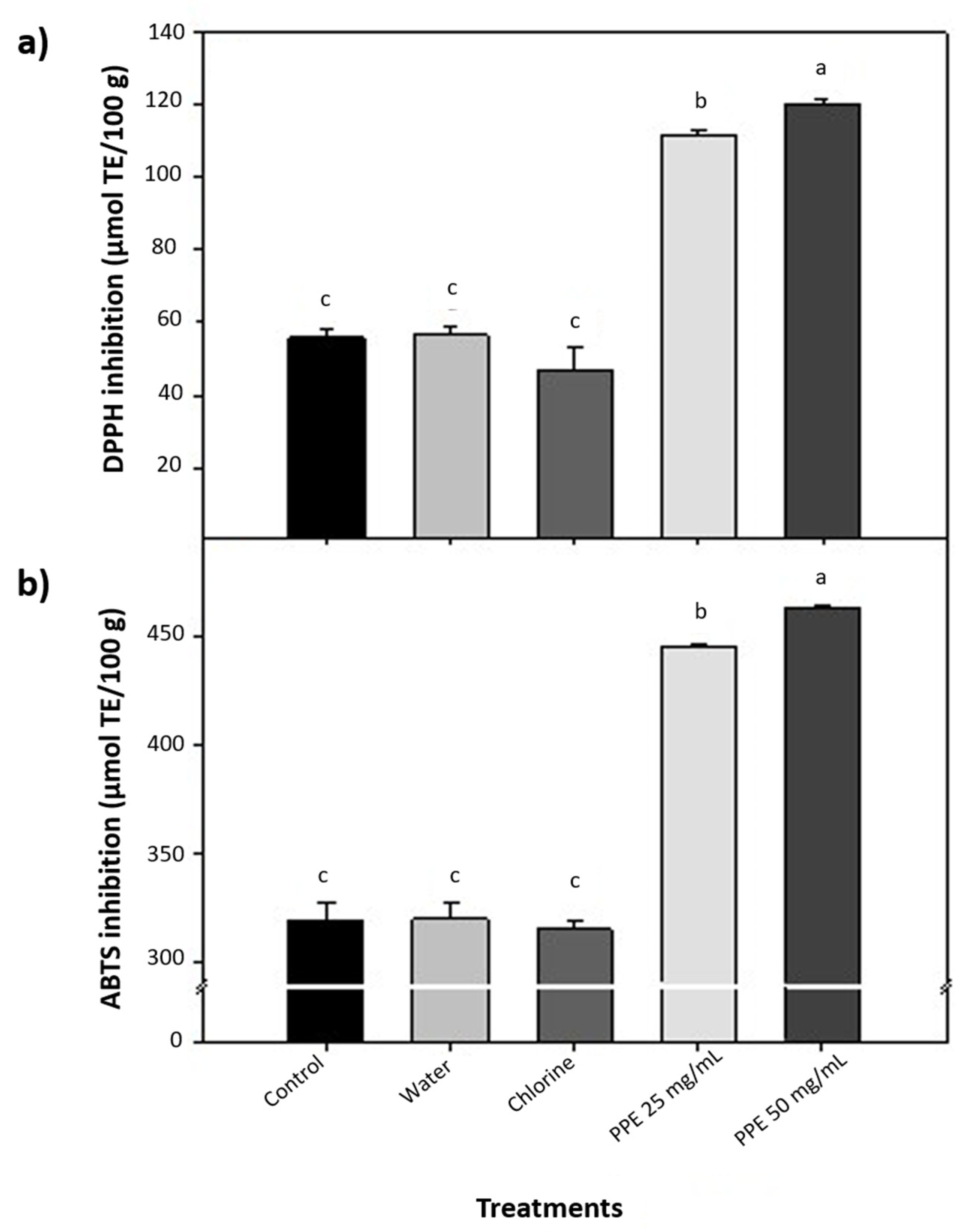
3.3. Antioxidant Status and Microbial Counts of Alfalfa Sprouts Treated with PPE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- USDA Alfalfa Seeds, Sprouted, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168384/nutrients (accessed on 7 April 2020).
- Rossi, F.; Lathrop, A. Effects of Lactobacillus plantarum, Pediococcus acidilactici, and Pediococcus pentosaceus on the growth of Listeria monocytogenes and Salmonella on alfalfa sprouts. J. Food Prot. 2019, 82, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Harfield, S.; Beazley, R.; Denehy, E.; Centofanti, A.; Dowsett, P.; Housen, T.; Flood, L. An outbreak and case-control study of Salmonella Havana linked to alfalfa sprouts in South Australia, 2018. Commun. Dis. Intell. 2019, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Abrol, G.S.; Dubey, N. Sodium and calcium hypochlorite as postharvest disinfectants for fruits and vegetables. In Postharvest Disinfection of Fruits and Vegetables; Wasim Siddiqui, M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 253–272. [Google Scholar]
- Yang, Y.; Komaki, Y.; Kimura, S.Y.; Hu, H.-Y.; Wagner, E.D.; Mariñas, B.J.; Plewa, M.J. Toxic impact of bromide and iodide on drinking water disinfected with chlorine or chloramines. Environ. Sci. Technol. 2014, 48, 12362–12369. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J. To regulate or not to regulate? What to do with more toxic disinfection by-products? J. Environ. Chem. Eng. 2020, 8, 103939. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Ahmadi Gavlighi, H. Antioxidant and antimicrobial activities of (-)-epigallocatechin-3-gallate (EGCG) and its potential to preserve the quality and safety of foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J. Food waste and byproducts: An opportunity to minimize malnutrition and hunger in developing countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Arun, K.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; Reshmy, R.; Sirohi, R. Remodeling agro-industrial and food wastes into value-added bioactives and biopolymers. Ind. Crops Prod. 2020, 154, 112621. [Google Scholar] [CrossRef]
- Drinić, Z.; Mudrić, J.; Zdunić, G.; Bigović, D.; Menković, N.; Šavikin, K. Effect of pomegranate peel extract on the oxidative stability of pomegranate seed oil. Food Chem. 2020, 333, 127501. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsiʼ pomegranate peel extracts. Ind. Crops Prod. 2018, 111, 345–352. [Google Scholar] [CrossRef]
- Alexandre, E.M.; Silva, S.; Santos, S.A.; Silvestre, A.J.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, L.; Gao, J.; Liu, X.; Feng, Y.; Wu, Q.; Baloch, A.B.; Cui, L.; Xia, X. Tannin-rich fraction from pomegranate rind inhibits quorum sensing in Chromobacterium violaceum and biofilm formation in Escherichia coli. Foodborne Pathog. Dis. 2016, 13, 28–35. [Google Scholar] [CrossRef]
- Belgacem, I.; Schena, L.; Teixidó, N.; Romeo, F.; Ballistreri, G.; Abadias, M. Effectiveness of a pomegranate peel extract (PGE) in reducing Listeria monocytogenes in vitro and on fresh-cut pear, apple and melon. Eur. Food Res. Technol. 2020, 246, 1765–1772. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Acevedo-Hernandez, C.; A Silva-Espinoza, B.; Cruz-Valenzuela, M.R.; A Gonzalez-Aguilar, G.; Nazzaro, F.; Siddiqui, M.W.; Ayala-Zavala, J.F.; Fratianni, F.; Vazquez-Armenta, F.J. Antioxidant and antimicrobial capacity of phenolic compounds of mango (Mangifera indica L.) seed depending upon the extraction process. J. Med. Plants By-Prod. 2018, 7, 209–219. [Google Scholar]
- Vazquez-Armenta, F.J.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J.F. Antibacterial and antioxidant properties of grape stem extract applied as disinfectant in fresh leafy vegetables. J. Food Sci. Technol. 2017, 54, 3192–3200. [Google Scholar] [CrossRef]
- Fratianni, F.; Coppola, R.; Nazzaro, F. Phenolic composition and antimicrobial and antiquorum sensing activity of an ethanolic extract of peels from the apple cultivar Annurca. J. Med. Food 2011, 14, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of single and combined use of catechin, protocatechuic, and vanillic acids as antioxidant and antibacterial agents against uropathogenic Escherichia coli at planktonic and biofilm levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control. 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Ayala-Zavala, J.F.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; de Miranda, M.R.A.; Silva-Espinoza, B.A. Using sensory evaluation to determine the highest acceptable concentration of mango seed extract as antibacterial and antioxidant agent in fresh-cut mango. Foods 2018, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Saad, H.; Charrier-El Bouhtoury, F.; Pizzi, A.; Rode, K.; Charrier, B.; Ayed, N. Characterization of pomegranate peels tannin extractives. Ind. Crops Prod. 2012, 40, 239–246. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Asghari Ghajari, M.; Tavasoli, S. Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 2019, 56, 2365–2372. [Google Scholar] [CrossRef]
- Cardenas-Toro, F.P.; Forster-Carneiro, T.; Rostagno, M.A.; Petenate, A.J.; Maugeri Filho, F.; Meireles, M.A.A. Integrated supercritical fluid extraction and subcritical water hydrolysis for the recovery of bioactive compounds from pressed palm fiber. J. Supercrit. Fluids 2014, 93, 42–48. [Google Scholar] [CrossRef]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.-S.; Cho, M.; Choi, H.-K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Caricapapaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Tehranifar, A.; Selahvarzi, Y.; Kharrazi, M.; Bakhsh, V.J. High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Ind. Crops Prod. 2011, 34, 1523–1527. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef]
- Chen, J.; Liao, C.; Ouyang, X.; Kahramanoğlu, I.; Gan, Y.; Li, M. Antimicrobial activity of pomegranate peel and its applications on food preservation. J. Food Qual. 2020, 2020, 8850339. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Cai, R.; Miao, M.; Yue, T.; Zhang, Y.; Cui, L.; Wang, Z.; Yuan, Y. Antibacterial activity and mechanism of cinnamic acid and chlorogenic acid against Alicyclobacillus acidoterrestris vegetative cells in apple juice. Int. J. Food Sci. Technol. 2019, 54, 1697–1705. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Sung, W.S.; Lee, D.G. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Ma, C.-M.; Abe, T.; Komiyama, T.; Wang, W.; Hattori, M.; Daneshtalab, M. Synthesis, anti-fungal and 1, 3-β-d-glucan synthase inhibitory activities of caffeic and quinic acid derivatives. Bioorganic Med. Chem. 2010, 18, 7009–7014. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.M.; Ferreira, T.R.B.; de Oliveira Biazotto, F.; dos Santos Dias, C.T. Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum. Nutr. 2012, 67, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Kumar, N.; Ojha, A.; Upadhyay, A.; Singh, R.; Kumar, S. Effect of active chitosan-pullulan composite edible coating enrich with pomegranate peel extract on the storage quality of green bell pepper. LWT 2021, 138, 110435. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Mujumdar, A.S.; Pan, Z.; Vidyarthi, S.K.; Xu, J.; Zielinska, M.; Xiao, H.-W. Emerging chemical and physical disinfection technologies of fruits and vegetables: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2481–2508. [Google Scholar] [CrossRef] [PubMed]
- Millan-Sango, D.; Sammut, E.; Van Impe, J.F.; Valdramidis, V.P. Decontamination of alfalfa and mung bean sprouts by ultrasound and aqueous chlorine dioxide. LWT 2017, 78, 90–96. [Google Scholar] [CrossRef]
- Weisman, R.J.; Heinrich, A.; Letkiewicz, F.; Messner, M.; Studer, K.; Wang, L.; Regli, S. Estimating national exposures and potential bladder cancer cases associated with chlorination DBPs in US drinking water. Environ. Health Perspect. 2022, 130, 087002. [Google Scholar] [CrossRef]
- Jaroni, D.; Ravishankar, S. Bactericidal effects of roselle (Hibiscus sabdariffa) against foodborne pathogens in vitro and on romaine lettuce and alfalfa sprouts. Qual. Assur. Saf. Crops Foods 2012, 4, 33–40. [Google Scholar] [CrossRef]
- Ruíz-Cruz, S.; Acedo-Félix, E.; Díaz-Cinco, M.; Islas-Osuna, M.A.; González-Aguilar, G.A. Efficacy of sanitizers in reducing Escherichia coli O157: H7, Salmonella spp. and Listeria monocytogenes populations on fresh-cut carrots. Food Control. 2007, 18, 1383–1390. [Google Scholar] [CrossRef]
| Phenolic Content (mg GAE/g dw) | Flavonoid Content (mg QE/g dw) | DPPH Inhibition (%) | ABTS Scavenging (mg TE/g) | |
|---|---|---|---|---|
| Aqueous PPE | 154.53 | 45.74 | 86.12 a | 958.21 a |
| Ethanolic PPE | 111.81 | 50.45 | 76.26 b | 767.17 b |
| L. monocytogenes | S. Typhimurium | C. tropicalis | C. violaceum | |
|---|---|---|---|---|
| Minimum Inhibitory Concentration (mg/mL) | ||||
| Aqueous PPE | 19 | 19 | 30 | 10 |
| Ethanolic PPE | 24 | 24 | 30 | 26 |
| Coliform | Psychrophilic | |||
|---|---|---|---|---|
| Log CFU/100 g | Reduction Log | Log CFU/100 g | Reduction Log | |
| Control | 5.72 a | - | 5.98 a | - |
| Water | 4.95 b | 0.94 | 5.69 a | 0.28 |
| Chlorine 200 ppm | 4.77 c | 0.76 | 4.91 b | 1.07 |
| Aqueous extract 25 mg/mL | 4.60 d | 1.12 | 4.75 c | 1.23 |
| Aqueous extract 50 mg/mL | 4.55 e | 1.16 | 4.39 d | 1.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Valenzuela, M.R.; Ayala-Soto, R.E.; Ayala-Zavala, J.F.; Espinoza-Silva, B.A.; González-Aguilar, G.A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Nazzaro, F.; Fratianni, F.; Tapia-Rodríguez, M.R.; et al. Pomegranate (Punica granatum L.) Peel Extracts as Antimicrobial and Antioxidant Additives Used in Alfalfa Sprouts. Foods 2022, 11, 2588. https://doi.org/10.3390/foods11172588
Cruz-Valenzuela MR, Ayala-Soto RE, Ayala-Zavala JF, Espinoza-Silva BA, González-Aguilar GA, Martín-Belloso O, Soliva-Fortuny R, Nazzaro F, Fratianni F, Tapia-Rodríguez MR, et al. Pomegranate (Punica granatum L.) Peel Extracts as Antimicrobial and Antioxidant Additives Used in Alfalfa Sprouts. Foods. 2022; 11(17):2588. https://doi.org/10.3390/foods11172588
Chicago/Turabian StyleCruz-Valenzuela, Manuel Reynaldo, Rosa E. Ayala-Soto, Jesus Fernando Ayala-Zavala, Brenda A. Espinoza-Silva, Gustavo A. González-Aguilar, Olga Martín-Belloso, Robert Soliva-Fortuny, Filomena Nazzaro, Florinda Fratianni, Melvin R. Tapia-Rodríguez, and et al. 2022. "Pomegranate (Punica granatum L.) Peel Extracts as Antimicrobial and Antioxidant Additives Used in Alfalfa Sprouts" Foods 11, no. 17: 2588. https://doi.org/10.3390/foods11172588
APA StyleCruz-Valenzuela, M. R., Ayala-Soto, R. E., Ayala-Zavala, J. F., Espinoza-Silva, B. A., González-Aguilar, G. A., Martín-Belloso, O., Soliva-Fortuny, R., Nazzaro, F., Fratianni, F., Tapia-Rodríguez, M. R., & Bernal-Mercado, A. T. (2022). Pomegranate (Punica granatum L.) Peel Extracts as Antimicrobial and Antioxidant Additives Used in Alfalfa Sprouts. Foods, 11(17), 2588. https://doi.org/10.3390/foods11172588










