Potential of Syzygnium polyanthum as Natural Food Preservative: A Review
Abstract
:1. Introduction
2. Food Spoilage
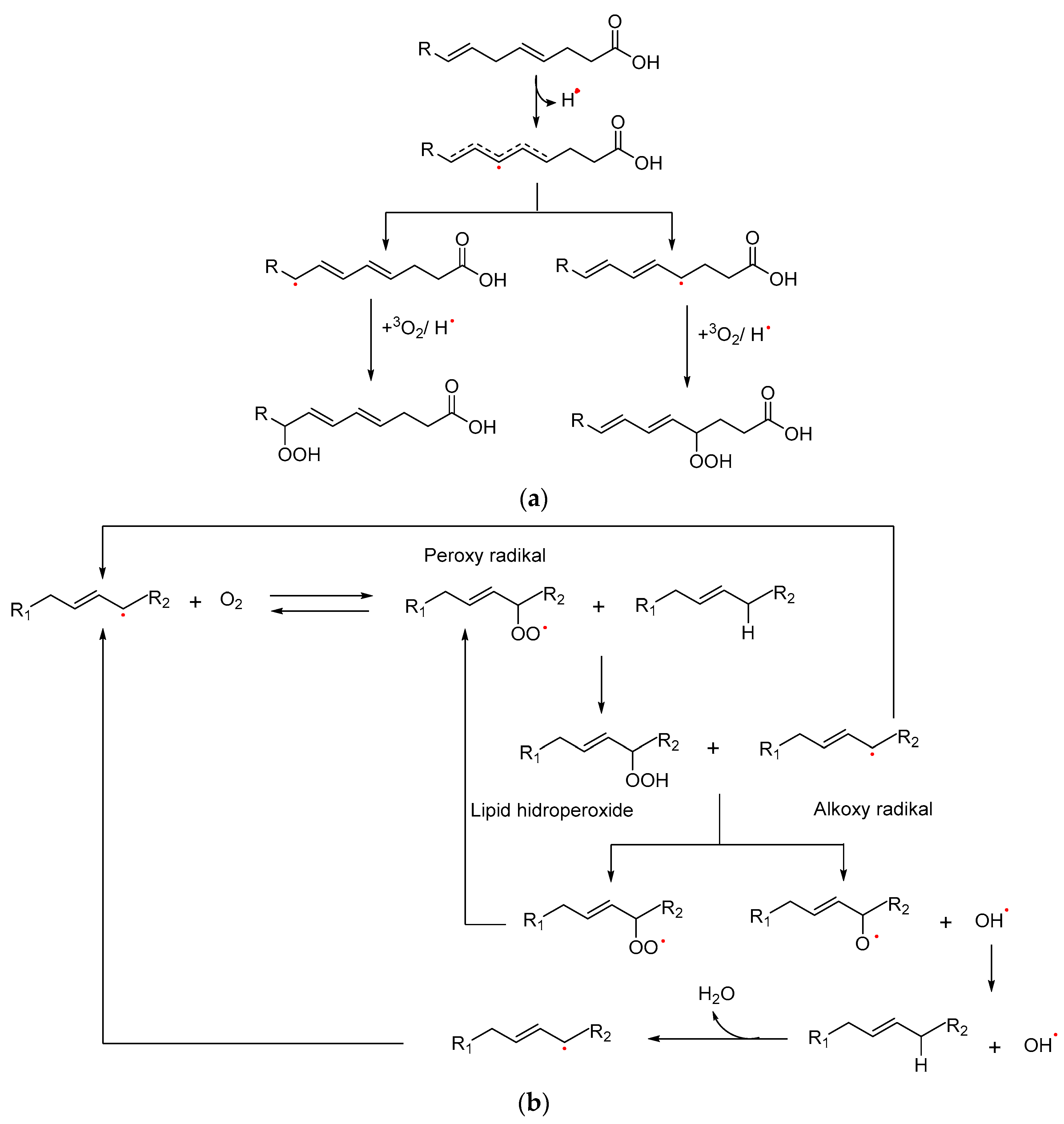
2.1. Oxidation Activity
2.2. Microbial Activity
3. Food Preservation
4. Syzygnium Polyanthum
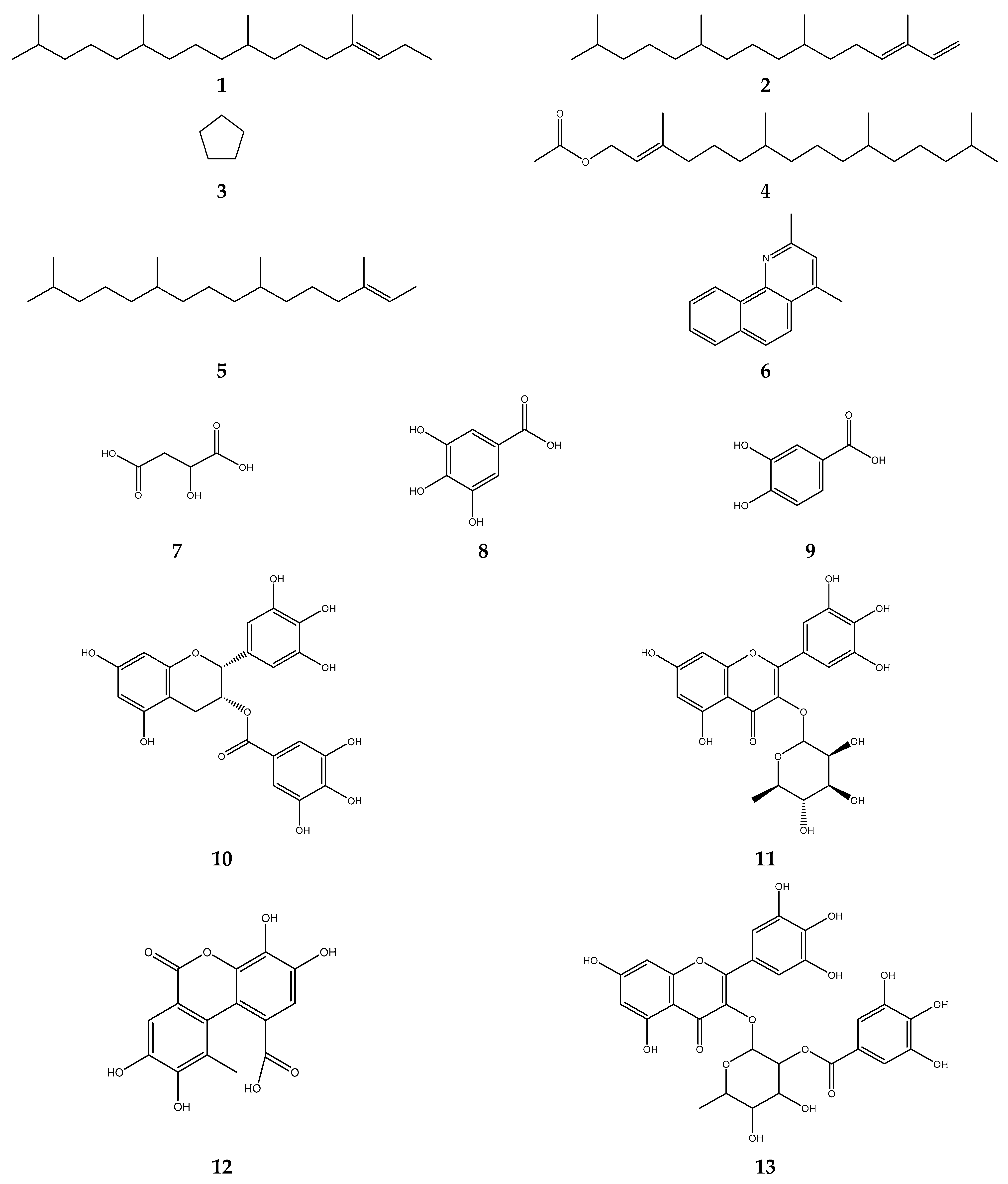
5. Bioactivities of S. polyanthum Leaves
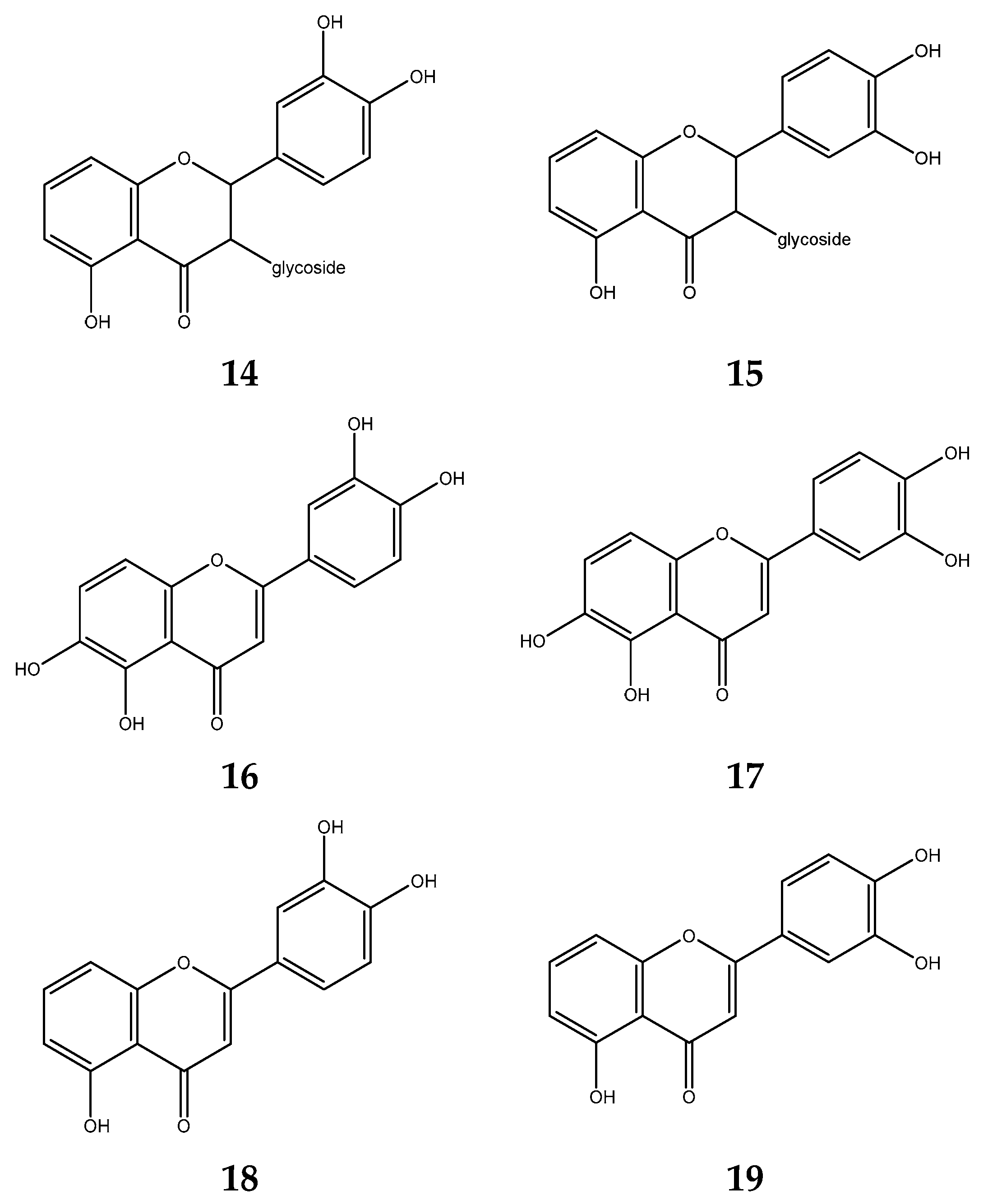
5.1. Antioxidant Activity of S. polyanthum Leaves
5.2. Antibacterial Activity of S. polyanthum Leaves
6. Natural Food Preservation
6.1. Food Antioxidant Preservative
6.2. Food Antimicrobial Preservative
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cichello, S.A. Oxygen absorbers in food preservation: A review. J. Food Sci. Technol. 2015, 52, 1889–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-Based Phenolic Molecules as Natural Preservatives in Comminuted Meats: A Review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, J.; Dong, M.; Zhang, H.; Li, L.; Wang, L. Food spoilage, bioactive food fresh-keeping films and functional edible coatings: Research status, existing problems and development trend. Trends Food Sci. Technol. 2022, 119, 122–132. [Google Scholar] [CrossRef]
- Tometri, S.S.; Ahmady, M.; Ariaii, P.; Soltani, M.S. Extraction and encapsulation of Laurus nobilis leaf extract with nano-liposome and its effect on oxidative, microbial, bacterial and sensory properties of minced beef. J. Food Meas. Charact. 2020, 14, 3333–3344. [Google Scholar] [CrossRef]
- Ismail, A.; Mohamed, M.; Sulaiman, S.A.; Wan Ahmad, W.A.N. Autonomic Nervous System Mediates the Hypotensive Effects of Aqueous and Residual Methanolic Extracts of Syzygium Polyanthum (Wight) Walp. Var. Polyanthum Leaves in Anaesthetized Rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 716532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabandar, C.W.; Jalil, J.; Ahmat, N.; Aladdin, N.-A.; Nik Abdullah Zawawi, N.K.; Sahidin, I. Anti-Inflammatory and Antioxidant Activity of Syzygium Polyanthum (Wight) Walp. Sains Malays. 2022, 51, 1475–1485. [Google Scholar] [CrossRef]
- Jumaat, S.R.; Tajuddin, S.N.; Sudmoon, R.; Chaveerach, A.; Abdullah, U.H.; Mohamed, R. Chemical Constituents and Toxicity Screening of Three Aromatic Plant Species from Peninsular Malaysia. BioResources 2017, 12, 5878–5895. [Google Scholar] [CrossRef] [Green Version]
- Berawi, K.N.; Shidarti, L.; Nurdin, S.U.; Lipoeto, N.I.; Wahid, I.; Jamsari; Nurcahyani, E. Comparison Effectiveness of Antidiabetic Activity Extract Herbal Mixture of Soursop Leaves (Annona Muricata), Bay Leaves (Syzygium Polyanthum) and Pegagan Leaves (Centella Asiatica). Biomed. Pharmacol. J. 2017, 10, 1481–1488. [Google Scholar] [CrossRef]
- Chen, J.; Liao, C.; Ouyang, X.; Kahramanoğlu, I.; Gan, Y.; Li, M. Antimicrobial Activity of Pomegranate Peel and Its Applications on Food Preservation. J. Food Qual. 2020, 2020, 8850339. [Google Scholar] [CrossRef]
- Durgawale, P.; Patil, M.; Joshi, S.; Korabu, K.; Datkhile, K. Studies on phytoconstituents, in vitro antioxidant, antibacterial, antiparasitic, antimicrobial, and anticancer potential of medicinal plant Lasiosiphon eriocephalus decne (Family: Thymelaeaceae). J. Nat. Sci. Biol. Med. 2019, 10, 38–47. [Google Scholar] [CrossRef]
- Scicutella, F.; Mannelli, F.; Daghio, M.; Viti, C.; Buccioni, A. Polyphenols and Organic Acids as Alternatives to Antimicrobials in Poultry Rearing: A Review. Antibiotics 2021, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.X.; Samsuri, S.N.; Yong, P.H. The antioxidant index and chemometric analysis of tannin, flavonoid, and total phenolic extracted from medicinal plant foods with the solvents of different polarities. J. Food Process. Preserv. 2020, 44, e14680. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. Chemical Deterioration and Physical Instability of Foods and Beverages. In The Stability and Shelf Life of Food; Elsevier: Amsterdam, The Netherlands, 2016; pp. 43–76. [Google Scholar]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.; Cabral, L.M. Grape By-Product Extracts against Microbial Proliferation and Lipid Oxidation: A Review: Grape by-Products with Antimicrobial and Antioxidant Potential. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Steele, R. Understanding and Measuring the Shelf-Life of Food; Woodhead Publishing: Sawston, UK, 2004. [Google Scholar]
- Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Kim, J.; Foley, A.; Rooney, D. Decarbonizing the food and beverages industry: A critical and systematic review of developments, sociotechnical systems and policy options. Renew. Sustain. Energy Rev. 2021, 143, 110856. [Google Scholar] [CrossRef]
- Karwowska, M.; Laba, S.; Szczepański, K. Food Loss and Waste in Meat Sector—Why the Consumption Stage Generates the Most Losses? Sustainability 2021, 13, 6227. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Addis, M. Major Causes of Meat Spoilage and Preservation Techniques: A. Changes 2015, 41, 101–114. [Google Scholar]
- Ding, Z.; Wei, Q.; Liu, C.; Zhang, H.; Huang, F. The Quality Changes and Proteomic Analysis of Cattle Muscle Postmortem during Rigor Mortis. Foods 2022, 11, 217. [Google Scholar] [CrossRef]
- Shahidi, F.; Abad, A. Lipid-Derived Flavours and Off-Flavours in Food. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 182–192. ISBN 978-0-12-814045-1. [Google Scholar]
- Nardella, S.; Conte, A.; Del Nobile, M.A. State-of-Art on the Recycling of By-Products from Fruits and Vegetables of Mediterranean Countries to Prolong Food Shelf Life. Foods 2022, 11, 665. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology application in food packaging: A plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef]
- Jacobsen, C.; Paiva-Martins, F.; Schwarz, K.; Bochkov, V. Lipid Oxidation and Antioxidants in Food and Nutrition. Eur. J. Lipid Sci. Technol. 2019, 121, 1900298. [Google Scholar] [CrossRef] [Green Version]
- Abdullahi, A.A.; Bala, J.D.; Kuta, F.A.; Adabara, N.U.; Liyasu, U.S.; Aliu, M.O. Microbial spoilage of food in industry: A review. FUW Trends in Science & Technology 2019, 4, 519–523. [Google Scholar]
- Pinu, F.R. Early detection of food pathogens and food spoilage microorganisms: Application of metabolomics. Trends Food Sci. Technol. 2016, 54, 213–215. [Google Scholar] [CrossRef]
- Wijaya, C.H.; Wijaya, W.; Mehta, B.M. General Properties of Major Food Components. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin, Heidelberg, 2015; pp. 1–32. ISBN 978-3-642-41609-5. [Google Scholar]
- Sartika, R.A.D. Pengaruh Asam Lemak Jenuh, Tidak Jenuh dan Asam Lemak Trans terhadap Kesehatan. Kesmas Natl. Public Health J. 2008, 2, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and Purification of High-Value Metabolites from Microalgae: Essential Lipids, Astaxanthin and Phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Zhao, J. Structure, inhibition, and regulation of essential lipid A enzymes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1424–1438. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of Lipids in Foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Johnson, D.R.; Decker, E.A. The Role of Oxygen in Lipid Oxidation Reactions: A Review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, P.A.; Hegeman, A.D.; Reed, G.H. Free Radical Mechanisms in Enzymology. Chem. Rev. 2006, 106, 3302–3316. [Google Scholar] [CrossRef]
- Heck, R.T.; Saldaña, E.; Lorenzo, J.M.; Correa, L.P.; Fagundes, M.B.; Cichoski, A.J.; de Menezes, C.R.; Wagner, R.; Campagnol, P.C.B. Hydrogelled emulsion from chia and linseed oils: A promising strategy to produce low-fat burgers with a healthier lipid profile. Meat Sci. 2019, 156, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- de Lima Júnior, D.M.; do Nascimento Rangel, A.H.; Urbano, S.A.; Moreno, G.M.B. Oxidação Lipídica e Qualidade Da Carne Ovina. Acta Vet. Bras. 2013, 7, 14–28. [Google Scholar]
- Van Hecke, T.; Van Camp, J.; De Smet, S. Oxidation during Digestion of Meat: Interactions with the Diet and Helicobacter Pylori Gastritis, and Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2017, 16, 214–233. [Google Scholar] [CrossRef] [Green Version]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Králová, M. The Effect of Lipid Oxidation on the Quality of Meat and Meat Products. Maso. Int. J. Food Sci. Technol. 2015, 2, 125–132. [Google Scholar]
- Chaijan, M.; Panpipat, W. Mechanism of Oxidation in Foods of Animal Origin. In Natural Antioxidants; Apple Academic Press, Inc.: Boca Raton, FL, USA, 2017; pp. 21–58. [Google Scholar]
- Amaral, A.B.; da Silva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, K.; Uemura, M.; Hosokawa, M. Effective Prevention of Oxidative Deterioration of Fish Oil: Focus on Flavor Deterioration. Annu. Rev. Food Sci. Technol. 2018, 9, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Adhikari, B.; Barrow, C.J. Optimisation of the microencapsulation of tuna oil in gelatin–sodium hexametaphosphate using complex coacervation. Food Chem. 2014, 158, 358–365. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.-S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [Green Version]
- Wali, M.K.; Abed, M.M. Antibacterial Activity of Acetic Acid against Different Types of Bacteria Causes Food Spoilage. Plant Arch. 2019, 19, 1827–1831. [Google Scholar]
- Shao, P.; Liu, L.; Yu, J.; Lin, Y.; Gao, H.; Chen, H.; Sun, P. An overview of intelligent freshness indicator packaging for food quality and safety monitoring. Trends Food Sci. Technol. 2021, 118, 285–296. [Google Scholar] [CrossRef]
- Blackburn, C.D.W. Managing microbial food spoilage: An overview. In Food Spoilage Microorganisms; Woodhead Publishing Ltd.: Cambridge, UK, 2006; pp. 147–170. [Google Scholar] [CrossRef]
- Bai A, J.; Rai Vittal, R. Quorum Sensing Regulation and Inhibition of Exoenzyme Production and Biofilm Formation in the Food Spoilage Bacteria Pseudomonas psychrophila PSPF19. Food Biotechnol. 2014, 28, 293–308. [Google Scholar] [CrossRef]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Snyder, A.B.; Worobo, R.W. Fungal Spoilage in Food Processing. J. Food Prot. 2018, 81, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Gammariello, D.; Conte, A.; Lucera, A.; Mastromatteo, M.; Del Nobile, M.A. Anti-yeast activity of natural compounds: In vitro and in vivo tests. Food Packag. Shelf Life 2014, 1, 30–37. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage Bacteria and Meat Quality. In Meat Quality Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 307–334. ISBN 978-0-12-819233-7. [Google Scholar]
- Deschuyffeleer, N.; Audenaert, K.; Samapundo, S.; Ameye, S.; Eeckhout, M.; Devlieghere, F. Identification and characterization of yeasts causing chalk mould defects on par-baked bread. Food Microbiol. 2011, 28, 1019–1027. [Google Scholar] [CrossRef]
- Saranraj, P.; Geetha, M. Microbial Spoilage of Bakery Products and Its Control by Preservatives. Int. J. Pharm. Biol. Arch. 2010, 3, 38–48. [Google Scholar]
- Hocking, A.D.; Faedo, M. Fungi causing thread mould spoilage of vacuum packaged Cheddar cheese during maturation. Int. J. Food Microbiol. 1992, 16, 123–130. [Google Scholar] [CrossRef]
- Snyder, A.B.; Worobo, R.W. The incidence and impact of microbial spoilage in the production of fruit and vegetable juices as reported by juice manufacturers. Food Control 2018, 85, 144–150. [Google Scholar] [CrossRef]
- Aljamali, N.; Najim, M.; Alabbasy, A. Review on Food Poisoning (Types, Causes, Symptoms, Diagnosis, Treatment). Global Acad. J. Pharm. Drug Res. 2021, 3, 54–61. [Google Scholar] [CrossRef]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The Food Poisoning Toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef]
- Panel, E.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Pathogenicity assessment of Shiga toxin-producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. EFSA J. 2020, 18, e05967. [Google Scholar] [CrossRef]
- Nurjayadi, M.; Pertiwi, Y.P.; Islami, N.; Azizah, N.; Efrianti, U.R.; Saamia, V.; Wiranatha, I.M.; Nastassya, L.; El-Enshasye, H.A. Detection of the Salmonella typhi bacteria in contaminated egg using real-time PCR to develop rapid detection of food poisoning bacteria. Biocatal. Agric. Biotechnol. 2019, 20, 101214. [Google Scholar] [CrossRef]
- Grispoldi, L.; Karama, M.; Armani, A.; Hadjicharalambous, C.; Cenci-Goga, B.T. Staphylococcus aureus enterotoxin in food of animal origin and staphylococcal food poisoning risk assessment from farm to table. Ital. J. Anim. Sci. 2021, 20, 677–690. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.M.; Moreno-Vilet, L.; Villanueva-Rodríguez, S.J. Current status of emerging food processing technologies in Latin America: Novel non-thermal processing. Innov. Food Sci. Emerg. Technol. 2019, 58, 102233. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Pinto, C.A.; Moreira, S.A.; Pintado, M.; Saraiva, J.A. 5-Nonthermal Food Processing/Preservation Technologies. In Saving Food; Galanakis, C.M., Ed.; Academic Press: Cambridge, CA, USA, 2019; pp. 141–169. ISBN 978-0-12-815357-4. [Google Scholar]
- Režek Jambrak, A.; Vukušić, T.; Donsi′, F.; Paniwnyk, L.; Djekic, I. Three Pillars of Novel Nonthermal Food Technologies: Food Safety, Quality, and Environment. J. Food Qual. 2018, 2018, 8619707. [Google Scholar] [CrossRef]
- Singh, V.P. Recent approaches in food bio-preservation—A review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, S. Basic Principles for Effective Food Preservation: A Review. Int. J. Pure App. Biosci. 2013, 1, 84–85. [Google Scholar]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Davidson, P.M.; Sofos, J.N.; Branen, A.L. Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Mohammadzadeh-Aghdash, H.; Akbari, N.; Esazadeh, K.; Ezzati Nazhad Dolatabadi, J. Molecular and technical aspects on the interaction of serum albumin with multifunctional food preservatives. Food Chem. 2019, 293, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Bugos, J. Assessing the Public Health Implications of the Food Preservative Propylparaben: Has This Chemical Been Safely Used for Decades. Curr. Environ. Health Rep. 2021, 8, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Linke, B.G.O.; Casagrande, T.A.C.; Cardoso, L.A.C. Food additives and their health effects: A review on preservative sodium benzoate. Afr. J. Biotechnol. 2018, 17, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Prajapati, P.; Vyas, N.; Malviya, S.; Kharia, A. A Review on Food Preservation. Asian J. Pharm. Pharmacol 2017, 3, 193–199. [Google Scholar]
- Hamid, A.; Risikat, A.; Sururah, A. Food: Its Preservatives, Additives and Applications. Int. J. Chem. Biochem. Sci. 2012, 1, 36–47. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Kim, J.-S.; An, J.H.; Sohn, J.H.; Choi, J.-S. Natural Food Additives and Preservatives for Fish-Paste Products: A Review of the Past, Present, and Future States of Research. J. Food Qual. 2017, 2017, 9675469. [Google Scholar] [CrossRef] [Green Version]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Papapostolou, M.; Nenadis, N.; Mantzouridou, F.T.; Tsimidou, M.Z. Bay Laurel (Laurus nobilis L.) Essential Oil as a Food Preservative Source: Chemistry, Quality Control, Activity Assessment, and Applications to Olive Industry Products. Foods 2022, 11, 752. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Harun, N.H.; Rasdi, N.A.; Salleh, R.M.; Safuan, S.; Ahmad, W.A.N.W.; Fuad, W.E.M. Subacute Toxicity Evaluation of Methanolic Extract of Syzygium Polyanthum in Rats. Trop. Life Sci. Res. 2021, 32, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Widyawati, T.; Yusoff, N.A.; Asmawi, M.Z.; Ahmad, M. Antihyperglycemic Effect of Methanol Extract of Syzygium polyanthum (Wight.) Leaf in Streptozotocin-Induced Diabetic Rats. Nutrients 2015, 7, 7764–7780. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.; Ahmad, W.A.N.W. Syzygium Polyanthum (Wight) Walp: A Potential Phytomedicine. Pharmacogn. J. 2019, 11, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Dewijanti, I.D.; Artanti, N.; Mangunwardoyo, W.; Hanafi, M.; Abbas, J.; Megawati, M.; Minarti, M.; Musdalifah, D.; Meilawati, L. Bioactivities of Syzygium Polyanthum (Wight) Walp leaf extract for decreasing diabetic risk. AIP Conf. Proc. 2018, 2024, 020011. [Google Scholar] [CrossRef]
- Widjajakusuma, E.C.; Jonosewojo, A.; Hendriati, L.; Wijaya, S.; Ferawati; Surjadhana, A.; Sastrowardoyo, W.; Monita, N.; Muna, N.M.; Fajarwati, R.P.; et al. Phytochemical screening and preliminary clinical trials of the aqueous extract mixture of Andrographis paniculata (Burm. f.) Wall. ex Nees and Syzygium Polyanthum (Wight.) Walp leaves in metformin treated patients with type 2 diabetes. Phytomedicine 2019, 55, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, R.; Gontar Alamsyah, S.; Lindarto, D. The effect of bay leaf extract (Syzygium Polyanthum) on vascular endothelial growth factor (VEGF) and CD31 (PECAM-1) expression in acute coronary syndrome. Med. Glas. Ljek. Komore Zenicko-Doboj. Kantona 2020, 17, 321–327. [Google Scholar] [CrossRef]
- Prianwari, C.; Syafril, S. Relationship Between Triglycerides And Glucose Index (TyG) And Lipid Profile In Patients With Dyslipidemia Treated With Bay Leaf Extract [Syzygium Polyanthum (Wight) Walp]. J. Endocrinol. Trop. Med. Infect. Dis. (JETROMI) 2020, 2, 177–182. [Google Scholar] [CrossRef]
- Komaria, R.; Lister, I.N.E.; Lie, S. Hematological Profiles of Benzene-Induced Rats Treated with Ethanolic Extract of Syzygium Polyanthum. In Proceedings of the 2021 IEEE International Conference on Health, Instrumentation & Measurement, and Natural Sciences (InHeNce), Medan, Indonesia, 14–16 July 2021; pp. 1–6. [Google Scholar]
- Muhammad, D.S.; Salim, H.M.; Virlliana, C.D.; Donastin, A.; Adriansyah, A.A. The effect of Syzygium Polyanthum (wight) extract in histological changes of kidney in diabetic mice model. Bali Med. J. 2022, 11, 1301–1304. [Google Scholar] [CrossRef]
- Suwito, B.E.; Shanty, L.M.; Gumilang, R.; Handayani, H.; Ulhaq, R.A. Effect of Bay Leaf (Syzygium Polyanthum) Extract on Antioxidant Activity, MDA Levels, and Liver Histopathology Feature of Ethambutol Induced Wistar Rats. Indones. J. Med. Lab. Sci. Technol. 2022, 4, 148–156. [Google Scholar] [CrossRef]
- Muliani, L.; Puspita, G.; Anggai, R.A. Test the Effectiveness of Bay Leaf Extract (Syzygium Polyanthum) in Lowering Uric Acid Levels in Male White Rats (Rattus Novergicus). J. Health Technol. Sci. (JHTS) 2021, 2, 43–52. [Google Scholar] [CrossRef]
- Sandikapura, M.J.; Nyamathulla, S.; Noordin, M.I. Comparative Antioxidant and Antidiabetic Effects of Syzygium Polyanthum Leaf and Momordica Charantia Fruit Extracts. Pak. J. Pharm. Sci. 2018, 31, 623–636. [Google Scholar] [PubMed]
- Ramadhania, N.R.; Purnomo, A.S.; Fatmawati, S. Antibacterial activities of Syzygium Polyanthum wight leaves. AIP Conf. Proc. 2018, 2049, 020024. [Google Scholar] [CrossRef]
- Uddin, A.B.M.N.; Hossain, F.; Reza, A.S.M.A.; Nasrin, M.S.; Alam, A.H.M.K. Traditional uses, pharmacological activities, and phytochemical constituents of the genus Syzygium: A review. Food Sci. Nutr. 2022, 10, 1789–1819. [Google Scholar] [CrossRef] [PubMed]
- Wahjuni, S.; Wita, I.W. Hypoglycemic and antioxidant effects of Syzygium Polyanthum leaves extract on alloxan induced hyperglycemic Wistar Rats. Bali Med. J. 2017, 6, 113. [Google Scholar] [CrossRef]
- Syabana, M.A.; Yuliana, N.D.; Batubara, I.; Fardiaz, D. α-glucosidase inhibitors from Syzygium Polyanthum (Wight) Walp leaves as revealed by metabolomics and in silico approaches. J. Ethnopharmacol. 2022, 282, 114618. [Google Scholar] [CrossRef] [PubMed]
- Sulistiyani; Falah, S.; Wahyuni, W.T.; Sugahara, T.; Tachibana, S.; Syaefudin. Cellular Mechanism of the Cytotoxic Effect of Extracts from Syzygium Polyanthum Leaves. Am. J. Drug Discov. Dev. 2014, 4, 90–101. [Google Scholar]
- Syarifah, A.L.; Retnowati, R.; Soebiantoro, S. Characterization of Secondary Metabolites Profile of Flavonoid from Salam Leaves (Eugenia Polyantha) Using TLC and UV Spectrophotometry. Pharm. Sci. Res. 2019, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Annisa, M.; Harsini, H.; Murti, Y.B. Potential Effect of Bay Leaf (Syzygium Polyanthum [Wight] Walp.) Essential Oil for Herbal Toothpaste Active Agent. Maj. Obat Tradis. 2022, 27, 126. [Google Scholar] [CrossRef]
- Hamad, A.; Mahardika, M.G.P.; Yuliani, I.; Hartanti, D. Chemical Constituents and Antimicrobial Activities of Essential Oils of Syzygium Polyanthum and Syzygium Aromaticum. Rasayan J. Chem. 2017, 10, 564–569. [Google Scholar]
- Kadir, N.; Salleh, W.M.N.H.W.; Ghani, N. A Systematic Review on Essential Oils and Biological Activities of the Genus Syzygium (Myrtaceae). Riv. Ital. Delle Sostanze Grasse 2022, 99, 165–178. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils-A Review. Food Chem. Toxicol. 2008, 46, 446. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Decker, E.A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Secci, G.; Parisi, G. From farm to fork: Lipid oxidation in fish products. A review. Ital. J. Anim. Sci. 2016, 15, 124–136. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Carsono, N.; Tumilaar, S.G.; Kurnia, D.; Latipudin, D.; Satari, M.H. A Review of Bioactive Compounds and Antioxidant Activity Properties of Piper Species. Molecules 2022, 27, 6774. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Sumiwi, S.A.; Zuhrotun, A.; Hendriani, R.; Rizal, M.; Levita, J.; Megantara, S. Subchronic Toxicity of Ethanol Extract of Syzygium Polyanthum (Wight) Walp. Leaves on Wistar Rat. Indones. Biomed. J. 2019, 11, 30–35. [Google Scholar] [CrossRef]
- Abd Rahim, E.N.A.; Ismail, A.; Omar, M.N.; Rahmat, U.N.; Wan Ahmad, W.A.N. GC-MS Analysis of Phytochemical Compounds in Syzygium Polyanthum Leaves Extracted using Ultrasound-Assisted Method. Pharmacogn. J. 2017, 10, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Umaru, I.J.; Umaru, K.I.; Umaru, H.A. Phytochemical Screening, Isolation, Characterization of Bioactive and Biological Activity of Bungkang, (Syzygium Polyanthum) Root-Bark Essential Oil. Korean J. Food Health Converg. 2020, 6, 5–21. [Google Scholar] [CrossRef]
- de Oliveira, V.S.; Ferreira, F.S.; Cople, M.C.R.; Labre, T.D.S.; Augusta, I.M.; Gamallo, O.D.; Saldanha, T. Use of Natural Antioxidants in the Inhibition of Cholesterol Oxidation: A Review: Inhibition of Cholesterol Oxidation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1465–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Andriani, R.; Subroto, T.; Ishmayana, S.; Kurnia, D. Enhancement Methods of Antioxidant Capacity in Rice Bran: A Review. Foods 2022, 11, 2994. [Google Scholar] [CrossRef]
- Perumal, S.; Mahmud, R.; Piaru, S.P.; Cai, L.W.; Ramanathan, S. Potential Antiradical Activity and Cytotoxicity Assessment of Ziziphus mauritiana and Syzygium Polyanthum. Int. J. Pharmacol. 2012, 8, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Hidayati, M.D.; Ersam, T.; Shimizu, K.; Fatmawati, S. Antioxidant Activity of Syzygium Polyanthum Extracts. Indones. J. Chem. 2017, 17, 49–53. [Google Scholar] [CrossRef]
- Pratama, B.P.; Supriyadi; Swasono, R.T.; Pranoto, Y. Different leaf maturities and withering durations affect the antioxidant potential and aroma compound of Indonesian bay leaf [Syzygium Polyanthum (Wight) Walp.]. Int. Food Res. J. 2021, 28, 1196–1203. [Google Scholar] [CrossRef]
- Mahmoud, A.D.; Ali, A.M.; Khandaker, M.M.; Fatihah, H.N.N.; Awang, N.A.; Mat, N. Discrimination of Syzygium Polyanthum Cultivars (Wight) Walp Based on Essential Oil Composition. J. Agrobiotechnol. 2019, 10, 1–9. [Google Scholar]
- Widyawati, T.; Roslan, N.A.B.; Yusoff, N.A. The Evaluation of Antioxidant and Free Radical Scavenging Activities of Eugenia Polyantha Leaves Extracts. Int. J. ChemTech Res. 2016, 9, 465–471. [Google Scholar]
- Ramadhania, Z.M.; Insanu, M.; Gunarti, N.S.; Wirasutisna, K.R.; Sukrasno, S.; Hartati, R. Antioxidant activity from ten species of myrtaceae. Asian J. Pharm. Clin. Res. 2017, 10, 5–7. [Google Scholar] [CrossRef]
- Fei, P.; Ali, M.A.; Gong, S.; Sun, Q.; Bi, X.; Liu, S.; Guo, L. Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control 2018, 94, 289–294. [Google Scholar] [CrossRef]
- ′Afifah, A.N.; Zuprizal; Dono, N.D. In vitro antibacterial activities of Syzygium Polyanthum leaves extract-nanoparticle against Salmonella typhimurium, Escherchia coli, and Lactobacillus acidophilus. IOP Conf. Ser. Earth Environ. Sci. 2021, 782, 022093. [Google Scholar] [CrossRef]
- Kusumaningrum, M.; Prasetiawan, H.; Setiyorini, N.; Anis, A.; Rengga, W.D.P. Optimization of Terpenoid Extraction from Bay (Syzygium Polyanthum) Leaves Using Ethanol as a Solvent by an Ultrasonic Wave Technology. Int. J. Res. Innov. Entrep. 2020, 1, 35–42. [Google Scholar]
- Windaryanti, D.; Gabriel, C.S.; Hidayat, I.W.; Zainuddin, A.; Dharsono, H.D.A.; Satari, M.H.; Kurnia, D. The Potential of 24-Propylcholestrol as Antibacterial Oral Bacteria of Enterococcus faecalis ATCC 29212 and Inhibitor Biofilms Formation: In vitro and in silico Study. Adv. Appl. Bioinform. Chem. 2022, 15, 99–111. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of Bactericidal Action of Cinnamaldehyde against Listeria Monocytogenes and of Eugenol against L. Monocytogenes and Lactobacillus Sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, R.; Zhang, K.; Chen, X.; Zhang, Y. Antioxidant, Hypoglycemic and Molecular Docking Studies of Methanolic Extract, Fractions and Isolated Compounds from Aerial Parts of Cymbopogon citratus (DC.) Stapf. Molecules 2022, 27, 2858. [Google Scholar] [CrossRef]
- Iskandi, S.; Fauziah, F.; Oktavia, S. Antibacterial Activity of Syzygium Polyanthum. Int. J. Pharm. Sci. Med. 2021, 6, 182–186. [Google Scholar] [CrossRef]
- Hakim, R.F.; Fakhrurrazi, F.; Ferisa, W. Pengaruh Air Rebusan Daun Salam (Eugenia Polyantha Wight) Terhadap Pertumbuhan Enterococcus Faecalis. J. Syiah Kuala Dent. Soc. 2016, 1, 21–28. [Google Scholar]
- Miksusanti, M.; Herlina, H.; Masril, K.M.K. Antibacterial and Antioxidant of Uwi (Dioscorea Alata L) Starch Edible Film Incorporated with Ginger Essential Oil. Int. J. Biosci. Biochem. Bioinforma. 2013, 3, 354–356. [Google Scholar]
- Sikkema, J.A.N.; de Bont, J.A.; Poolman, B. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Satta, G.; Cornaglia, G.; Mazzariol, A.; Golini, G.; Valisena, S.; Fontana, R. Target for bacteriostatic and bactericidal activities of beta-lactam antibiotics against Escherichia coli resides in different penicillin-binding proteins. Antimicrob. Agents Chemother. 1995, 39, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Tortora, M.L.; Díaz-Ricci, J.C.; Pedraza, R.O. Protection of strawberry plants (Fragaria ananassa Duch.) against anthracnose disease induced by Azospirillum brasilense. Plant Soil 2011, 356, 279–290. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O′Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konaté, K.; Hilou, A.; Mavoungou, J.F.; Lepengué, A.N.; Souza, A.; Barro, N.; Datté, J.Y.; M′batchi, B.; Nacoulma, O.G. Antimicrobial Activity of Polyphenol-Rich Fractions from Sida Alba L.(Malvaceae) against Co-Trimoxazol-Resistant Bacteria Strains. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Putri, S.A.; Nur Shadrina, A.A.; Julaeha, E.; Kurnia, D. Potential Nevadensin from Ocimum basilicum as Antibacterial Agent against Streptococcus mutans: In Vitro and In Silico Studies. Comb. Chem. High Throughput Screen. 2023, 26, 1746–1754. [Google Scholar] [CrossRef]
- Lau, K.Y.; Rukayadi, Y. Screening of Tropical Medicinal Plants for Sporicidal Activity. Int. Food Res. J. 2015, 22, 421. [Google Scholar]
- Nurjanah, N.; Herijulianti, E.; Putri, M.H.; Sukmasari, S. Eugenia polyantha (Wight) infusion against oral microorganisms on toothbrushes. Sci. Dent. J. 2020, 4, 105. [Google Scholar] [CrossRef]
- Pavase, T.R.; Lin, H.; Shaikh, Q.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent advances of conjugated polymer (CP) nanocomposite-based chemical sensors and their applications in food spoilage detection: A comprehensive review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Neelofar, K.; Shreaz, S.; Rimple, B.; Muralidhar, S.; Nikhat, M.; Khan, L.A. Curcumin as a promising anticandidal of clinical interest. Can. J. Microbiol. 2011, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Siswina, T.; Rustama, M.M.; Sumiarsa, D.; Kurnia, D. Phytochemical profiling of Piper crocatum and its antifungal activity as Lanosterol 14 alpha demethylase CYP51 inhibitor: A review. F1000Research 2022, 11, 1115. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Gabriel, A.A.; Nakano, H. Antimicrobial efficacies of plant extracts and sodium nitrite against Clostridium botulinum. Food Control 2010, 21, 1030–1036. [Google Scholar] [CrossRef]
- Lau, K.Y.; Zainin, N.S.; Abas, F.; Rukayadi, Y. Antibacterial and Sporicidal Activity of Eugenia Polyantha Wight against Bacillus Cereus and Bacillus Subtilis. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 499–510. [Google Scholar]
- Abd Wahab, N.Z.; Aqilah Ja’afar, N.S. Phytochemical Composition and Antibacterial Activities of Syzygium Polyanthum Methanolic Leaves Extract. Pharmacogn. J. 2021, 13, 1355–1358. [Google Scholar] [CrossRef]
- Wong, J.X.; Ramli, S.; Rukayadi, Y.; Juhari, N.K.K.; Radu, S.; Abd Wahid, N.B. Antifungal Activity of Ethanolic Extract of Syzygium Polyanthum (Wight) Walp. Leaves Extract Against Several Types of Filamentous Fungi and Candida Species. Malays. J. Microsc. 2022, 18, 136–146. [Google Scholar]
- Fitri, K.S.A.; Ramdhani, D.; Mustarichie, R. Comparative Study on Activities of Anti Bacillary Dysentery Shigella Dysenteriae of Syzygium Polyanthum and Dracaena Angustifolia Leaves Ethanol Extracts. Asian J. Pharm. Clin. Res. 2017, 10, 348–352. [Google Scholar]
- Ramli, S.; Radu, S.; Shaari, K.; Rukayadi, Y. Antibacterial Activity of Ethanolic Extract of Syzygium polyanthum L. (Salam) Leaves against Foodborne Pathogens and Application as Food Sanitizer. BioMed Res. Int. 2017, 2017, 9024246. [Google Scholar] [CrossRef] [Green Version]
- Kusuma, S.A.F.; Purnamasari, E.; Herawati, I.E. Syzygium Polyanthum (Wight) Walp. Leaves Extract as the Antifungal Agent for Oral Candidiasis. Drug Invent. Today 2019, 12, 1339–1342. [Google Scholar]
- Aldhaher, Z.A.; Merza, W.M.; Almelan, M.F.; Shaker, R.M.; Yas, L.S. Effectiveness of Bay Leaves Aqueous Extract on Streptococcus Mutans in Comparision to Chlorhexidine Gluconate. IOSR-J. Pharm. Bio. Sci. 2017, 12, 12–16. [Google Scholar]
- Hamad, A.; Djali, A.D.; Dewi, D.Y.S.; Saputri, E.Y.; Nurlaeli, E.; Fadlilah, I.N.; Mentari, M.; Hartanti, D. The microbial growth inhibition profile of selected indonesian spices essential oils on tofu during 8-day storage. J. Chem. Process Mater. Technol. 2022, 1, 15–27. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and Their Prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Silva, M.M.; Lidon, F. Food preservatives—An overview on applications and side effects. Emir. J. Food Agric. 2016, 28, 366–373. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Bhagwat, P.K.; Ozogul, F.; Benjakul, S.; Pillai, S.; Annapure, U.S. Cold plasma for the preservation of aquatic food products: An overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4407–4425. [Google Scholar] [CrossRef] [PubMed]
- Daniloski, D.; Gjorgjijoski, D.; Trajkovska Petkoska, A. Advances in Active Packaging: Perspectives in Packaging of Meat and Dairy Products. Adv. Mater. Lett. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Roopa, H.; Panghal, A.; Kumari, A.; Chhikara, N.; Sehgal, E.; Rawat, K. Active Packaging in Food Industry. In Novel Technologies in Food Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 375–404. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Campêlo, M.C.S.; Medeiros, J.M.S.; Silva, J.B.A. Natural Products in Food Preservation. Int. Food Res. J. 2019, 26, 41–46. [Google Scholar]
- Dewijanti, I.D.; Mangunwardoyo, W.; Dwianti, A.; Hanafi, M.; Artanti, N.; Mozef, T.; Devi, A.F. Antimicrobial activity of bay leaf (Syzygium Polyanthum (wight) walp) extracted using various solvent. AIP Conf. Proc. 2019, 2175, 020021. [Google Scholar] [CrossRef]
- Antolak, H.; Kregiel, D. Food Preservatives from Plants. Food Addit. 2017, 1, 45–85. [Google Scholar] [CrossRef] [Green Version]
- Sahari, M.A.; Asgari, S. Effects of Plants Bioactive Compounds on Foods Microbial Spoilage and Lipid Oxidation. Food Sci. Technol. 2013, 1, 52–61. [Google Scholar] [CrossRef]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A.M. Antioxidants: An overview on the natural and synthetic types. Eur. Chem. Bull. 2017, 6, 365–375. [Google Scholar] [CrossRef]
- Shahidi, F. Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- García-Pérez, P.; Losada-Barreiro, S.; Bravo-Díaz, C.; Gallego, P.P. Exploring the Use of Bryophyllum as Natural Source of Bioactive Compounds with Antioxidant Activity to Prevent Lipid Oxidation of Fish Oil-In-Water Emulsions. Plants 2020, 9, 1012. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Kamal-Eldin, A.; Lundin, E.A.; Zhang, J.-X.; Hallmans, G.; Åman, P. Cereal Alkylresorcinols Are Absorbed by Humans. J. Nutr. 2003, 133, 2222–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, H.M.; Johnson, E.J. Nutrients for the aging eye. Clin. Interv. Aging 2013, 8, 741–7418. [Google Scholar] [CrossRef] [Green Version]
- Noon, J.; Mills, T.B.; Norton, I.T. The use of natural antioxidants to combat lipid oxidation in O/W emulsions. J. Food Eng. 2020, 281, 110006. [Google Scholar] [CrossRef]
- Decker, E.A.; Warner, K.; Richards, M.P.; Shahidi, F. Measuring Antioxidant Effectiveness in Food. J. Agric. Food Chem. 2005, 53, 4303–4310. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Shahidi, F. Lipid-Derived Flavors in Meat Products. In Meat Process Improving Quality; Kerry, J., Kerry, J., Ledward, D., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2002; pp. 105–121. [Google Scholar]
- Damayanti, A.N.; Riyadi, P.H.; Dewi, E.N. Characteristic and boactive potential of brewed Sargassum sp. with the additional bay leaf (Syzygium Polyanthum). IOP Conf. Ser. Earth Environ. Sci. 2021, 890, 012044. [Google Scholar] [CrossRef]
- Jati, P.Z. Pengaruh Perendaman Jus Daun Salam Syzygium Polyanthum (Wight) terhadap Kandungan Asam Lemak Bebas, Total Volatile Bases dan Antioksidan Pada Telur Asin ASAP. J. Tek. Ind. Terintegrasi 2021, 4, 31–37. [Google Scholar] [CrossRef]
- Agustina, K.K.; Sari, P.H.; Suada, I.K. Pengaruh Perendaman pada Infusa Daun Salam terhadap Kualitas dan Daya Tahan Daging Babi. Bul. Vet. Udayana 2017, 9, 34–41. [Google Scholar]
- Suada, I.K.; Purnama, D.I.D.; Agustina, K.K. Infusa Daun Salam Mempertahankan Kualitas Dan Daya Tahan Daging Sapi Bali. Bul. Vet. Udayana 2018, 10, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Rasini Pirimoy, P.C.; Rohadi, R.; Iswoyo, I. Penggunaan Ekstrak Etanol Daun Salam (Syzygium Polyanthum Wight) pada Sosis Daging Sapi untuk Penghambatan Kerusakan Oksidatif. J. Teknol. dan Ind. Pertan. Indones. 2019, 11, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Aini, V.A.W.A.N.; Dewi, D.P.A.R.K. Uji Aktivitas Antioksidan Fraksi Etil Asetat Daun Salam (Syzygium Polyanthum) Dan Uji Bilangan Peroksida-Nya Terhadap Minyak Goreng Curah. Food Technol. Halal Sci. J. 2018, 1, 55–63. [Google Scholar]
- Wahyudi, V.A.; Aini, A.N.; Puspita, D.; Dewi, A.R.K. The Bay Leaves Active Compounds and Its Lipid Oxidative Inhibition Activity in Bulk Cooking Oil. Planta Trop. J. Agrosains J. Agro Sci. 2021, 9, 71–81. [Google Scholar] [CrossRef]
- Lelono, R.A.A. Potential Antioxidative and Antifungal Activities From Eugenia polyantha Wight. Widyariset 2012, 15, 437–445. [Google Scholar]
- Agustina, K.K.; Mardyawati, I.A.P.A.; Suada, I.K. The Quality and Antioxidant Content of Salted Eggs Made By Addition of Bay Leave Crude Extract on the Salting Media. Adv. Trop. Biodivers. Environ. Sci. 2019, 3, 37–40. [Google Scholar] [CrossRef]
- Sari, I.P.L. Analisis Mutu Aktivitas Antioksidan Fraksi Daun Salam (Syzygium Polyanthum) Terhadap Masa Simpan Permen Jelly. Ph.D. Thesis, Stikes Karya Putra Bangsa Tulungagung, Tulungagung Regency, Indonesia, 2022. [Google Scholar]
- Nazir, F.; Salim, R.; Yousf, N.; Bashir, M.; Naik, H.R.; Hussain, S.Z. Natural Antimicrobials for Food Preservation. J. Pharmacogn. Phytochem. 2017, 6, 2078–2082. [Google Scholar]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial Activities of Plant Essential Oils and Their Components against Escherichia coli O157:H7 and Salmonella enterica in Apple Juice. J. Agric. Food Chem. 2004, 52, 6042–6048. [Google Scholar] [CrossRef]
- Suryanto, E.; Jamhari, J.; Afidah, U.; Utami, N.A. Physical and Microbial Quality of Broiler Chicken Meat Soaked in Syzygium Polyanthum Infusion with Different Storage Time. In Proceedings of the 9th International Seminar on Tropical Animal Production (ISTAP 2021), Yogyakarta, Indonesia, 21–22 September 2021. [Google Scholar]
- Kusumaningrum, A.; Widiyaningrum, P.; Mubarok, I. Penurunan Total Bakteri Daging Ayam dengan Perlakuan Perendaman Infusa Daun Salam (Syzygium Polyanthum). Indones. J. Math. Nat. Sci. 2013, 36, 14–19. [Google Scholar]
- Hartanti, D.; Djalil, A.D.; Hamad, A.; Yulianingsih, N. The Effect of Infusion of Syzygium Polyanthum (Wight) Walp. Leaves as Natural Preservative Chicken Meats. J. Kefarmasian Indones. 2019, 9, 19–27. [Google Scholar] [CrossRef]
- Ramli, S.; Lim, L.Y.; Samsudin, N.I.P.; Rukayadi, Y. Effect of Salam [Syzygium Polyanthum (Wigt) Walp.] Leaves Extract on the Microorganism Population in Chicken Meat and Shrimp and Their Sensory. Int. Food Res. J. 2018, 25, 928–935. [Google Scholar]
- Naja, B.K. Pengaruh Berbagai Konsentrasi Ekstrak Daun Salam (Syzygium Polyanthum W.) Dan Lama Penyimpanan Terhadap Kualitas Ikan Nila Segar Sebagai Sumber Belajar Biologi. Doctoral Dissertation, University of Muhammadiyah Malang, Malang, Indonesia, 2019. [Google Scholar]
- Iriani, Y.; Ramona, Y.; Astiti, N.P.A. Efektifitas daun salam dalam mengurangi cemaran mikroba penyebab busuk telur itik. Intisari Sains Medis 2022, 13, 352–361. [Google Scholar] [CrossRef]

| Solvent | Method | IC50 | Standard | IC50 | Reference |
|---|---|---|---|---|---|
| Methanol | DPPH | 20.90 µg/mL | BHT | 18.50 µg/mL | [127] |
| FRAP | 77.55 µg/mL | Not detected | |||
| DPPH | 2.82 µg/mL | [6] | |||
| DPPH | 44.35 µg/mL | Trolox | 3.09 µg/mL | [128] | |
| ABTS | 17.69 µg/mL | 4.11 µg/mL | |||
| DPPH | 77.06 mg TEAC/g | [129] | |||
| FRAP | 7.92 mg TEAC/g | ||||
| ABTS | 83.19 mg TEAC/g | ||||
| Ethanol | DPPH | 10.89 µg/mL | Quercetin | 5.24 µg/mL | [130] |
| FRAP | 27.76 mmol/g | 27.03 mmol/g | |||
| Chloroform | DPPH | 0.029 mg/mL | Quercetin | 0.0080 mg/mL | [131] |
| ABTS | 1.91 mmol TEAC | ||||
| Petroleum ether | DPPH | 0.023 mg/mL | Quercetin | 0.0080 mg/mL | [131] |
| ABTS | 0.32 mmol TEAC | ||||
| Hexane | DPPH | 3121.73 µg/mL | Ascorbic acid | 693.30 µg/mL | [132] |
| Ethyl acetate | DPPH | 73.15 µg/mL | Ascorbic acid | 3.94 µg/mL | [132] |
| Essential oil | DPPH | 2.08 µg/mL | Ascorbic acid | 3.73 µg/mL | [109] |
| FRAP | 3.28 µg/mL | 10.24 µg/mL |
| Source | Microbial | MIC | MBC/MFC | Reference |
|---|---|---|---|---|
| Methanol extract | C. botulinum | >5000 mg/L | - | [153] |
| Bacillus cereus | 0.31 mg/mL | 2.50 mg/mL | [154] | |
| Bacillus subtilis | 0.63 mg/mL | 2.50 mg/mL | ||
| S. aureus | 6.25 mg/mL | - | [155] | |
| S. pyogenes | 6.25 mg/mL | - | ||
| Methicillin-resistant | 6.25 mg/mL | - | ||
| K. pneumiae | 6.25 mg/mL | - | ||
| E. coli | 12.15 mg/mL | - | ||
| C. albicans | 1.25 µg/mL | 1.25 µg/mL | [156] | |
| Ethanol extract | Shigella dysenteriae | 20% b/v | 20% b/v | [157] |
| E.coli | 1.25 mg/L | 2.50 mg/L | [158] | |
| K. pneumoniae | 1.25 mg/L | 2.50 mg/L | ||
| S. aureus | 0.63 mg/L | 1.25 mg/L | ||
| S. typhimurium | 1.25 mg/L | 1.25 mg/L | ||
| S. typhimurium | 0.63 mg/L | 0.63 mg/L | ||
| C. albicans | 0.16% w/v | 0.16% w/v | [159] | |
| Water extract | S. mutans | 30 mg/mL | [160] | |
| Essential oils | B. subtilis | 31.25 µg/mL | - | [161] |
| E. coli | >1000 µg/mL | - | ||
| S. aureus | >1000 µg/mL | - | ||
| S. typhimurium | >1000 µg/mL | - | ||
| V. cholera | >1000 µg/mL | - |
| Food | Preservative | Methods | Conclusions | References |
|---|---|---|---|---|
| Sargassum tea | Simplisia | Water infusion | The addition of S. polyanthum contributed to a reduction in IC50 concentration. IC50 measurements were carried out using the ABTS method. | [183] |
| Salted egg | Simplisia | Water infusion | The concentration of antioxidant IC50 reduced from 89.92 to 88.58 mg/g after soaking salted egg at varying concentrations 0–10%. S. polyanthum addition. | [184] |
| Pork | Simplisia | Water infusion | There is a significant color difference between pork immersed in 0 and 10% S. polyanthum leaf. After soaking S. polyanthum for 6 h, significant differences were also observed. Another significant difference between the control and 5% S. polyanthum immersion was the difference in meat texture. | [185] |
| Beef | Simplisia | Water infusion | Various concentrations until 15% of S. polyanthum leaf infusion had significant effects on the odor, color, texture, shelf life at room temperature, pH, and water content of Bali beef but had no significant effect on the beef’s ability to retain water. | [186] |
| Beef sausage | Extract | Extract addition | S. polyanthum possesses antioxidative properties and could be utilized as a natural antioxidant to prevent lipid oxidation and oily food products. The addition of S. polyanthum at a concentration of 1.50 ppm inhibits oxidative damage in beef sausages. | [187] |
| Bulk cooking oil | Extract | Extract addition | The addition of the ethyl acetate fraction lowered the peroxide values. The addition of 1.0% S. polyanthum lowered the peroxide value of bulk cooking oil from 7.75 to 5.04 meq O2/kg. Using 0.2% TBHQ as a control, the peroxide value of bulk cooking oil was reduced to 4.14 meq O2/kg. | [188] |
| Bulk cooking oil | Extract | Extract addition | The optimal amount of S. polyanthum extract added to cooking oil is 0.8%. The addition of S. polyanthum altered the iodine value and acid value from 42.9 to 48.2 g I2/100 mL and from 0.42 to 0.34 KOH/g, respectively. As 0.2% TBHQ was added as a control, the iodine value and acid value changed to 48.7 g I2/100 mL and 0.19 KOH/g. | [189] |
| Meat | Extract | Extract addition | The tested extracts were methanol, methanol–water, and water. Using the TBARS technique, the addition of 3% S. polyanthum extract to raw and cooked meat inhibited fat oxidation. The meat samples were stored at 4 °C for seven days prior to testing. Based on the results of the tests, it was determined that the best extract to inhibit fat oxidation in meat was water extract. The lipid protections of water extract on raw meat and cooked meat are up to 58 and 68%, respectively. | [190] |
| Salted egg | Extract | Extract addition | The concentration of extract addition on salted egg duck media is 25 and 50%. The antioxidant capacity of standard salted eggs, 25% extract addition and 50% extract, were measured to be 4.45, 30.85, and 44.32%. The quality of salted eggs is similar to that of standard salted eggs, despite an increase in albumin index and Haugh unit values to 0.053–0.060 and 44–47, respectively. | [191] |
| Jelly candy | Extract | Extract addition | S. polyanthum extract addition decreases jelly oxidation by up to 50%. Experiments are held for up to 12 days of storage. | [192] |
| Food | Preservative | Methods | Conclusions | References |
|---|---|---|---|---|
| Chicken meat | Simplicia | Water infusion | Soaking and storage time had no effect on the physical characteristics of chicken meat, but it could reduce the color value of raw meat while increasing the aroma value, as well as the tenderness and aroma of cooked meat. Soaking chicken meat in S. polyanthum leaf infusion with varying storage times can increase tenderness and inhibit microbial growth until the fourth day. However, S. polyanthum leaf infusion had no effect on the pH or cooking loss of chicken meat. S. polyanthum infusion can reduce the total number of microbes in chicken meat during refrigerator storage. | [195] |
| Chicken meat | Extract | Water infusion | Variations in the concentration of S. polyanthum leaf infusion and the length of observation at room temperature had a substantial impact on the total number of bacteria in fresh chicken meat. | [196] |
| Chicken meat | Simplicia | Water infusion | At the optimal concentration of 10%, S. polyanthum leaf infusion can inhibit bacteria growth on chicken meat during storage, extending its shelf life by up to three days at 3–7 °C. | [197] |
| Shrimp and chicken | Extract | Dilution | Chicken and shrimp were treated with S. polyanthum leaf extract at various concentrations, 0.0, 0.1, and 1.00%, and exposure periods of 5 and 10 min. In untreated chicken samples, S. aureus TPC values were determined to be 6.66 and 8.66 CFU/mL. In untreated shrimp samples, S. aureus TPC values were determined to be and 7.25 and 6.54 CFU/mL. However, neither sample contained E. coli, Salmonella spp., or Vibrio cholerae. The number of S. aureus TPCs in chicken meat and shrimp began to decrease significantly after 5 min of exposure to S. polyanthum leaf extract at a concentration of 0.01%. There were no statistically significant differences between exposure times. TPC was reduced from 6.66 to 0.00 CFU/mL and from 8.66 to 4.88 CFU/mL in shrimp, whereas TPC S. aureus was reduced from 7.25 to 3.88 CFU/mL and from 6.54 to 4.92 CFU/mL in chicken and shrimp, respectively, following treatment with 1.0% extract for 10 min. | [198] |
| Tilapian fish | Extract | Extract addition | Using 15% S. polyanthum extract and storing for seven days was able to maintain the number of bacterial colonies below the national regulator requirement. | [199] |
| Dug eggs | Simplicia | Water infusion | Using 5% (v/v) S. polyanthum addition to salted duck eggs can inhibit Proteus mirabilis growth significantly. It is shown by the total plate number decreasing. | [200] |
| Tofu | Essential oil | Water infusion | Essential oil is added to tofu bacterial growth media. Essential oil concentrations are 0.063, 0.313, and 1.563 mg/mL. Tofu and bacteria are incubated for 2, 4, 6, and 8 days. Bacterial growth is monitored visually. Preservation of essential oil is equal to its concentration. Essential oil optimally preserved tofu in 6-day incubation. | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julizan, N.; Ishmayana, S.; Zainuddin, A.; Van Hung, P.; Kurnia, D. Potential of Syzygnium polyanthum as Natural Food Preservative: A Review. Foods 2023, 12, 2275. https://doi.org/10.3390/foods12122275
Julizan N, Ishmayana S, Zainuddin A, Van Hung P, Kurnia D. Potential of Syzygnium polyanthum as Natural Food Preservative: A Review. Foods. 2023; 12(12):2275. https://doi.org/10.3390/foods12122275
Chicago/Turabian StyleJulizan, Nur, Safri Ishmayana, Achmad Zainuddin, Pham Van Hung, and Dikdik Kurnia. 2023. "Potential of Syzygnium polyanthum as Natural Food Preservative: A Review" Foods 12, no. 12: 2275. https://doi.org/10.3390/foods12122275
APA StyleJulizan, N., Ishmayana, S., Zainuddin, A., Van Hung, P., & Kurnia, D. (2023). Potential of Syzygnium polyanthum as Natural Food Preservative: A Review. Foods, 12(12), 2275. https://doi.org/10.3390/foods12122275