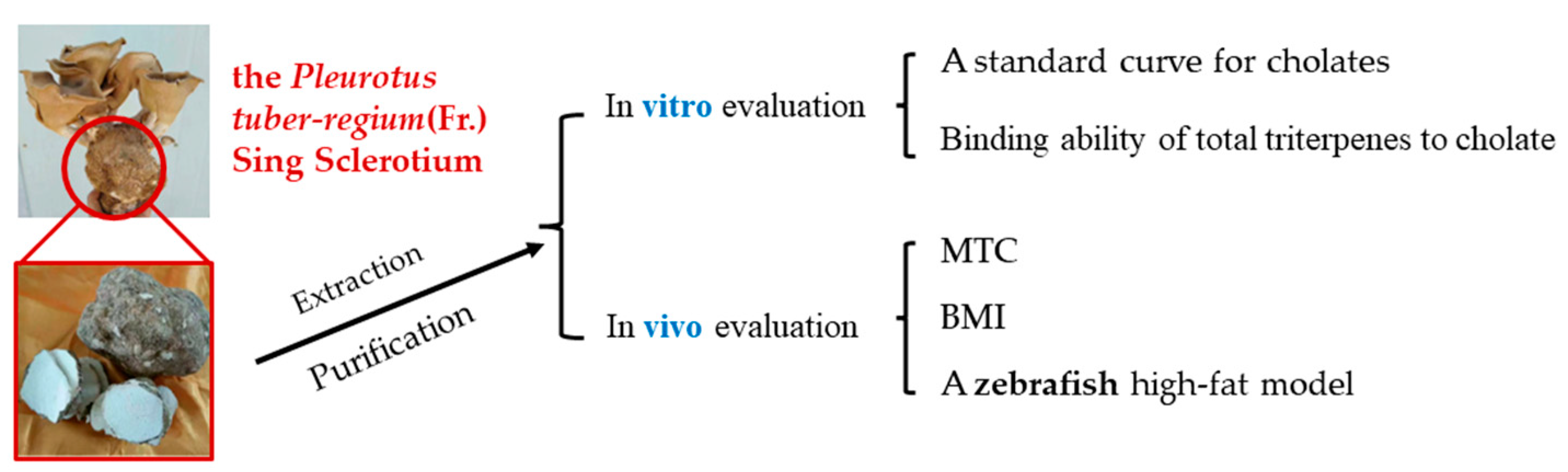
Extraction of a Triterpene Solution and Evaluation of the Hypolipidemic Efficacy of the Pleurotus tuber-regium (Fr.) Sing Sclerotium
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatuses
2.2. Extraction of the Triterpene Solution
2.3. Establishment of a Standard Curve for Cholates
2.4. Binding Ability of Total Triterpenes to Cholate
2.5. Zebrafish Breeding
2.6. Measurement of Maximum Tolerated Concentration (MTC) and Body Mass Index (BMI)
2.7. Construction of a High-Fat Zebrafish Model
2.8. Statistical Analysis Methods
3. Results
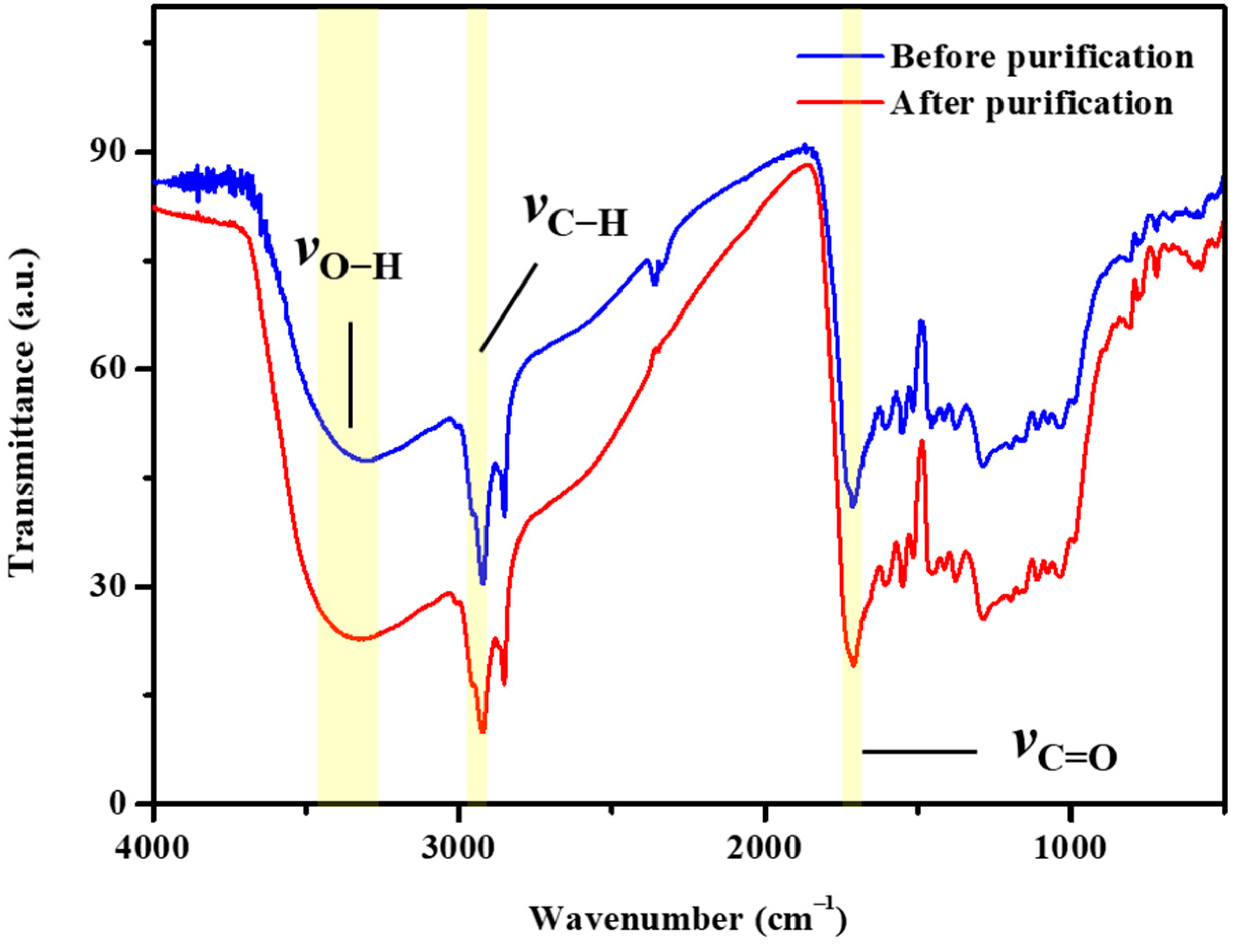
3.1. Analysis of Infrared Spectrograms
3.2. Analysis of the In Vitro Evaluation
3.3. Analysis of the In Vivo Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, X.; Xu, W.; Zhang, L.; Li, X.; Wang, R.; Wu, S. Impact of Gut Microbiota and Microbiota-Related Metabolites on Hyperlipidemia. Front. Cell. Infect. Microbiol. 2021, 11, 634780. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, K.; Mishra, N. Nanoparticulate carrier system: A novel treatment approach for hyperlipidemia. Drug Deliv. 2016, 23, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, Y.; Gu, W.; Xu, Q. Response of vascular mesenchymal stem/progenitor cells to hyperlipidemia. Cell. Mol. Life Sci. 2018, 75, 4079–4091. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D. Lipid-Lowering drugs in the management of hyperlipidaemia. Pharmacol. Ther. 1998, 79, 205–230. [Google Scholar] [CrossRef]
- Rauf, A.; Akram, M.; Anwar, H.; Daniyal, M.; Munir, N.; Bawazeer, S.; Bawazeer, S.; Rebezov, M.; Bouyahya, A.; Shariati, M.A.; et al. Therapeutic potential of herbal medicine for the management of hyperlipidemia: Latest updates. Environ. Sci. Pollut. R. 2022, 29, 40281–40301. [Google Scholar] [CrossRef]
- Jakopovic, B.; Oršolić, N.; Jakopovich, I. Proteomic Research on the Antitumor Properties of Medicinal Mushrooms. Molecules 2021, 26, 6708. [Google Scholar] [CrossRef]
- De Silva, D.D.; Rapior, S.; Hyde, K.D.; Bahkali, A.H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012, 56, 1–29. [Google Scholar] [CrossRef]
- Jeitler, M.; Michalsen, A.; Frings, D.; Hübner, M.; Fischer, M.; Koppold-Liebscher, D.A.; Murthy, V.; Kessler, C.S. Significance of medicinal mushrooms in integrative oncology: A narrative review. Front. Pharmacol. 2020, 11, 580656. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Yap, H.Y.; Muria-Gonzalez, M.J.; Kong, B.; Stubbs, K.A.; Tan, C.; Ng, S.; Tan, N.; Solomon, P.S.; Fung, S.; Chooi, Y. Heterologous expression of cytotoxic sesquiterpenoids from the medicinal mushroom Lignosus rhinocerotis in yeast. Microb. Cell Fact. 2017, 16, 103. [Google Scholar] [CrossRef]
- Jiang, N.; Hu, S.; Peng, B.; Li, Z.; Yuan, X.; Xiao, S.; Fu, Y. Genome of ganoderma species provides insights into the evolution, conifers substrate utilization, and terpene synthesis for ganoderma tsugae. Front. Microbiol. 2021, 12, 724451. [Google Scholar] [CrossRef] [PubMed]
- Vahid, H.; Rakhshandeh, H.; Ghorbani, A. Antidiabetic properties of Capparis spinosa L. And its components. Biomed. Pharmacother. 2017, 92, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, C.; Liang, Y.; Yeh, W.; Wang, H.; Yu, T.; Peng, R.Y. Alpinia zerumbet Potentially Elevates High-Density Lipoprotein Cholesterol Level in Hamsters. J. Agr. Food Chem. 2008, 56, 4435–4443. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Frota, J.T.; Arruda, B.R.; de Melo, T.S.; Da, S.A.; Brito, G.A.; Chaves, M.H.; Rao, V.S. Antihyperglycemic and hypolipidemic effects of alpha, beta-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis. 2012, 11, 98. [Google Scholar] [CrossRef]
- Andreadou, I.; Mitakou, S.; Paraschos, S.; Efentakis, P.; Magiatis, P.; Kaklamanis, L.; Halabalaki, M.; Skaltsounis, L.; Iliodromitis, E.K. “Pistacia lentiscus L.” reduces the infarct size in normal fed anesthetized rabbits and possess antiatheromatic and hypolipidemic activity in cholesterol fed rabbits. Phytomedicine 2016, 23, 1220–1226. [Google Scholar] [CrossRef]
- Amoakwah, E.; Arthur, E.; Frimpong, K.A.; Lorenz, N.; Rahman, M.A.; Nziguheba, G.; Islam, K.R. Biochar amendment impacts on microbial community structures and biological and enzyme activities in a weathered tropical sandy loam. Appl. Soil Ecol. 2022, 172, 104364. [Google Scholar] [CrossRef]
- Pandit, A.; Kochar, M.; Srivastava, S.; Johny, L.; Adholeya, A. Diversity and functionalities of unknown mycorrhizal fungal microbiota. Microbiol. Res. 2022, 256, 126940. [Google Scholar] [CrossRef]
- Afifa; Halabalaki, M.; Baqar, Z.; Mumtaz, M.; El-Sappah, A.H.; Show, P.L.; Iqbal, H.M.N.; Varjani, S.; Bilal, M. Bioprospecting fungal-derived value-added bioproducts for sustainable pharmaceutical applications. Sustain. Chem. Pharm. 2022, 29, 100755. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Contreras, M.D.M.; Romero, I.; Castro, E. Sequential extraction of hydroxytyrosol, mannitol and triterpenic acids using a green optimized procedure based on ultrasound. Antioxidants 2021, 10, 1781. [Google Scholar] [CrossRef]
- Oludemi, T.; Barros, L.; Prieto, M.A.; Heleno, S.A.; Barreiro, M.F.; Ferreira, I. Extraction of triterpenoids and phenolic compounds from Ganoderma lucidum: Optimization study using the response surface methodology. Food Funct. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Puttarak, P.; Panichayupakaranant, P. A new method for preparing pentacyclic triterpene rich Centella asiatica extracts. Nat. Prod. Res. 2013, 27, 684–686. [Google Scholar] [CrossRef]
- Charles Dorni, A.I.; Peter, G.; Jude, S.; Arundhathy, C.A.; Jacob, J.; Amalraj, A.; Pius, A.; Gopi, S. UHPLC-Q-ToF-MS-guided enrichment and purification of triterpenoids from Centella asiatica (L.) extract with macroporous resin. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 13–25. [Google Scholar] [CrossRef]
- Li, M.; Guo, L.; Zhu, R.; Yang, D.; Xiao, Y.; Wu, Y.; Zhong, K.; Huang, Y.; Gao, H. Effect of fixation methods on biochemical characteristics of green teas and their Lipid-Lowering effects in a zebrafish larvae model. Foods 2022, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhong, K.; Bai, J.; Wu, Y.; Zhang, J.; Gao, H. The biochemical characteristics of a novel fermented loose tea by Eurotium cristatum (MF800948) and its hypolipidemic activity in a zebrafish model. LWT 2020, 117, 108629. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Y.; Zhong, K.; Gao, H. Comprehensive evaluation of the composition of Mingshan Laochuancha green tea and demonstration of hypolipidemic activity in a zebrafish obesity model. RSC Adv. 2019, 9, 41269–41279. [Google Scholar] [CrossRef]
- Dai, X.; Pu, D.; Wang, L.; Cheng, X.; Liu, X.; Yin, Z.; Wang, Z. Emergence of breeding tubercles and puberty onset in male zebrafish: Evidence for a dependence on body growth. J. Fish Biol. 2021, 99, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Yoganantharjah, P.; Byreddy, A.R.; Fraher, D.; Puri, M.; Gibert, Y. Rapid quantification of neutral lipids and triglycerides during zebrafish embryogenesis. Int. J. Dev. Biol. 2017, 61, 105–111. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Ren, W.; Liu, J.; Irudayaraj, J. Optical clearing delivers ultrasensitive hyperspectral Dark-Field imaging for Single-Cell evaluation. ACS Nano 2016, 10, 3132–3143. [Google Scholar] [CrossRef]
- Schlegel, A. Zebrafish models for dyslipidemia and atherosclerosis research. Front. Endocrinol. 2016, 7, 159. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Qi, Z.; Liu, X.; Li, W.; Wang, S. A study of Ganoderma lucidum spores by FTIR microspectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 91, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Ma, Y.; Muhammad, M.; Huang, Q. Understanding the infrared and Raman spectra of ganoderic acid a: An experimental and DFT study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, Z.; Mo, L.; Wu, T.; Tan, C. Near-Infrared spectroscopy as a diagnostic tool for distinguishing between normal and malignant colorectal tissues. Biomed Res. Int. 2015, 2015, 472197. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yan, N.; Li, G. The effect of in vitro gastrointestinal digestion on the antioxidants, antioxidant activity, and hypolipidemic activity of green jujube vinegar. Foods 2022, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Gao, W.; Li, X.; Luo, S.; Chye, F.Y. Study on the hypolipidemic properties of garlic polysaccharide in vitro and in normal mice as well as its dyslipidemia amelioration in type2 diabetes mice. Food Biosci. 2022, 47, 101683. [Google Scholar] [CrossRef]
- Zeng, X.; Sheng, Z.; Li, X.; Fan, X.; Jiang, W. In vitro studies on the interactions of blood lipid level-related biological molecules with gallic acid and tannic acid. J. Sci. Food Agr. 2019, 99, 6882–6892. [Google Scholar] [CrossRef]


| Sodium Glycocholate (μmol/100 mg) | Binding Rate (%) | Sodium Taurocholate (μmol/100 mg) | Binding Rate (%) | Sodium Cholate (μmol/100 mg) | Binding Rate (%) | |
|---|---|---|---|---|---|---|
| PTRSS | 1.39 ± 0.07 | 50.93 ± 3.34 | 1.42 ± 0.07 | 52.14 ± 4.07 | 1.68 ± 0.03 | 43.06 ± 1.71 |
| Cholestyramine | 2.73 ± 0.04 | 100.00 ± 1.57 | 2.73 ± 0.09 | 100.00 ± 3.11 | 3.89 ± 0.08 | 100.00 ± 2.02 |
| Group | Concentration of the Total Triterpenoids (µg/mL) | Number of Deaths | Mortality (%) | Body Changes |
|---|---|---|---|---|
| Blank | - | 0 | 0 | - |
| Triterpenes | 100.00 | 0 | 0 | - |
| 300.00 | 0 | 0 | - | |
| 500.00 | 0 | 0 | - | |
| 700.00 | 1 | 6.67 | - | |
| 900.00 | 6 | 40.00 | Bend |
| Group | Concentration of the Total Triterpenoids (µg/mL) | Body Weight (mg) | Body Length (mm) | BMI (mg/mm2) |
|---|---|---|---|---|
| Blank | - | 20.29 ± 0.73 d | 10.97 ± 0.68 b | 0.17 ± 0.02 b |
| High-fat | - | 22.65 ± 0.65 a | 11.23 ± 0.54 a | 0.18 ± 0.02 a |
| High-fat triterpene | 100.00 | 21.17 ± 0.73 b | 10.85 ± 0.31 c | 0.18 ± 0.01 a |
| 300.00 | 20.73 ± 0.47 c | 10.71 ± 0.25 c | 0.18 ± 0.01 a | |
| 500.00 | 20.36 ± 0.72 d | 10.84 ± 0.38 c | 0.17 ± 0.02 b |
| Group | Concentration of the Total Triterpenoids (µg/mL) | Area | Integrated Optical Density (IOD) | Average Integrated Optical Density (AIOD) | Lipid-Lowering Effect (%) |
|---|---|---|---|---|---|
| High-fat | 0 | 159,371 ± 261.34 | 1053.34 ± 23.68 | 0.0066 ± 0.00014 a | - |
| High-fat triterpene | 100.00 | 134,051 ± 458.87 | 783.81 ± 34.89 | 0.0058 ± 0.00019 b | 11.54 ± 1.05 a |
| 300.00 | 126,218 ± 404.06 | 683.51 ± 33.17 | 0.0054 ± 0.00027 b | 19.27 ± 4.16 a | |
| 500.00 | 124,247 ± 539.63 | 573.59 ± 32.93 | 0.0046 ± 0.00028 c | 32.96 ± 3.29 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, Y.; Lan, Y.; Yuan, J. Extraction of a Triterpene Solution and Evaluation of the Hypolipidemic Efficacy of the Pleurotus tuber-regium (Fr.) Sing Sclerotium. Foods 2022, 11, 2881. https://doi.org/10.3390/foods11182881
Wang C, Liu Y, Lan Y, Yuan J. Extraction of a Triterpene Solution and Evaluation of the Hypolipidemic Efficacy of the Pleurotus tuber-regium (Fr.) Sing Sclerotium. Foods. 2022; 11(18):2881. https://doi.org/10.3390/foods11182881
Chicago/Turabian StyleWang, Chao, Yuan Liu, Yuanhong Lan, and Jianing Yuan. 2022. "Extraction of a Triterpene Solution and Evaluation of the Hypolipidemic Efficacy of the Pleurotus tuber-regium (Fr.) Sing Sclerotium" Foods 11, no. 18: 2881. https://doi.org/10.3390/foods11182881
APA StyleWang, C., Liu, Y., Lan, Y., & Yuan, J. (2022). Extraction of a Triterpene Solution and Evaluation of the Hypolipidemic Efficacy of the Pleurotus tuber-regium (Fr.) Sing Sclerotium. Foods, 11(18), 2881. https://doi.org/10.3390/foods11182881





