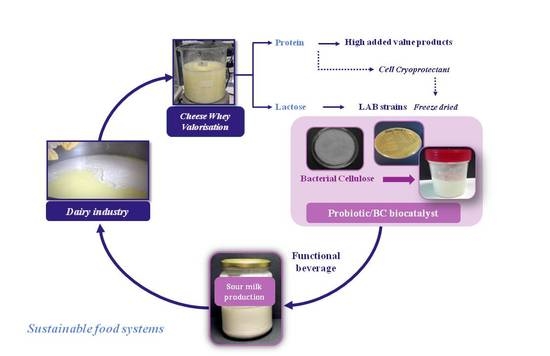
Novel Probiotic/Bacterial Cellulose Biocatalyst for the Development of Functional Dairy Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Submerged Fermentations
2.3. Freeze-Dried Biomass Production
2.4. Immobilized Biocatalysts Production
2.5. Sour Milk Production
2.6. Microbiological Analysis
2.7. Physicochemical Analysis
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results
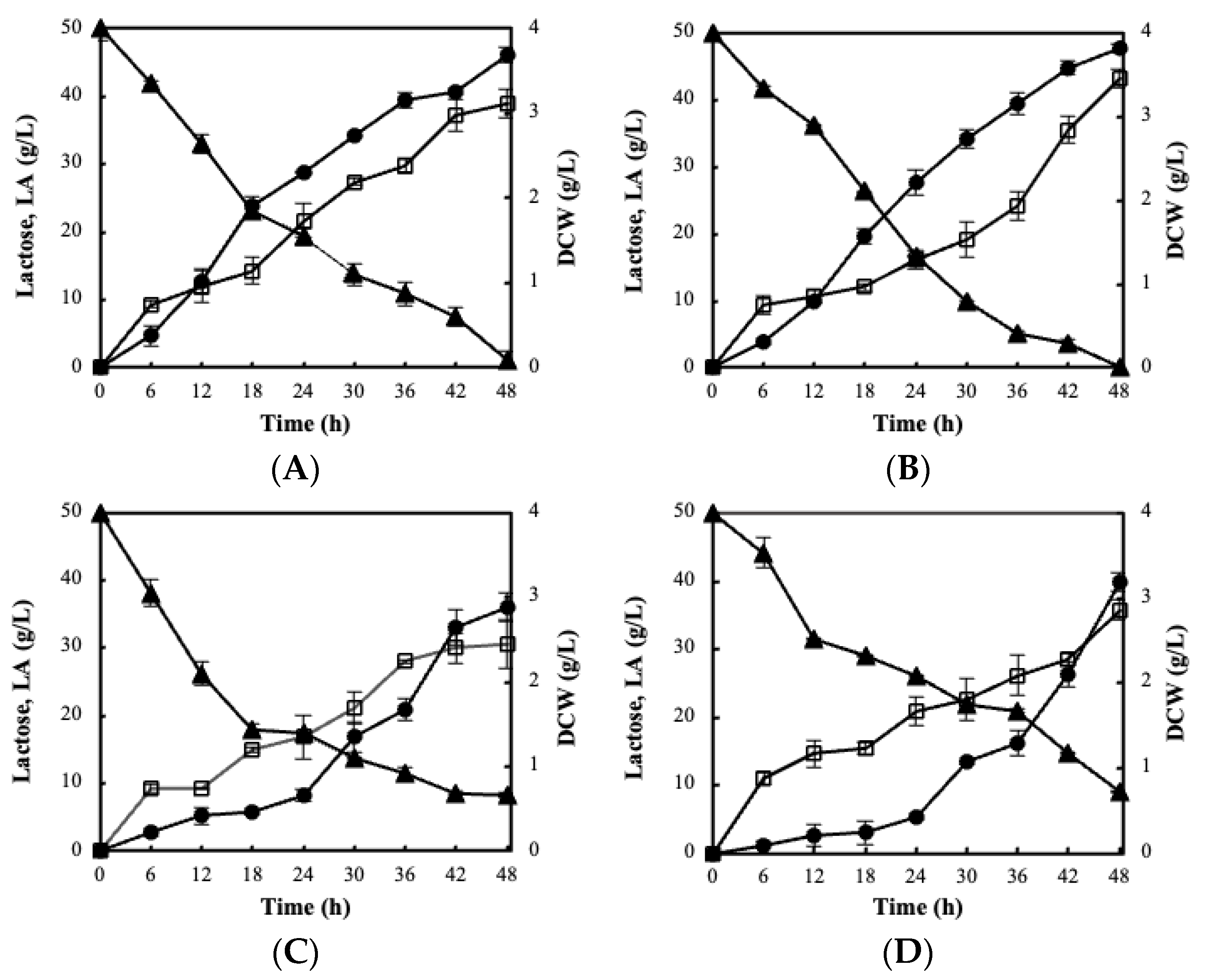
3.1. Evaluation of Probiotics on CWP Fermentations
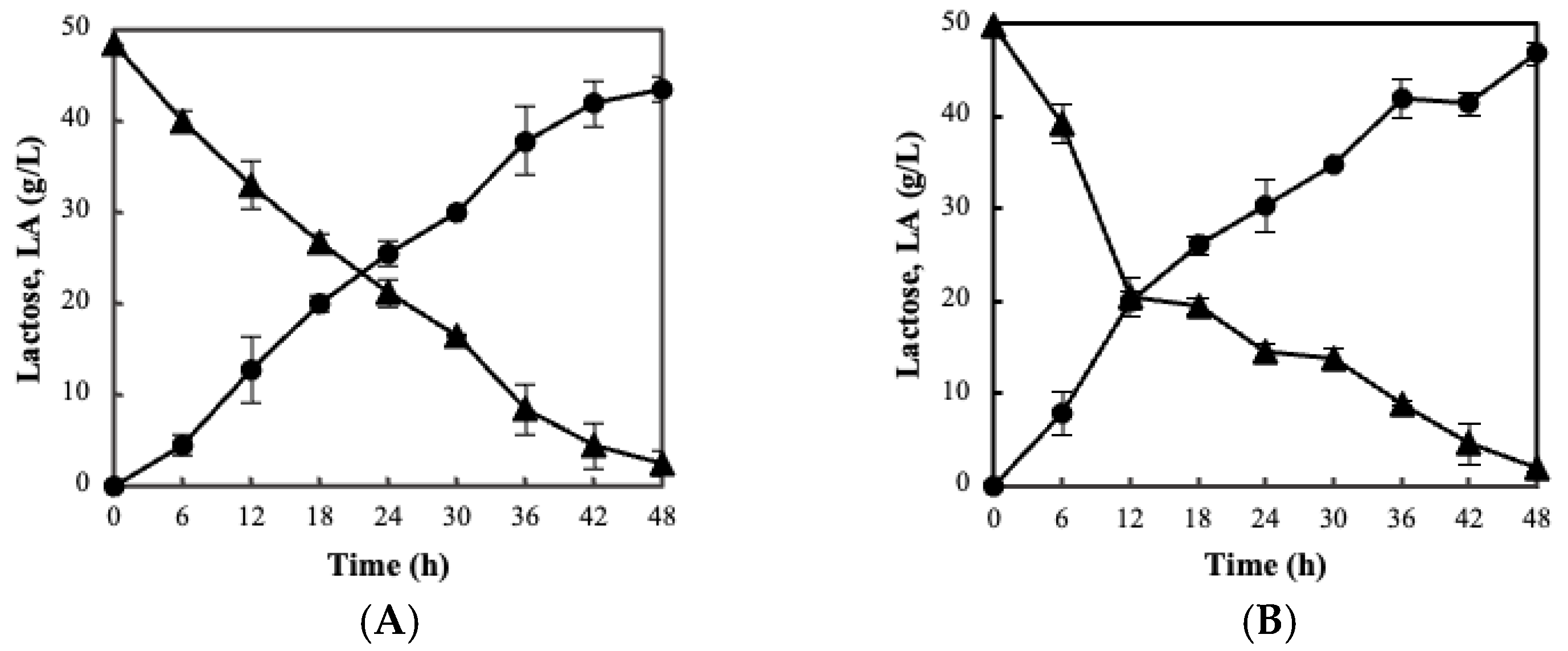
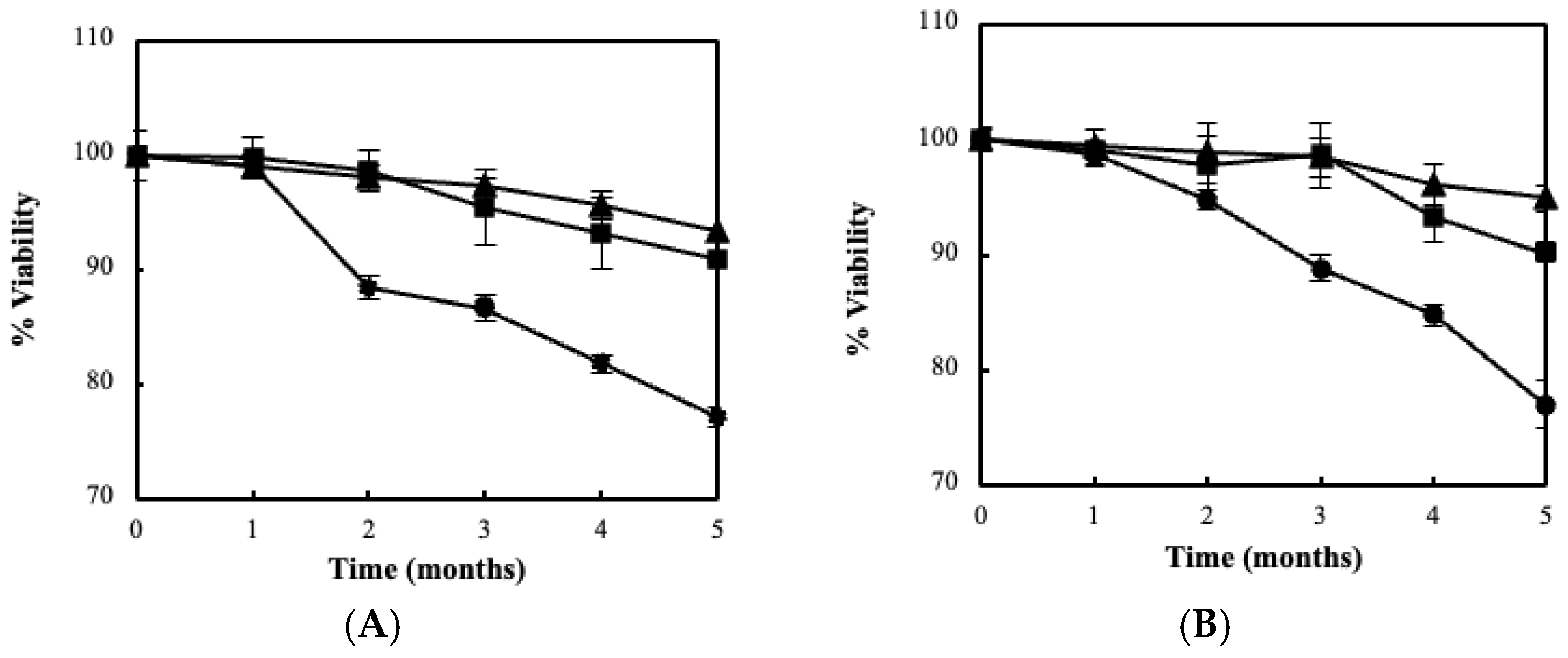
3.2. Fermentation Kinetics, Bacterial Viability and Storage Stability Using the BC/Biocatalyst
3.3. Sour Milk Production Using Probiotic Cellulose Biocatalyst
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantzourani, I.; Terpou, A.; Bekatorou, A.; Mallouchos, A.; Alexopoulos, A.; Kimbaris, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem. 2019, 308, 125658. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.S.; de Oliveira, V.M.; Pasquali, M.A.d.B. Probiotics in Citrus Fruits Products: Health Benefits and Future Trends for the Production of Functional Foods—A Bibliometric Review. Foods 2022, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Hadi, J.; Gutierrez-Maddox, N.; Li, Y.; Leung, I.K.H.; Gao, Y.; Shu, Q.; Quek, S.-Y. Sensory, Microbiological and Physicochemical Characterisation of Functional Manuka Honey Yogurts Containing Probiotic Lactobacillus reuteri DPC16. Foods 2020, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Tenea, G.N.; Suárez, J. Probiotic Potential and Technological Properties of Bacteriocinogenic Lactococcus lactis Subsp. Lactis UTNGt28 from a Native Amazonian Fruit as a Yogurt Starter Culture. Microorganisms 2020, 8, 733. [Google Scholar] [CrossRef]
- Katarzyna, S.; Agata, Z.; Piotr, K. Sensory and textural properties of fermented milk with viability of Lactobacillus rhamnosus and Bifidobacterium animalis ssp. lactis Bb-12 and increased calcium concentration. Int. J. Food Prop. 2020, 23, 582–598. [Google Scholar]
- Zheng, Y.; Fei, Y.; Yang, Y.; Jin, Z.; Yu, B.; Li, L. A potential flavor culture: Lactobacillus harbinensis M1 improves the organoleptic quality of fermented soymilk by high production of 2,3-butanedione and acetoin. Food Microbiol. 2020, 91, 103540. [Google Scholar] [CrossRef]
- Terpou, A.; Mantzourani, I.; Galanis, A.; Kanellaki, M.; Bezirtzoglou, E.; Bekatorou, A.; Koutinas, A.A.; Plessas, S. Employment of L. paracasei K5 as a novel potentially pobiotic freeze-dried starter for feta-Type cheese production. Microorganisms 2019, 7, 3. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Salamoura, C.; Kontogianni, A.; Katsipi, D.; Kandylis, P.; Zakynthinos, G.; Varzakas, T. Effect of Milk Type on the Microbiological, Physicochemical and Sensory Characteristics of Probiotic Fermented Milk. Microorganisms 2019, 7, 274. [Google Scholar] [CrossRef]
- Kariyawasam, K.M.G.M.M.; Lee, N.-K.; Paik, H.-D. Synbiotic yoghurt supplemented with novel probiotic Lactobacillus brevis KU200019 and fructooligosaccharides. Food Biosci. 2020, 39, 100835. [Google Scholar] [CrossRef]
- Ali, H.I.; Dey, M.; Alzubaidi, A.K.; Alneamah, S.J.A.; Altemimi, A.B.; Pratap-Singh, A. Effect of Rosemary (Rosmarinus officinalis L.) Supplementation on Probiotic Yoghurt: Physicochemical Properties, Microbial Content, and Sensory Attributes. Foods 2021, 10, 2393. [Google Scholar] [CrossRef] [PubMed]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 61, 1515–1536. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible Films and Coatings as Carriers of Living Microorganisms: A New Strategy Towards Biopreservation and Healthier Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef]
- Sidira, M.; Karapetsas, A.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effective survival of immobilized Lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of the microbial dynamics. Meat Sci. 2014, 96, 948–955. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Hu, Y.; Catchmark, J.M. In vitro biodegradability and mechanical properties of bioabsorbable bacterial cellulose incorporating cellulases. Acta Biomater. 2011, 7, 2835–2845. [Google Scholar] [CrossRef]
- Oliveira-Alcântara, A.V.; Abreu, A.A.S.; Gonçalves, C.; Fuciños, P.; Cerqueira, M.A.; da Gama, F.; Pastrana, L.M.; Rodrigues, S.; Azeredo, H.M. Bacterial cellulose/cashew gum films as probiotic carriers. LWT 2020, 130, 109699. [Google Scholar] [CrossRef]
- Tsouko, E.; Papadaki, A.; Papapostolou, H.; Ladakis, D.; Natsia, A.; Koutinas, A.; Kampioti, A.; Eriotou, E.; Kopsahelis, N. Valorization of Zante currant side-streams for the production of phenolic-rich extract and bacterial cellulose: A novel biorefinery concept. J. Chem. Technol. Biotechnol. 2019, 95, 427–438. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Papadaki, A.; Stamatiou, A.; Ladakis, D.; Eriotou, E.; Kopsahelis, N. A Comprehensive Bioprocessing Approach to Foster Cheese Whey Valorization: On-Site β-Galactosidase Secretion for Lactose Hydrolysis and Sequential Bacterial Cellulose Production. Fermentation 2021, 7, 184. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Zhang, Y.; Sun, S.; Ullah, M.W.; Xu, W. Biotransformation of nylon-6,6 hydrolysate to bacterial cellulose. Green Chem. 2021, 23, 7805–7815. [Google Scholar] [CrossRef]
- Lappa, I.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef]
- Rollini, M.; Musatti, A.; Cavicchioli, D.; Bussini, D.; Farris, S.; Rovera, C.; Romano, D.; De Benedetti, S.; Barbiroli, A. From cheese whey permeate to Sakacin-A/bacterial cellulose nanocrystal conjugates for antimicrobial food packaging applications: A circular economy case study. Sci. Rep. 2020, 10, 21358. [Google Scholar] [CrossRef]
- Permeate Market: Global Industry Analysis 2017–2021 and Opportunity Assessment 2022–2032; Future Market Insights: London, UK, 2022; Available online: https://www.futuremarketinsights.com/reports/permeate-market (accessed on 15 June 2022).
- Probiotics Market Size, Share & Trends Analysis Report by Product (Probiotic Food & Beverages, Probiotic Dietary Supplements), by Ingredient (Bacteria, Yeast), by End Use, by Distribution Channel, and Segment Forecasts, 2021–2030. Available online: https://www.researchandmarkets.com/reports/3972861/probiotics-market-size-share-and-trends-analysis (accessed on 13 June 2022).
- Lavari, L.; Páez, R.; Cuatrin, A.; Reinheimer, J.; Vinderola, G. Use of cheese whey for biomass production and spray drying of probiotic lactobacilli. J. Dairy Res. 2014, 81, 267–274. [Google Scholar] [CrossRef]
- Cerdeira, V.; Bravo-Ferrada, B.M.; Semorile, L.; Tymczyszyn, E. Design of a low-cost culture medium based in whey permeate for biomass production of enological Lactobacillus plantarum strains. Biotechnol. Prog. 2019, 35, e2791. [Google Scholar] [CrossRef]
- Malvido, M.C.; González, E.A.; Tantaleán, D.L.B.; Jácome, R.J.B.; Guerra, N.P. Batch and fed-batch production of probiotic biomass and nisin in nutrient-supplemented whey media. Braz. J. Microbiol. 2019, 50, 915–925. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Papadaki, A.; Lappa, I.; Papastergiou, S.; Kleisiari, D.; Kopsahelis, N. Biosurfactant Production from Lactobacilli: An Insight on the Interpretation of Prevailing Assessment Methods. Appl. Biochem. Biotechnol. 2021, 194, 882–900. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; Shin, H.S.; Lee, H.W.; Hong, D.; Park, H.; Holzapfel, W.; Kim, E.B.; Huh, C.S. Determination of Optimized Growth Medium and Cryoprotective Additives to Enhance the Growth and Survival of Lactobacillus salivarius. J. Microbiol. Biotechnol. 2018, 28, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Bodzen, A.; Jossier, A.; Dupont, S.; Mousset, P.-Y.; Beney, L.; Lafay, S.; Gervais, P. Design of a new lyoprotectant increasing freeze-dried Lactobacillus strain survival to long-term storage. BMC Biotechnol. 2021, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Żywicka, A.; Wenelska, K.; Junka, A.; Chodaczek, G.; Szymczyk, P.; Fijałkowski, K. Immobilization pattern of morphologically different microorganisms on bacterial cellulose membranes. World J. Microbiol. Biotechnol. 2019, 35, 11. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, R.F.; Nabeshima, E.H.; Iamanaka, B.T.; Ludwig, A.; Fries, L.L.M.; Bernardi, A.O.; Copetti, M.V. Survival and stability of Lactobacillus fermentum and Wickerhamomyces anomalus strains upon lyophilisation with different cryoprotectant agents. Food Res. Int. 2018, 115, 90–94. [Google Scholar] [CrossRef]
- Terpou, A.; Gialleli, A.-I.; Bekatorou, A.; Dimitrellou, D.; Ganatsios, V.; Barouni, E.; Koutinas, A.A.; Maria, Μ. Sour milk production by wheat bran supported probiotic biocatalyst as starter culture. Food Bioprod. Process. 2017, 101, 184–192. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y. Assessment of Freeze-Dried Immobilized Lactobacillus casei as Probiotic Adjunct Culture in Yogurts. Foods 2019, 8, 374. [Google Scholar] [CrossRef]
- Manzoor, A.; Qazi, J.I.; Haq, I.U.; Mukhtar, H.; Rasool, A. Significantly enhanced biomass production of a novel bio-therapeutic strain Lactobacillus plantarum (AS-14) by developing low cost media cultivation strategy. J. Biol. Eng. 2017, 11, 17. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Flores, S.H.; Brinques, G.B.; Ayub, M.A.Z. Viability and alternative uses of a dried powder, microencapsulated Lactobacillus plantarum without the use of cold chain or dairy products. LWT 2016, 71, 54–59. [Google Scholar] [CrossRef]
- Prajapati, J.B.S.; Hati, S.; Sreeja, V.; Trivedi., J. Deproteinated Cheese Whey Medium for Biomass Production of Probiotic Lactobacillus helveticus MTCC 5463. Int. Curr. Microbiol. App. Sci. 2017, 6, 174–187. [Google Scholar] [CrossRef][Green Version]
- Huang, S.; Méjean, S.; Rabah, H.; Dolivet, A.; Le Loir, Y.; Chen, X.D.; Jan, G.; Jeantet, R.; Schuck, P. Double use of concentrated sweet whey for growth and spray drying of probiotics: Towards maximal viability in pilot scale spray dryer. J. Food Eng. 2016, 196, 11–17. [Google Scholar] [CrossRef]
- Hayek, S.A.; Gyawali, R.; Aljaloud, S.O.; Krastanov, A.; Ibrahim, S.A. Cultivation media for lactic acid bacteria used in dairy products. J. Dairy Res. 2019, 86, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Papizadeh, M.; Rohani, M.; Hosseini, S.N.; Shojaosadati, S.A.; Nahrevanian, H.; Talebi, M.; Pourshafie, M.R. Screening for efficient nitrogen sources for overproduction of the biomass of the functionally probiotic L. plantarum strain RPR42 in a cane molasses-based medium. AMB Express 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Panesar, P.S.; Kennedy, J.F.; Knill, C.J.; Kosseva, M. Production of L(+) lactic acid using Lactobacillus casei from whey. Braz. Arch. Biol. Technol. 2010, 53, 219–226. [Google Scholar] [CrossRef]
- Samedi, L.; Charles, A.L. Viability of 4 Probiotic Bacteria Microencapsulated with Arrowroot Starch in the Simulated Gastrointestinal Tract (GIT) and Yoghurt. Foods 2019, 8, 175. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Calabuig-Jiménez, L.; Barrera, C.; Rosa, M.D. Effect of Drying Process, Encapsulation, and Storage on the Survival Rates and Gastrointestinal Resistance of L. salivarius spp. salivarius Included into a Fruit Matrix. Microorganisms 2020, 8, 654. [Google Scholar] [CrossRef]
- Gadhiya, D.; Shah, N.; Patel, A.; Prajapati, J. Preparation and shelf life study of probiotic chocolate manufactured using Lactobacillus helveticus MTCC 5463. Acta Aliment. 2018, 47, 350–358. [Google Scholar] [CrossRef]
- Khem, S.; Small, D.M.; May, B.K. The behavior of whey protein isolate in protecting Lactobacillus plantarum. Food Chem. 2016, 190, 717–723. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Wang, H.; Sun, X.; Guo, M.; Hou, J. Effects of polymerized whey protein on survivability of Lactobacillus acidophilus LA-5 during freeze-drying. Food Sci. Nutr. 2019, 7, 2708–2715. [Google Scholar] [CrossRef]
- Wu, S.C.; Wu, S.M.; Su, F.M. Novel process for immobilizing an enzyme on a bacterial cellulose membrane through repeated absorption. J. Chem. Technol. Biotechnol. 2017, 92, 109–114. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Andrade, F.K.; Vieira, L.D.A.P.; Vieira, R.S.; Vaz, J.M.; Chevallier, P.; Mantovani, D.; Borges, M.D.F.; Rosa, M.D.F. Oxidized bacterial cellulose membrane as support for enzyme immobilization: Properties and morphological features. Cellulose 2020, 27, 3055–3083. [Google Scholar] [CrossRef]
- Sabio, L.; González, A.; Ramírez-Rodríguez, G.B.; Gutiérrez-Fernández, J.; Bañuelo, O.; Olivares, M.; Gálvez, N.; Delgado-López, J.M.; Dominguez-Vera, J.M. Probiotic cellulose: Antibiotic-free biomaterials with enhanced antibacterial activity. Acta Biomater. 2021, 124, 244–253. [Google Scholar] [CrossRef]
- Phromthep, K.; Leenanon, B. Survivability of immobilized Lactobacillus plantarum cells within bacterial cellulose in mamao juice. Int. Food. Res. J. 2017, 24, 939–949. [Google Scholar]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Żywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Savitskaya, I.S.; Shokatayeva, D.H.; Kistaubayeva, A.S.; Ignatova, L.V.; Digel, I.E. Antimicrobial and wound healing properties of a bacterial cellulose based material containing B. subtilis cells. Heliyon 2019, 5, e02592. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Manikas, A.C.; Papazoglou, E.; Kachrimanidou, V.; Lappa, I.; Galiotis, C.; Mandala, I.; Kopsahelis, N. Whey protein films reinforced with bacterial cellulose nanowhiskers: Improving edible film properties via a circular economy approach. Food Chem. 2022, 385, 132604. [Google Scholar] [CrossRef]
- Gao, C.; Wan, Y.; Yang, C.; Dai, K.; Tang, T.; Luo, H.; Wang, J. Preparation and characterization of bacterial cellulose sponge with hierarchical pore structure as tissue engineering scaffold. J. Porous Mater. 2010, 18, 139–145. [Google Scholar] [CrossRef]
- Schär-Zammaretti, P.; Ubbink, J. The Cell Wall of Lactic Acid Bacteria: Surface Constituents and Macromolecular Conformations. Biophys. J. 2003, 85, 4076–4092. [Google Scholar] [CrossRef]
- Żur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic Responses of Bacterial Cells to Immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.; Kachrimanidou, V.; Bosnea, L.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
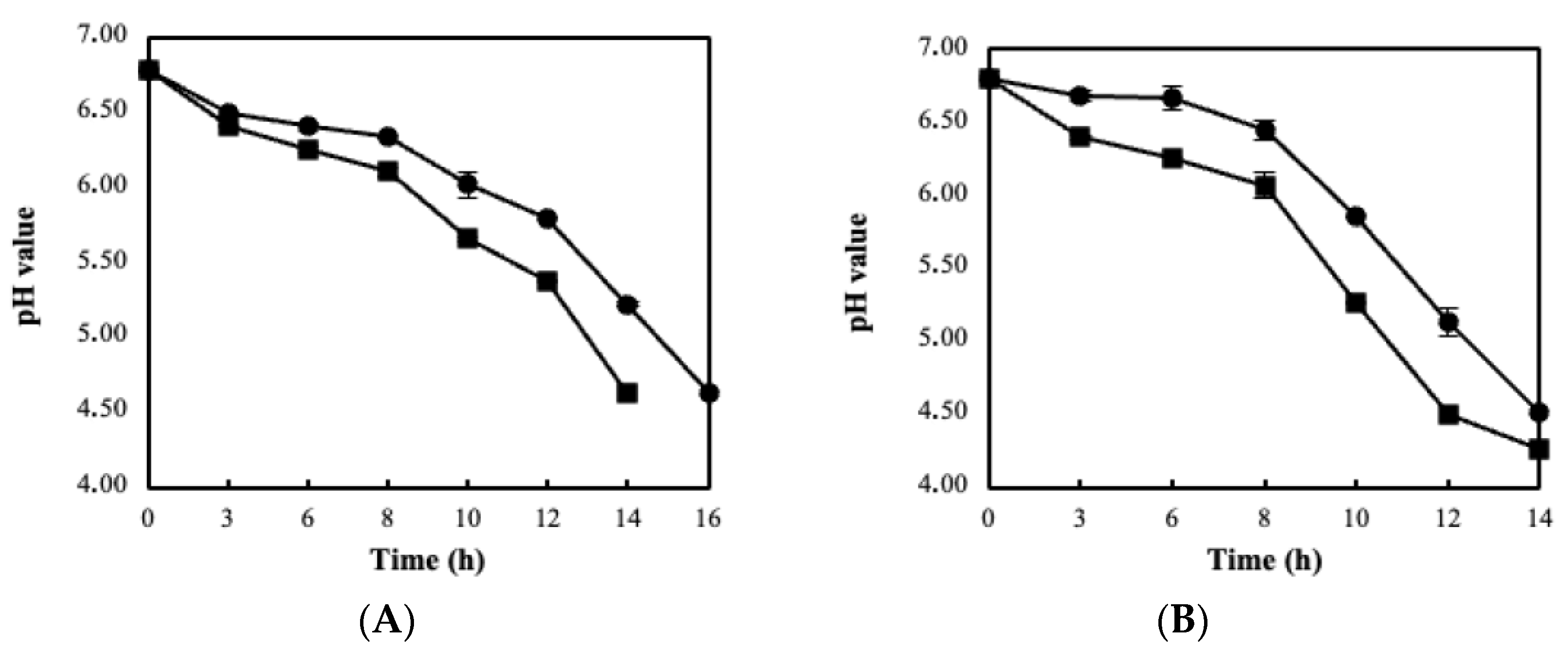
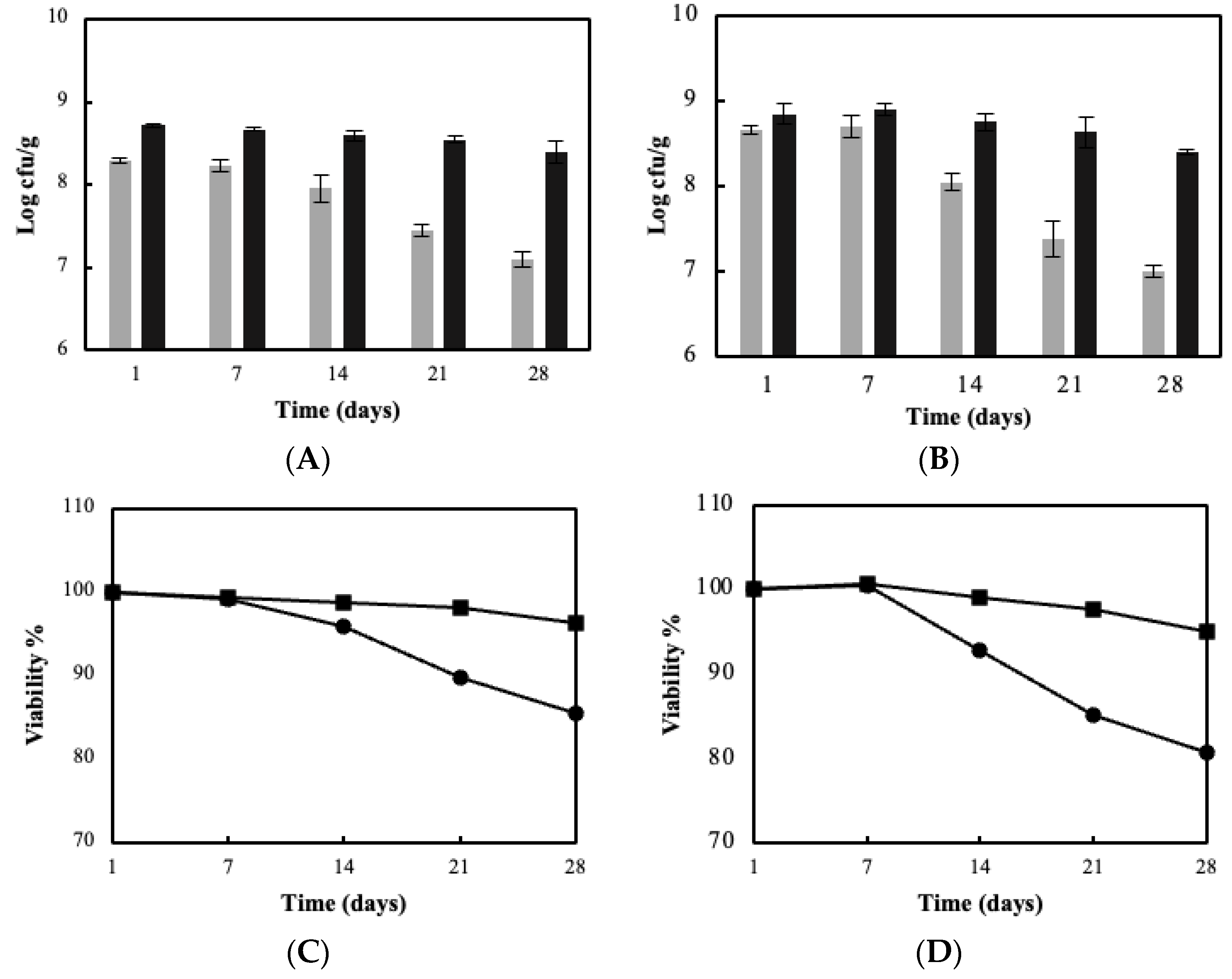
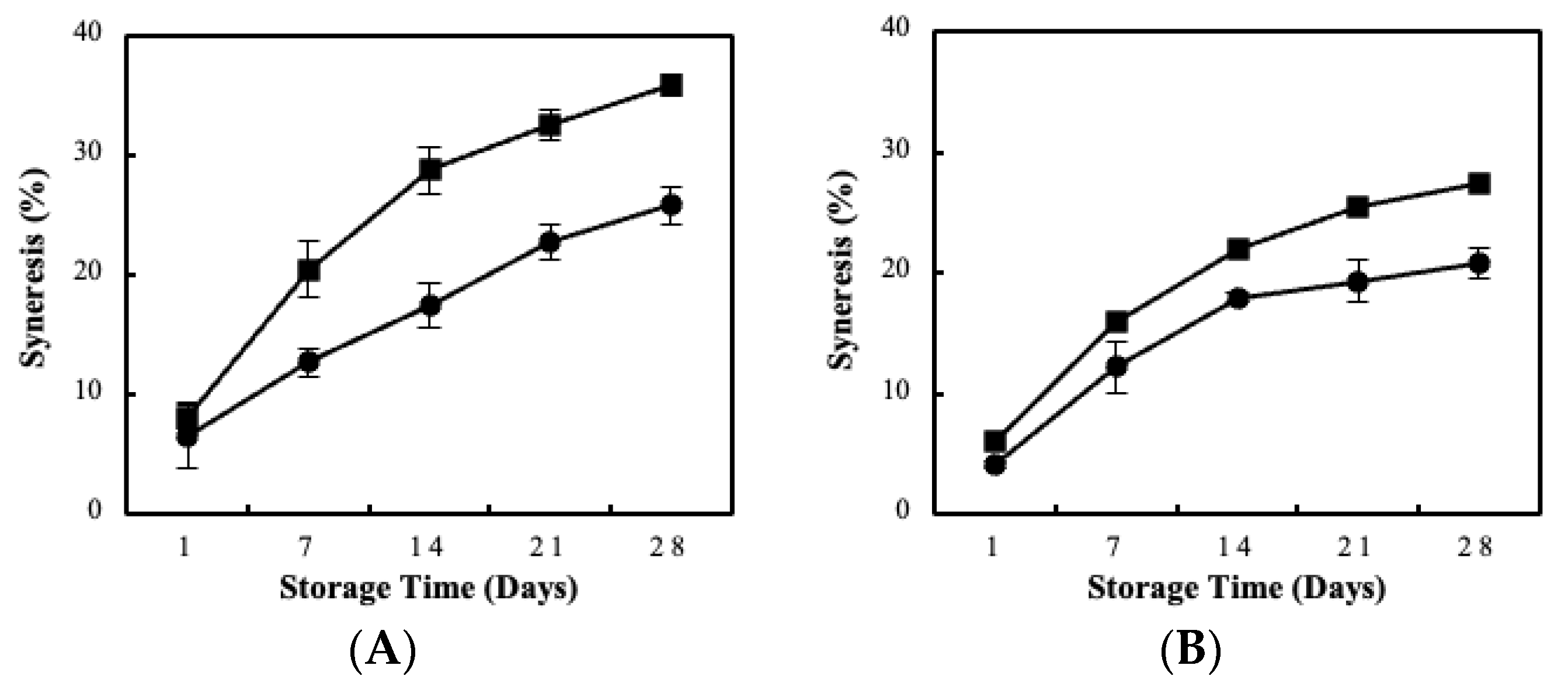
| Immobilization Substrate | Strains | log cfu/g (Wet Culture) | log cfu/g (Freeze-Dried Culture) |
|---|---|---|---|
| BC film | B329 | 8.80 ± 0.09 a | 8.74 ± 0.06 a |
| blended BC | B329 | 8.33 ± 0.04 b | 8.06 ± 0.03 b |
| BC film | 820 | 8.83 ± 0.05 a | 8.58 ± 0.37 a |
| blended BC | 820 | 8.78 ± 0.28 b | 8.43 ± 0.05 b |
| Storage Time | Free Cells | Blended BC | ||
|---|---|---|---|---|
| L. pentosus | L. plantarum | L. pentosus | L. plantarum | |
| pH value | ||||
| 1 | 4.50 | 4.40 | 4.52 | 4.22 |
| 7 | 4.46 | 4.12 | 4.44 | 3.82 |
| 14 | 4.44 | 4.07 | 4.41 | 3.75 |
| 21 | 4.35 | 3.94 | 4.37 | 3.56 |
| 28 | 4.31 | 3.90 | 4.23 | 3.53 |
| Titratable Acidity (% expressed as Lactic Acid) | ||||
| 1 | 0.58 | 0.60 | 0.63 | 0.66 |
| 7 | 0.64 | 0.67 | 0.69 | 0.70 |
| 14 | 0.66 | 0.71 | 0.72 | 0.82 |
| 21 | 0.68 | 0.82 | 0.76 | 1.10 |
| 28 | 0.71 | 1.02 | 0.88 | 1.24 |
| L. pentosus B329 | Color | Sour Odor | Flavor | Smoothness | Overall Acceptance |
| C1 | 8.2 ± 0.2 a | 7.2 ± 0.4 a | 6.9 ± 0.2 a | 7.6 ± 0.4 a | 7.5 ± 0.3 a |
| B2 | 8.1 ± 0.2 a | 7.0 ± 0.3 a | 7.6 ± 0.5 ac | 8.4 ± 0.2 c | 7.6 ± 0.2 ab |
| L. plantarum 820 | Color | Sour Odor | Flavor | Smoothness | Overall Acceptance |
| C1 | 8.9 ± 0.2 b | 8.4 ± 0.5 a | 8.0 ± 0.3 a | 8.6 ± 0.4 ad | 8.4 ± 0.2 a |
| B2 | 8.9 ± 0.2 b | 7.8 ± 0.5 a | 7.5 ± 0.3 ab | 8.7 ± 0.3 a | 8.2 ± 0.2 a |
| Commercial | 8.8 ± 0.2 b | 8.5 ± 0.3 a | 8.7 ± 0.2 d | 8.8 ± 0.2 d | 8.6 ± 0.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lappa, I.K.; Kachrimanidou, V.; Alexandri, M.; Papadaki, A.; Kopsahelis, N. Novel Probiotic/Bacterial Cellulose Biocatalyst for the Development of Functional Dairy Beverage. Foods 2022, 11, 2586. https://doi.org/10.3390/foods11172586
Lappa IK, Kachrimanidou V, Alexandri M, Papadaki A, Kopsahelis N. Novel Probiotic/Bacterial Cellulose Biocatalyst for the Development of Functional Dairy Beverage. Foods. 2022; 11(17):2586. https://doi.org/10.3390/foods11172586
Chicago/Turabian StyleLappa, Iliada K., Vasiliki Kachrimanidou, Maria Alexandri, Aikaterini Papadaki, and Nikolaos Kopsahelis. 2022. "Novel Probiotic/Bacterial Cellulose Biocatalyst for the Development of Functional Dairy Beverage" Foods 11, no. 17: 2586. https://doi.org/10.3390/foods11172586
APA StyleLappa, I. K., Kachrimanidou, V., Alexandri, M., Papadaki, A., & Kopsahelis, N. (2022). Novel Probiotic/Bacterial Cellulose Biocatalyst for the Development of Functional Dairy Beverage. Foods, 11(17), 2586. https://doi.org/10.3390/foods11172586