Evaluation of Biochemical Properties, Antioxidant Activities and Phenolic Content of Two Wild-Grown Berberis Fruits: Berberis nummularia and Berberis atrocarpa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Proximate Composition Analysis of B. nummularia and B. atrocarpa Fruits
2.3. Determination of Organic Acids
2.4. Mineral Content Analysis
2.5. Extraction Process for Total Phenolic Content (TPC), Total Flavonoids Content (TFC) and Antioxidant Capacity
2.5.1. Total Phenolic Content (TPC)
2.5.2. Total Flavonoids Content (TFC)
2.6. Antioxidant Activity
2.6.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.6.2. The Ferric Reducing Antioxidant Power (FRAP) Assay
2.6.3. Assay of ABTS Radical Scavenging Activity
2.6.4. Measurement of Oxygen Radical Absorbance Capacity (ORAC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Composition of Fruits of B. nummularia and B. atrocarpa
3.2. Mineral Contents
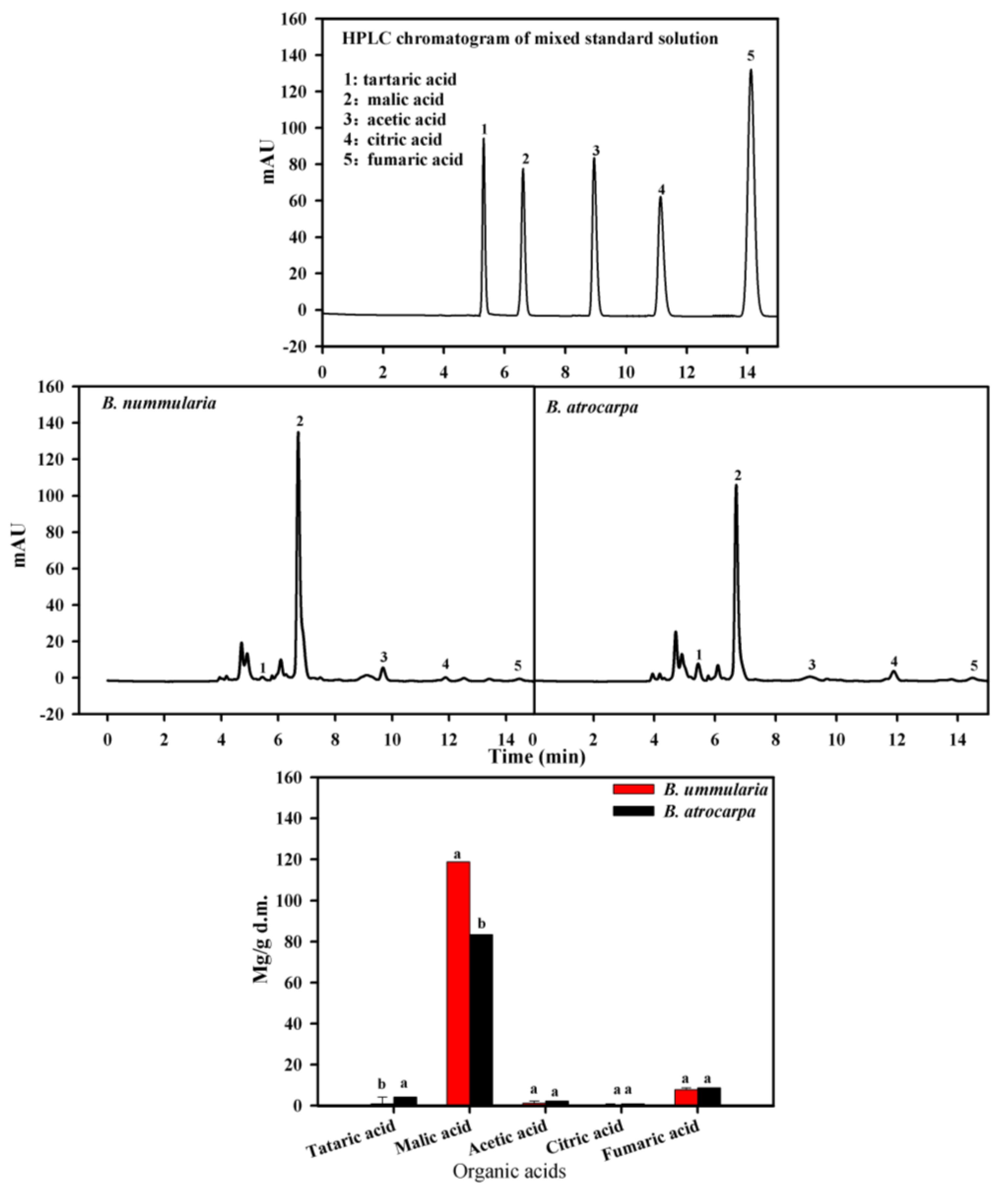
3.3. The Organic Acids Contents
3.4. Total Phenolic Content (TPC) and Total Flavonoids Content (TFC)
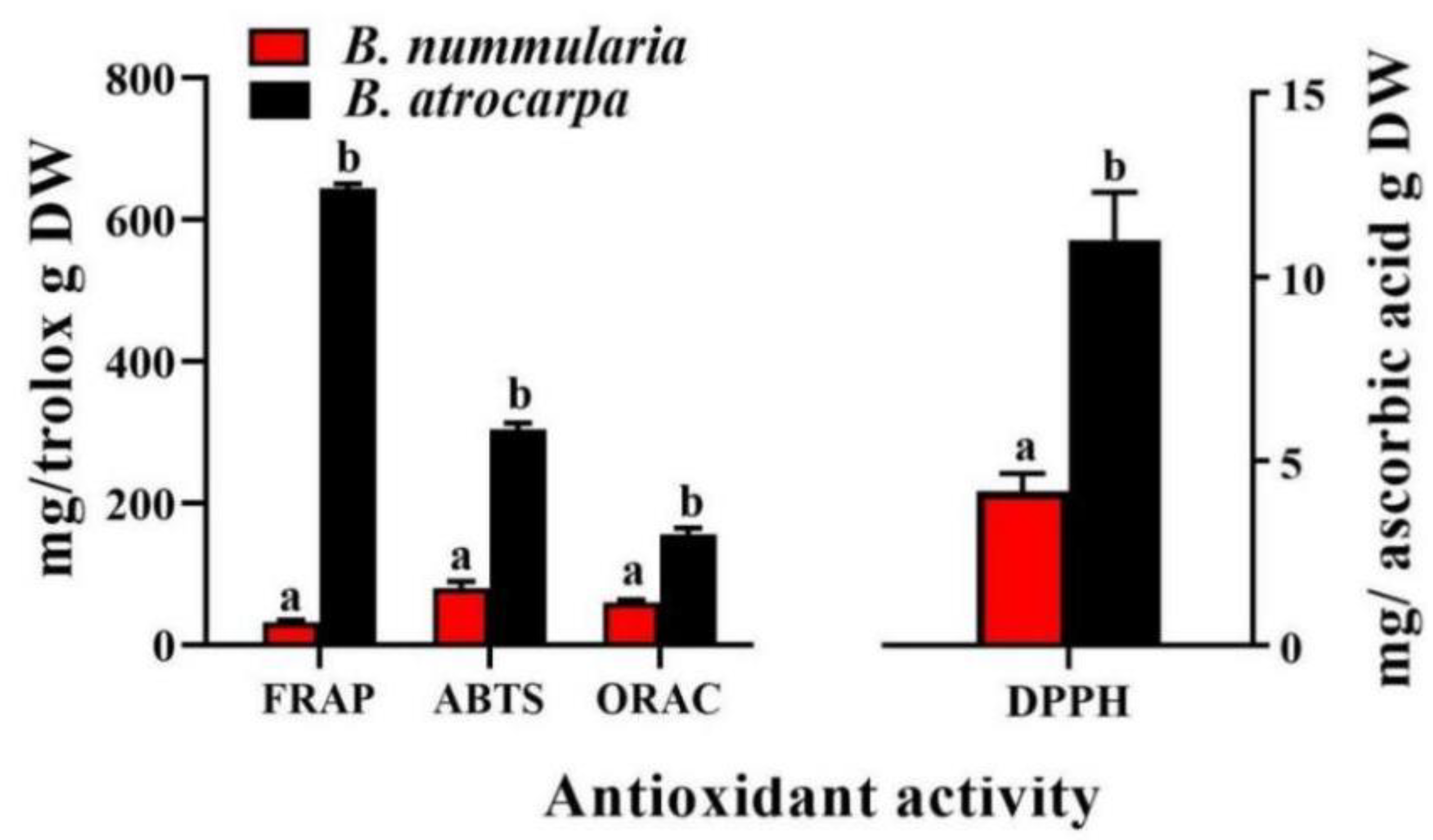
3.5. Antioxidant Activity of B. nummularia and B. atrocarpa Fruit Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gıdık, B. Antioxidant, Antimicrobial activities and fatty acid compositions of wild Berberis spp. By different techniques combined with chemometrics (PCA and HCA). Molecules 2021, 26, 7448. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Q.; Li, J.; Liu, J.; Xu, F. Nutritional composition and antioxidant properties of the fruit of Berberis heteropoda Schrenk. PLoS ONE 2022, 17, e0262622. [Google Scholar] [CrossRef]
- Arancibia-Avila, P.; Namiesnik, J.; Toledo, F.; Werner, E.; Martinez-Ayala, A.M.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Gorinstein, S. The influence of different time durations of thermal processing on berries quality. Food Control. 2012, 26, 587–593. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosin-Gutierrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; VonBaer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Dienait, L.; Pukalskas, A.; Pukalskien, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Phytochemical composition, antioxidant and antiproliferative activities of defatted Sea Buckthorn (Hippophaë rhamnoides L.) berry pomace fractions consecutively recovered by pressurized ethanol and water. Antioxidants 2020, 9, 274. [Google Scholar] [CrossRef]
- Rop, O.; Reznicek, V.; Mlcek, J.; Juríková, T.; Balík, J. Nutritional values of new Czech cultivars of Saskatoon berries (Amelanchier alnifolia Nutt.). Hortic. Sci. 2012, 39, 123–128. [Google Scholar] [CrossRef]
- Sun, L.L.; Gao, W.; Zhang, M.M.; Li, C.; Wang, A.G.; Su, Y.L.; Ji, T.F. Composition and antioxidant activity of the anthocyanins of the fruit of Berberis heteropoda Schrenk. Molecules 2014, 19, 19078–19096. [Google Scholar] [CrossRef]
- Di, X.; Liu, C.T.; Gao, L.; Abbas, A. Research on Berberis nummularia Bge. Pigment extraction and its stability. Food Ferm. Ind. 2010, 36, 184–189. [Google Scholar]
- Garcia-Diaz, D.F.; Jimenez, P.; Reyes-Farias, M.; Soto-Covasich, J.; Costa, A.G.V. A review of the potential of Chilean Native Berries in the treatment of obesity and its related features. Plant Food. Hum. Nutr. 2019, 74, 277–286. [Google Scholar] [CrossRef]
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Barrio, D.A.; Carrillo, W. Argentine Patagonia barberry chemical composition and evaluation of its antioxidant capacity. J. Food Biochem. 2020, 44, e13254. [Google Scholar] [CrossRef]
- Fredes, C.; Parada, A.; Salinas, J.; Rober, P. Phytochemicals and traditional use of two Southernmost Chilean berry fruits: Murta (Ugni molinae Turcz) and Calafate (Berberis buxifolia Lam.). Foods 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turahun, Y.; Muhammad, T. Ultrasonic extraction technology and DPPH·scavenging properties of red pigment in Berberis iliensis Popov. fruit. Food Res. Dev. 2013, 34, 45–49. [Google Scholar]
- Li, L.Y.; Fang, Z.Y.; Alkebaier, M.; Zhou, L.; Lu, B. Effects of natural drought stress on growth, physiological and biochemical indexes of black fruit and red fruit berberis seedling. N. Hortic. 2020, 4, 80–86. [Google Scholar]
- Yakup, O.; Paitulamu, M.; Feng, C.R.; Abulimiti, R.; Zhang, X.H.; Abulaiti, D.; Shayibuzhati, M. Protective effect of Berberis atrocarpa on liver injury caused by carbon tetrachloride in mice. AHFS 2017, 38, 1–3. [Google Scholar]
- Sun-Waterhouse, D.; Teoh, A.; Massarotto, C.; Wibisono, R.; Wadhwa, S. Comparative analysis of fruit-based functional snack bars. Food Chem. 2010, 119, 1369–1379. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, J.; Liu, C.; Jiang, J.; Yang, H.; Li, J. Genetic characteristics and QTL analysis of the soluble sugar content in ripe tomato fruits. Sci. Hortic. 2021, 276, 109785. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Ramchoun, M.; Alem, C.; Ghafoor, K.; Ennassir, J.; Zegzouti, Y.F. Functional composition and antioxidant activities of eight Moroccan date fruit varieties (Phoenix dactylifera L.). J. Saudi Soc. Agric. Sci. 2017, 16, 257–264. [Google Scholar] [CrossRef]
- Wu, J.; Fan, J.; Li, Q.; Jia, L.; Xu, L.; Wu, X.; Yin, H. Variation of organic acids in mature fruits of 193 pear (Pyrus spp.) cultivars. J. Food Compos. Ana. 2022, 109, 104483. [Google Scholar] [CrossRef]
- Kookal, S.K.; Thimmaiah, A. Nutritional composition of staple food Bananas of three cultivars in India. Am. J. Plant Sci. 2018, 9, 2480–2493. [Google Scholar] [CrossRef]
- Pu, Y.; Ding, T.; Wang, W.; Xiang, Y.; Ye, X.; Li, M.; Liu, D. Effect of harvest, drying and storage on the bitterness, moisture, sugars, free amino acids and phenolic compounds of jujube fruit (Zizyphus jujuba cv. Junzao). J. Sci. Food Agric. 2018, 98, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.; Lv, F.; Chen, S.; Chen, J.; Liu, D.; Ye, X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purplefleshed potatoes. Food Chem. 2016, 197, 1264–1270. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, Y.; Lü, Y.; Ma, G.; Chen, J.; Liu, D.; Ye, X. Phenolic compounds and antioxidant capacities of bayberry juices. Food Chem. 2009, 113, 884–888. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Q.; Rakariyatham, K.; Cao, Y.; Goulette, T.; Liu, X.; Xiao, H. Antioxidation and anti-ageing activities of different stereoisomeric astaxanthin in vitro and in vivo. J. Func. Foods 2016, 25, 50–61. [Google Scholar] [CrossRef]
- Deng, L.L.; Pan, X.Q.; Sheng, J.P.; Shen, L. Optimization of experimental conditions for the determination of water soluble protein in apple pulp using Coomassie Brilliant Blue Method. Food Sci. 2012, 33, 185–189. [Google Scholar]
- Çakır, Ö.; Karabulut, A. Comparison of two wild-grown Berberis varieties based on biochemical characterization. J. Food Process. Preserv. 2020, 44, e14844. [Google Scholar] [CrossRef]
- Rybicka, I.; Kiewlicz, J.; Kowalczewski, P.Ł.; Gliszczyńska-Świgło, A. Selected dried fruits as a source of nutrients. Eur. Food Res. Technol. 2021, 247, 2409–2419. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef]
- Ye, X.Q.; Ren, Y.M.; Chen, J.L.; Pan, H.B.; Tian, J.H.; Chen, S.G. The new nutritional function of fruit and vegetable: Prebiotic. J. Chin. Inst. Food Sci. Tech. 2021, 21, 1–10. [Google Scholar]
- Rahimi-Madiseh, M.; Gholami-Arjenaki, M.; Bahmani, M.; Mardani, G.; Farzan, M.; RafieianKopaei, M. Evaluation of minerals, phenolics and anti-radical activity of three species of Iranian berberis fruit. Der Pharma Chem. 2016, 8, 191–197. [Google Scholar]
- Chen, L.F.; Sun, Y.P.; Niu, G.H.; Liu, Q.; Altland, J. Relative salt tolerance of eight Japanese barberry cultivars. HortScience 2017, 52, 1810–1815. [Google Scholar] [CrossRef]
- Zhou, X.L.; Liang, J.Y.; Zhou, Z.L. Effects of salt stress on seed germination of Berberis nummularia Bge. Heilongjiang Agric. Sci. 2017, 2, 111–116. [Google Scholar]
- Daood, H.G.; Biacs, P.A.; Dakar, M.A.; Hajdu, F. Ion-pair chromatography and photodiode-array detection of Vitamin C and organic acids. J. Chromatogr. Sci. 1994, 32, 481–487. [Google Scholar] [CrossRef]
- Scherer, R.; Rybka, A.C.P.; Ballus, C.A.; Meinhart, A.D.; Filho, J.T.; Godoy, H.T. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012, 135, 150–154. [Google Scholar] [CrossRef]
- Ma, B.Q.; Chen, J.; Zheng, H.Y.; Fang, T.; OgutU, C.; Li, S.H.; Han, Y.P.; Wu, B.H. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Zhou, G.; Wei, Q.; Hu, H.; Peng, S. Identification of organic acid-related genes and their expression profiles in two pear (Pyrus pyrofolia) cultivars with difference in predominant acid type at fruit ripening stage. Sci. Hortic. 2011, 129, 680–687. [Google Scholar] [CrossRef]
- Glew, R.H.; Ayaz, F.A.; Sanz, C.; VanderJagt, D.J.; Huang, H.S.; Chuang, L.T.; Strnad, M. Effect of postharvest period on sugars, organic acids and fatty acids composition in commercially sold medlar (Mespilus germanica Dutch) fruit. Eur. Food Res. Technol. 2003, 216, 390–394. [Google Scholar] [CrossRef]
- Aliakbarlu, J.; Ghiasi, S.; Bazargani-Gilani, B. Effect of extraction conditions on antioxidant activity of barberry (Berberis vulgaris L.) fruit extracts. Vet. Res. Forum. 2018, 9, 361–365. [Google Scholar]
- Okatan, V.; Çolak, A.M. Chemical and phytochemicals content of barberry (Berberis vulgaris L.) fruit genotypes from Sivasli district of Usak province of western Turkey. Pak. J. Bot. 2019, 51, 165–170. [Google Scholar] [CrossRef]
- Ruiz, A.; Zapata, M.; Sabando, C.; Bustamante, L.; von Baer, D.; Vergara, C.; Mardones, C. Flavonols, alkaloids, and antioxidant capacity of edible wild Berberis species from patagonia. J. Agric. Food Chem. 2014, 62, 12407–12417. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh-Moghadam, N.; Hosseini, B.; Alirezalu, A. Classification of barberry genotypes by multivariate analysis of biochemical constituents and HPLC profiles. Phytochem. Anal. 2019, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, H.; Alizadeh, S. Evaluation of phenolic compound, antioxidant activities and antioxidant enzymes of barberry genotypes in Iran. Sci. Hortic. 2016, 200, 125–130. [Google Scholar] [CrossRef]
- Zovko Končić, M.; Kremer, D.; Schühly, W.; Brantner, A.; Karlović, K.; Kalođera, Z. Chemical differentiation of Berberis croatica and B. vulgaris using HPLC fingerprinting. Croat. Chem. Acta 2010, 83, 451–456. [Google Scholar]
- Ismail, B.B.; Pu, Y.; Guo, M.; Ma, X.; Liu, D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chem. 2019, 277, 279–288. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Desseva, I.; Manolov, I.; Petkova, N.; Vrancheva, R.; Peltekov, A.; Slavov, A.; Zhivondov, A. Comprehensive evaluation of late season peach varieties (Prunus persica L.): Fruit nutritional quality and phytochemicals. Molecules 2021, 26, 2818. [Google Scholar] [CrossRef]
- Wang, R.M.; Wang, L.; Zhang, L.; Wan, S.T.; Li, C.F.; Liu, S.X. Solvents effect on phenolics, iridoids, antioxidant activity, antibacterial activity, and pancreatic lipase inhibition activity of noni (Morinda citrifolia L.) fruit extract. Food Chem. 2022, 377, 131989. [Google Scholar] [CrossRef]


| Fruits | B. nummularia | B. atrocarpa |
|---|---|---|
| Protein [g/100 g d.m. 1] | 3 ± 0.1 a | 4 ± 0.1 b |
| Fat [g/100 g d.m. 1] | 7 ± 0.4 a | 5 ± 0.1 b |
| Fiber [g/100 g d.m. 2] | 16 ± 1.0 a | 18 ± 1.4 a |
| Ash [g/100 g d.m. 1] | 1 ± 0.1 a | 1 ± 0.0 a |
| Soluble sugar [g/100 g d.m. 1] | 23 ± 0.6 a | 12 ± 1.5 b |
| Fructose (mg/g d.m. 1) | 36 ± 0.2 a | 6 ± 0.1 b |
| Glucose (mg/g d.m. 1) | 29 ± 0.1 a | 10 ± 0.0 b |
| Titratable acid (mg/g d.m. 1) | 18 ± 2.5 a | 14 ± 1.3 b |
| Carbohydrates [g/100 g d.m. 2] | 57 ± 1.8 a | 56 ± 1.8 a |
| Energy value [Kcal/100 g d.m. 3] | 330.86 a | 314.41 b |
| Fruits | Macroelements [mg/100 g d.m. 1] | Microelements [mg/100 g d.m. 1] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | K | Na | P | Mg | Zn | Cd | Fe | Cu | Mn | |
| 2 NRV | 800 | 2000 | - | 700 | 375 | 15 | - | 15 | 1 | 2 |
| B. nummularia | 160 ± 16.7 a | 1380 ± 13.6 a | 198 ± 16.6 a | 109 ± 4.3 a | 109± 1.5 a | 6.4 ± 0.4 a | 0 | 0 b | 0 b | 1 ± 0.0 a |
| B. atrocarpa | 132 ± 11.7 b | 1225 ± 10.6 b | 18 ± 1.4 b | 93 ± 1.3 b | 58± 0.9 b | 2 ± 0.3 b | 0 | 10 ± 0.3 a | 1 ± 0.0 a | 1 ± 0.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudureheman, B.; Zhou, X.; Shu, X.; Chai, Z.; Xu, Y.; Li, S.; Tian, J.; Pan, H.; Ye, X. Evaluation of Biochemical Properties, Antioxidant Activities and Phenolic Content of Two Wild-Grown Berberis Fruits: Berberis nummularia and Berberis atrocarpa. Foods 2022, 11, 2569. https://doi.org/10.3390/foods11172569
Abudureheman B, Zhou X, Shu X, Chai Z, Xu Y, Li S, Tian J, Pan H, Ye X. Evaluation of Biochemical Properties, Antioxidant Activities and Phenolic Content of Two Wild-Grown Berberis Fruits: Berberis nummularia and Berberis atrocarpa. Foods. 2022; 11(17):2569. https://doi.org/10.3390/foods11172569
Chicago/Turabian StyleAbudureheman, Buhailiqiemu, Xinyue Zhou, Xipan Shu, Ziqi Chai, Yongping Xu, Shuying Li, Jinhu Tian, Haibo Pan, and Xingqian Ye. 2022. "Evaluation of Biochemical Properties, Antioxidant Activities and Phenolic Content of Two Wild-Grown Berberis Fruits: Berberis nummularia and Berberis atrocarpa" Foods 11, no. 17: 2569. https://doi.org/10.3390/foods11172569
APA StyleAbudureheman, B., Zhou, X., Shu, X., Chai, Z., Xu, Y., Li, S., Tian, J., Pan, H., & Ye, X. (2022). Evaluation of Biochemical Properties, Antioxidant Activities and Phenolic Content of Two Wild-Grown Berberis Fruits: Berberis nummularia and Berberis atrocarpa. Foods, 11(17), 2569. https://doi.org/10.3390/foods11172569






