Characterization of Botanical Origin of Italian Honey by Carbohydrate Composition and Volatile Organic Compounds (VOCs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey Collection and Sampling
2.2. Chemicals and Reagents
2.3. Melissopalynological Analysis
2.4. Sugars Analysis
2.5. Volatile Organic Compounds Analysis
2.5.1. Extraction Conditions and Sample Preparation
2.5.2. Gas Chromatography–Mass Spectrometry
2.6. Statistical Analysis
3. Results
3.1. Melissopalynological Data
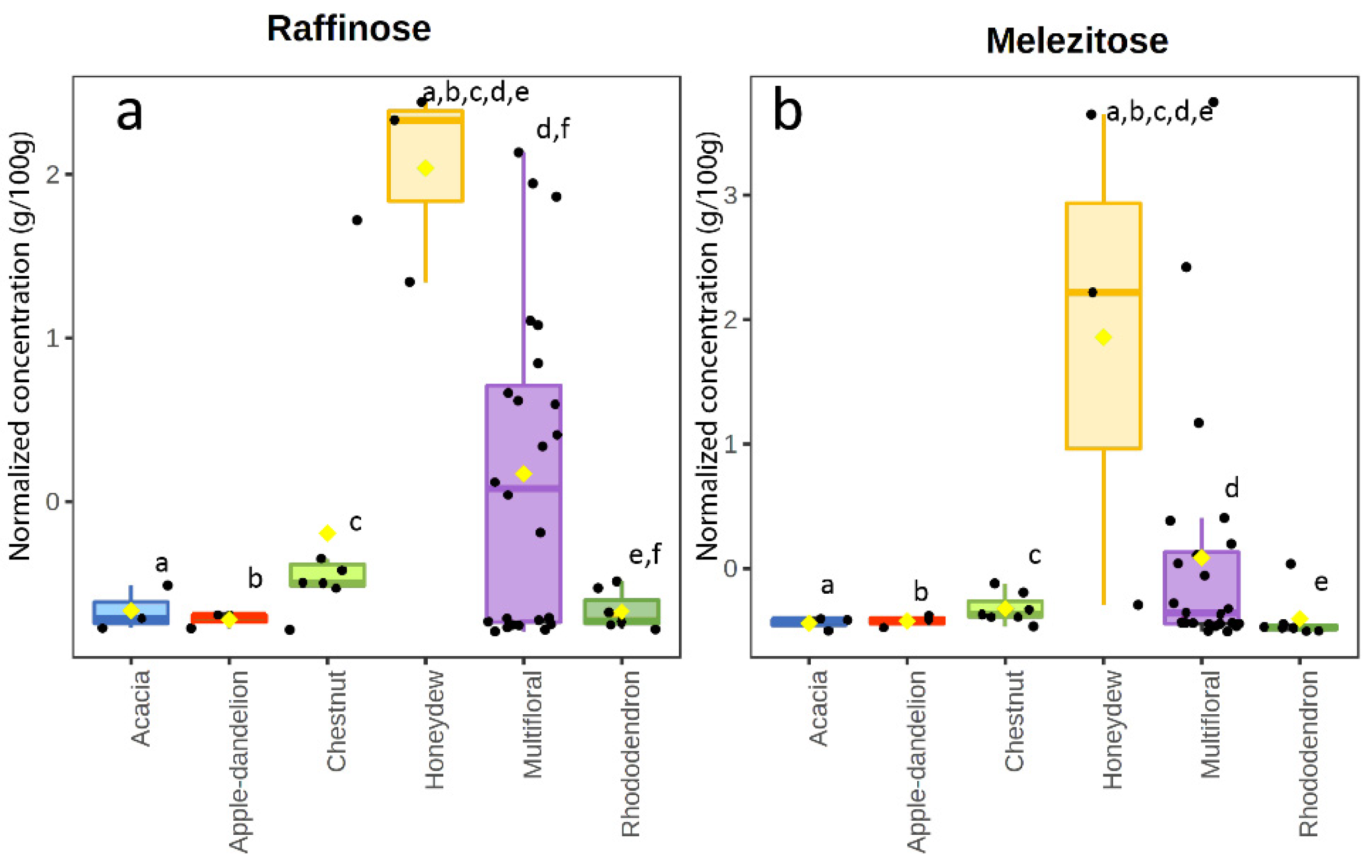
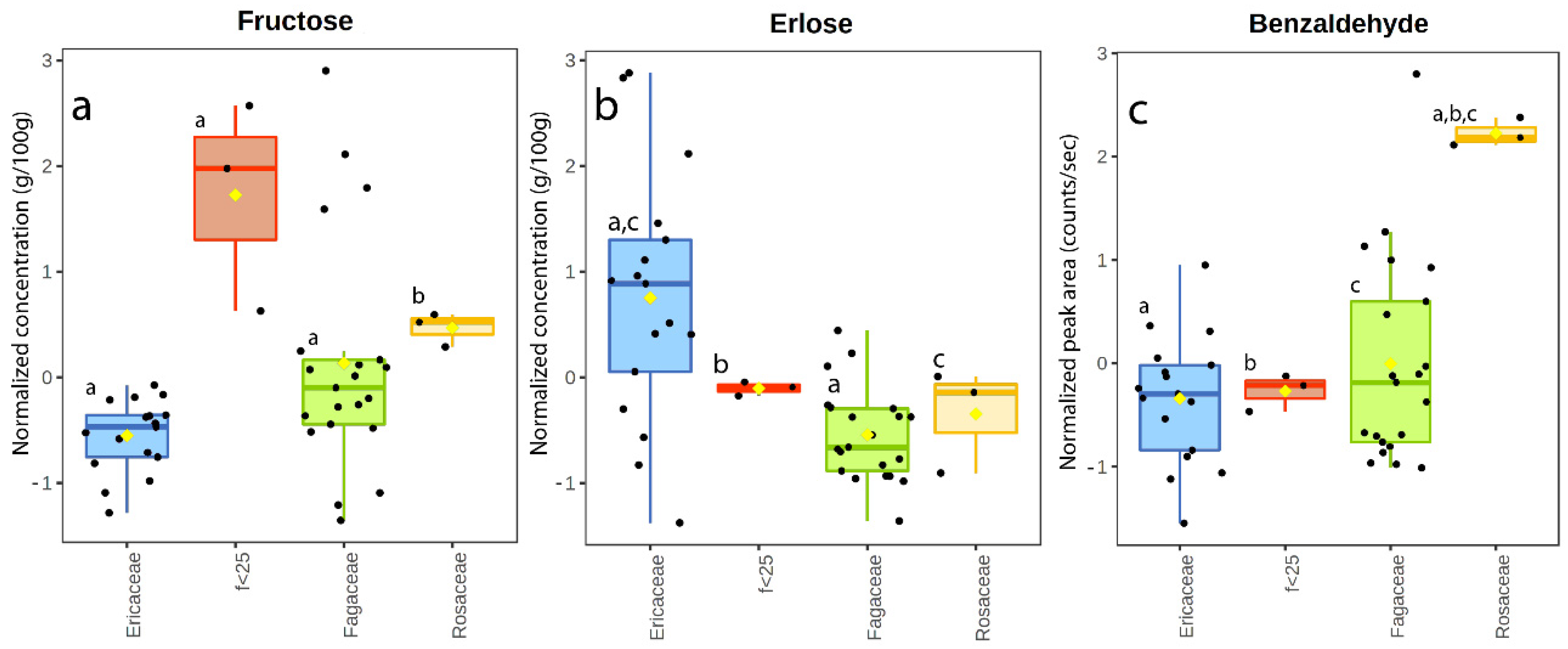
3.2. Sugar Content
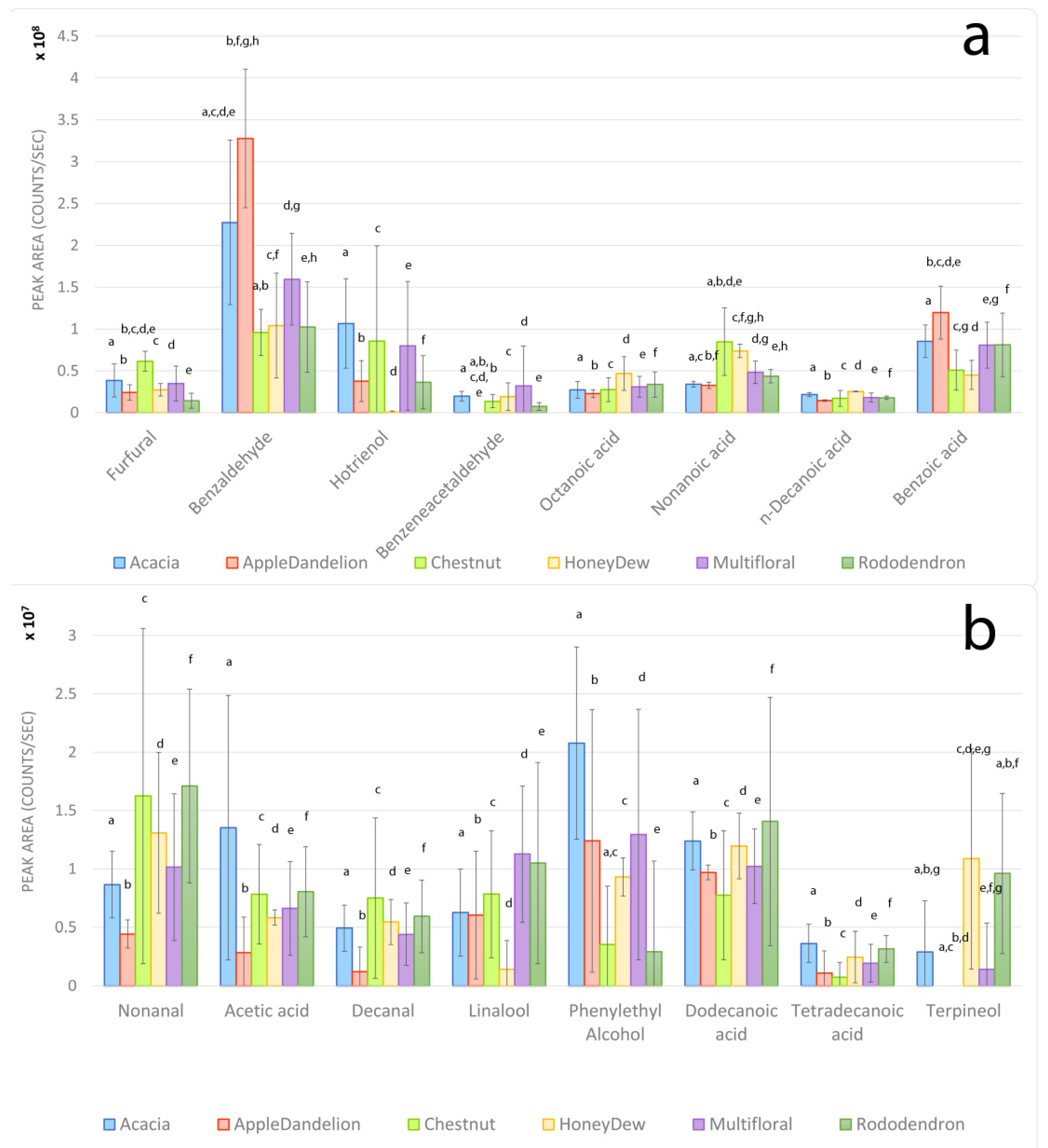
3.3. Volatile Organic Compounds
4. Discussion
4.1. Acacia Honeys
4.2. Apple–Dandelion Honeys
4.3. Rhododendron Honeys
4.4. Honeydew Honeys
4.5. Chestnut Honeys
4.6. Multifloral Honeys
4.7. Overall Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Qassemi, R.; Robinson, R.K. Some Special Nutritional Properties of Honey—A Brief Review. Nutr. Food Sci. 2003, 33, 254–260. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Codex Alimentarius: Standards for Honey; FAO: Roma, Italy, 1981. [Google Scholar]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of Honey Criteria and Standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Venskutonis, P.R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Di Marco, G.; Manfredini, A.; Leonardi, D.; Canuti, L.; Impei, S.; Gismondi, A.; Canini, A. Geographical, Botanical and Chemical Profile of Monofloral Italian Honeys as Food Quality Guarantee and Territory Brand. Plant Biosyst. 2017, 151, 450–463. [Google Scholar] [CrossRef]
- Beckh, G.; Camps, G. Neue Spezifikationen Für Trachthonige. Dtsch. Leb. 2009, 105, 105–110. [Google Scholar]
- Persano Oddo, L.; Piazza, M.G.; Sabatini, A.G.; Accorti, M. Review Article Characterization of Unifloral Honeys. Apidologie 1995, 26, 453–465. [Google Scholar] [CrossRef]
- Conti, M.E.; Stripeikis, J.; Campanella, L.; Cucina, D.; Tudino, M.B. Characterization of Italian Honeys (Marche Region) on the Basis of Their Mineral Content and Some Typical Quality Parameters. Chem. Cent. J. 2007, 1, 14. [Google Scholar] [CrossRef]
- Ente nazionale italiano di unificazione UNI 11382:2010 Miele Di Acacia (Robinia pseudoacacia L.)—Definizione, Requisiti e Metodi Di Analisi 2010.
- European Commission Council Directive 2001/110/EC. Off. J. Eur. Communities 2002. Available online: https://www.fao.org/faolex/results/details/zh/c/LEX-FAOC037441/ (accessed on 15 December 2021).
- Tornuk, F.; Karaman, S.; Ozturk, I.; Toker, O.S.; Tastemur, B.; Sagdic, O.; Dogan, M.; Kayacier, A. Quality Characterization of Artisanal and Retail Turkish Blossom Honeys: Determination of Physicochemical, Microbiological, Bioactive Properties and Aroma Profile. Ind. Crops Prod. 2013, 46, 124–131. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Giampieri, F.; Battino, M. Honey as a Source of Dietary Antioxidants: Structures, Bioavailability and Evidence of Protective Effects Against Human Chronic Diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, A.; Chamunorwa, J.P.; Erlwanger, K.H. Nutraceutical Values of Natural Honey and Its Contribution to Human Health and Wealth. Nutr. Metab. 2012, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Schievano, E.; Morelato, E.; Facchin, C.; Mammi, S. Characterization of Markers of Botanical Origin and Other Compounds Extracted from Unifloral Honeys. J. Agric. Food Chem. 2013, 61, 1747–1755. [Google Scholar] [CrossRef]
- Opsenica, D.M.; Lušíc, D.; Teší, Ž. Modern Analytical Techniques in the Assessment of the Authenticity of Serbian Honey. Arh. Hig. Rada Toksikol. 2015, 66, 233–241. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; González-Paramás, A.M.; Santos-Buelga, C.; Morroni, G.; Simoni, S.; Forbes-Hernández, T.Y.; et al. Apis Mellifera vs Melipona Beecheii Cuban Polifloral Honeys: A Comparison Based on Their Physicochemical Parameters, Chemical Composition and Biological Properties. Lwt 2018, 87, 272–279. [Google Scholar] [CrossRef]
- Tedesco, R.; Barbaro, E.; Zangrando, R.; Rizzoli, A.; Malagnini, V.; Gambaro, A.; Fontana, P.; Capodaglio, G. Carbohydrate Determination in Honey Samples by Ion Chromatography–Mass Spectrometry (HPAEC-MS). Anal. Bioanal. Chem. 2020, 412, 5217–5227. [Google Scholar] [CrossRef]
- Da Costa Leite, J.M.; Trugo, L.C.; Costa, L.S.M.; Quinteiro, L.M.C.; Barth, O.M.; Dutra, V.M.L.; De Maria, C.A.B. Determination of Oligosaccharides in Brazilian Honeys of Different Botanical Origin. Food Chem. 2000, 70, 93–98. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of Botanical Origin and Sugar Composition of Honeys on the Crystallization Phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Kaškoniene, V.; Venskutonis, P.R.; Čeksteryte, V. Composition of Volatile Compounds of Honey of Various Floral Origin and Beebread Collected in Lithuania. Food Chem. 2008, 111, 988–997. [Google Scholar] [CrossRef]
- Patrignani, M.; Fagúndez, G.A.; Tananaki, C.; Thrasyvoulou, A.; Lupano, C.E. Volatile Compounds of Argentinean Honeys: Correlation with Floral and Geographical Origin. Food Chem. 2018, 246, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterization and Classification of Thymus capitatus (L.) Honey According to Geographical Origin Based on Volatile Compounds, Physicochemical Parameters and Chemometrics. Food Res. Int. 2014, 55, 363–372. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterisation and Classification of Greek Pine Honeys According to Their Geographical Origin Based on Volatiles, Physicochemical Parameters and Chemometrics. Food Chem. 2014, 146, 548–557. [Google Scholar] [CrossRef]
- Osservatorio nazionale miele Andamento Produttivo e Di Mercato Per La Stagione 2018; Il Valore Della Terra: Bologna, Italy, 2019; Volume 1.
- Bogdanov, S. Harmonised Methods of the International IHC. Bee Prod. Sci. 2009, 1–62. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 15 December 2021).
- Robotti, E.; Campo, F.; Riviello, M.; Bobba, M.; Manfredi, M.; Mazzucco, E.; Gosetti, F.; Calabrese, G.; Sangiorgi, E.; Marengo, E. Optimization of the Extraction of the Volatile Fraction from Honey Samples by SPME-GC-MS, Experimental Design, and Multivariate Target Functions. J. Chem. 2017, 2017, 6437857. [Google Scholar] [CrossRef]
- Bianchi, F.; Mangia, A.; Mattarozzi, M.; Musci, M. Characterization of the Volatile Profile of Thistle Honey Using Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Food Chem. 2011, 129, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Chong, J.; Xia, J. Comprehensive Meta-Analysis of Covid-19 Global Metabolomics Datasets. Metabolites 2021, 11, 44. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- PERSANO ODDO, L.; BOGDANOV, S. Field Trial of Honey Bee Colonies Bred for Mechanisms of Resistance against Varroa Destructor. Apidologie 2004, 35, S2–S3. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Brokl, M.; Soria, A.C.; Sanz, M.L.; Martínez-Castro, I. Gas Chromatographic-Mass Spectrometric Characterisation of Tri- and Tetrasaccharides in Honey. Food Chem. 2010, 120, 637–642. [Google Scholar] [CrossRef]
- Nayik, G.A.; Dar, B.N.; Nanda, V. Physico-Chemical, Rheological and Sugar Profile of Different Unifloral Honeys from Kashmir Valley of India. Arab. J. Chem. 2019, 12, 3151–3162. [Google Scholar] [CrossRef]
- Terrab, A.; Vega-Pérez, J.M.; Díez, M.J.; Heredia, F.J. Characterisation of Northwest Moroccan Honeys by Gas Chromatographic-Mass Spectrometric Analysis of Their Sugar Components. J. Sci. Food Agric. 2002, 82, 179–185. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Schweitzer, P.; Bachir Bey, M.; Djoudad-Kadji, H.; Louaileche, H. HPLC Sugar Profiles of Algerian Honeys. Food Chem. 2010, 121, 561–568. [Google Scholar] [CrossRef]
- De La Fuente, E.; Ruiz-Matute, A.I.; Valencia-Barrera, R.M.; Sanz, J.; Martínez Castro, I. Carbohydrate Composition of Spanish Unifloral Honeys. Food Chem. 2011, 129, 1483–1489. [Google Scholar] [CrossRef]
- Tezcan, F.; Kolayli, S.; Ulusoy, H.S.E.; Erim, F.B. Evaluation of Organic Acid, Saccharide Composition and Antioxidant Properties of Some Authentic Turkish Honeys. J. Food Nutr. Res. 2011, 50, 33–40. [Google Scholar]
- Cotte, J.F.; Casabianca, H.; Chardon, S.; Lheritier, J.; Grenier-Loustalot, M.F. Chromatographic Analysis of Sugars Applied to the Characterisation of Monofloral Honey. Anal. Bioanal. Chem. 2004, 380, 698–705. [Google Scholar] [CrossRef]
- Val, A.; Huidobro, J.F.; Sánchez, M.P.; Muniategui, S.; Fernández-Muiño, M.A.; Sancho, M.T. Enzymatic Determination of Galactose and Lactose in Honey. J. Agric. Food Chem. 1998, 46, 1381–1385. [Google Scholar] [CrossRef]
- Mateo, R.; Bosch-Reig, F. Sugar Profiles of Spanish Unifloral Honeys. Food Chem. 1997, 60, 33–41. [Google Scholar] [CrossRef]
- Lazarević, K.B.; Jovetić, M.S.; Tešić, Ž.L. Physicochemical Parameters as a Tool for the Assessment of Origin of Honey. J. AOAC Int. 2017, 100, 840–851. [Google Scholar] [CrossRef]
- Jerković, I.; Kuś, P.M. Terpenes in Honey: Occurrence, Origin and Their Role as Chemical Biomarkers. RSC Adv. 2014, 4, 31710–31728. [Google Scholar] [CrossRef]
- Tasdemir, D.; Demirci, B.; Demirci, F.; Dönmez, A.A.; Can Baser, K.H.; Rüedi, P. Analysis of the Volatile Components of Five Turkish Rhododendron Species by Headspace Solid-Phase Microextraction and GC-MS (HS-SPME-GC-MS). Z. Naturforsch.—Sect. C J. Biosci. 2003, 58, 797–803. [Google Scholar] [CrossRef]
- Vasić, V.; Đurđić, S.; Tosti, T.; Radoičić, A.; Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Two Aspects of Honeydew Honey Authenticity: Application of Advance Analytical Methods and Chemometrics. Food Chem. 2020, 305, 125457. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Vázquez, M. Differences between Honeydew and Blossom Honeys: A Review. Trends Food Sci. Technol. 2017, 59, 79–87. [Google Scholar] [CrossRef]
- Bogdanov, S.; Ruoff, S.; Oddo, L.P. Physico-Chemical Methods for the Characterisation of Unifloral Honeys: A Review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef]
- Doner, L.W. The Sugars of Honey—A Review. J. Sci. Food Agric. 1977, 28, 443–456. [Google Scholar] [CrossRef]
- Lombard, A.; Buffa, M.; Manino, A.; Patetta, A. Identification of Raffinose in Honeydew. Experientia 1984, 40, 178–180. [Google Scholar] [CrossRef]
- Piasenzotto, L.; Gracco, L.; Conte, L. Solid Phase Microextraction (SPME) Applied to Honey Quality Control. J. Sci. Food Agric. 2003, 83, 1037–1044. [Google Scholar] [CrossRef]
- Yang, Y.; Battesti, M.J.; Djabou, N.; Muselli, A.; Paolini, J.; Tomi, P.; Costa, J. Melissopalynological Origin Determination and Volatile Composition Analysis of Corsican “Chestnut Grove” Honeys. Food Chem. 2012, 132, 2144–2154. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.; Harizanis, P.C.; Polissiou, M. Investigation of Organic Extractives from Unifloral Chestnut (Castanea Sativa L.) and Eucalyptus (Eucalyptus Globulus Labill.) Honeys and Flowers to Identification of Botanical Marker Compounds. LWT—Food Sci. Technol. 2011, 44, 1042–1051. [Google Scholar] [CrossRef]
- Radovic, B.S.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Anklam, E. Contribution of Dynamic Headspace GC–MS Analysis of Aroma Compounds to Authenticity Testing of Honey. Food Chem. 2001, 72, 511–520. [Google Scholar] [CrossRef]
- Soria, A.C.; Martínez-Castro, I.; Sanz, J. Some Aspects of Dynamic Headspace Analysis of Volatile Components in Honey. Food Res. Int. 2008, 41, 838–848. [Google Scholar] [CrossRef]
- Pérez, R.A.; Sánchez-Brunete, C.; Calvo, R.M.; Tadeo, J.L. Analysis of Volatiles from Spanish Honeys by Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2002, 50, 2633–2637. [Google Scholar] [CrossRef] [PubMed]
- Apriceno, A.; Girelli, A.M.; Scuto, F.R.; Tarola, A.M. Determination of Furanic Compounds and Acidity for Italian Honey Quality. Flavour Fragr. J. 2018, 33, 411–419. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gonzaga, L.V.; de Azevedo, M.S.; Biluca, F.C.; Schulz, M.; Costa, A.C.O.; Fett, R. Stability of Volatile Compounds of Honey during Prolonged Storage. J. Food Sci. Technol. 2020, 57, 1167–1182. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Hegić, G.; Marijanović, Z.; Bubalo, D. Organic Extractives from Mentha Spp. Honey and the Bee-Stomach: Methyl Syringate, Vomifoliol, Terpenediol I, Hotrienol and Other Compounds. Molecules 2010, 15, 2911–2924. [Google Scholar] [CrossRef]
- Jerković, I.; Obradović, M.; Kuś, P.M.; Šarolić, M. Bioorganic Diversity of Rare Coriandrum Sativum L. Honey: Unusual Chromatographic Profiles Containing Derivatives of Linalool/Oxygenated Methoxybenzene. Chem. Biodivers. 2013, 10, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Nikolaou, C.; Karabagias, V.K. Volatile Fingerprints of Common and Rare Honeys Produced in Greece: In Search of PHVMs with Implementation of the Honey Code. Eur. Food Res. Technol. 2019, 245, 23–39. [Google Scholar] [CrossRef]
- Guyot, C.; Scheirman, V.; Collin, S. Floral Origin Markers of Heather Honeys: Calluna Vulgaris and Erica Arborea. Food Chem. 1999, 64, 3–11. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Falcão, S.I.; Escuredo, O.; Seijo, M.C.; Vilas-Boas, M. Description of the Volatile Fraction of Erica Honey from the Northwest of the Iberian Peninsula. Food Chem. 2021, 336, 127758. [Google Scholar] [CrossRef]





| Pollen Grain | Honey Type | Italy | Germany | Greece | Croatia | Serbia |
|---|---|---|---|---|---|---|
| Robinia pseudoacacia | Locust or acacia | 15 | 20 | 20 | 20 | |
| Citrus spp. | Orange | 10 | 20 | 3 | 10 | |
| Taraxacum spp. | Dandelion | 5 | 15 | 20 | ||
| Lavandula spp. | Lavander | 15 | 10 | |||
| Rosmarius officinalis | Rosemary | 20 | 20 | |||
| Thymus spp. | Thyme | 15 | 20 | 18 | ||
| Tilia spp. | Linden or lime | 20 | 25 | 25 | ||
| Castanea sativa | Chestnut | 90 | 90 | 87 | 85 | 85 |
| Rhododendron | Snowrose or alpenrose | 25 |
| Multifloral Honey | Monofloral and Honeydew Honey | ||||||
|---|---|---|---|---|---|---|---|
| Sample Code | Botanical Origin | Geographical Origin | Harvest Year | Sample Code | Botanical Origin | Geographical Origin | Harvest Year |
| M36 | Multifloral | Val di Fiemme | 2017 | A1-18 | Acacia | Valsugana | 2018 |
| M37 | Multifloral | Val di Fiemme | 2017 | A11-18 | Acacia | Valsugana | 2018 |
| M38 | Multifloral | Val di Fiemme | 2017 | A28-18 | Acacia | Val di Non | 2018 |
| M39 | Multifloral | Val di Fiemme | 2017 | AD43 | Apple–dandelion | Val di Non | 2018 |
| M41 | Multifloral | Val di Cembra | 2017 | AD45 | Apple–dandelion | Val d’Adige | 2017 |
| M42 | Multifloral | Val di Fassa | 2017 | AD25-18 | Apple–dandelion | Val di Non | 2018 |
| M44 | Multifloral | Val di Fiemme | 2017 | R2-18 | Rhododendron | Valsugana | 2018 |
| H3-18 | Multifloral | Val di Non | 2018 | R4-18 | Rhododendron | Val di Non | 2018 |
| M5-18 | Multifloral | Val di Non | 2018 | R14-18 | Rhododendron | Val di Fiemme | 2018 |
| M6-18 | Multifloral | Val di Non | 2018 | R17-18 | Rhododendron | Val di Fiemme | 2018 |
| M7-18 | Multifloral | Valsugana | 2018 | R18-18 | Rhododendron | Val di Fiemme | 2018 |
| M9-18 | Multifloral | Valsugana | 2018 | R24-18 | Rhododendron | Valsugana | 2018 |
| M16-18 | Multifloral | Val di Fiemme | 2018 | R27-18 | Rhododendron | Val di Non | 2018 |
| M19-18 | Multifloral | Val di Fiemme | 2018 | HD15-18 | Honeydew | Val di Fiemme | 2018 |
| M20-18 | Multifloral | Val di Fiemme | 2018 | HD26-18 | Honeydew | Val di Non | 2018 |
| M21-18 | Multifloral | Val di Fiemme | 2018 | HD29-18 | Honeydew | Val di Non | 2018 |
| M22-18 | Multifloral | Val d’Adige | 2018 | C8-18 | Chestnut | Valsugana | 2018 |
| M23-18 | Multifloral | Valsugana | 2018 | C12-18 | Chestnut | Valsugana | 2018 |
| M46-C | Multifloral | Val d’Adige | 2018 | C13-18 | Chestnut | Valsugana | 2018 |
| M46-D | Multifloral | Val d’Adige | 2018 | C40 | Chestnut | Val di Fiemme | 2017 |
| M46-P | Multifloral | Val d’Adige | 2018 | C52-C | Chestnut | Val d’Adige | 2018 |
| M47-C | Multifloral | Val d’Adige | 2018 | C52-D | Chestnut | Val d’Adige | 2018 |
| M47-D | Multifloral | Val d’Adige | 2018 | C52-P | Chestnut | Val d’Adige | 2018 |
| M47-P | Multifloral | Val d’Adige | 2018 | ||||
| Sample | Floral Type | Honeydew Elements (HDE/P) | PG/g |
|---|---|---|---|
| HD15-18 | Honeydew | 3.13 | 19,602 |
| HD26-18 | Honeydew | 3.57 | 35,281 |
| HD29-18 | Honeydew | 7.61 | 15,964 |
| Family Classification | Genus Classification | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Floral origin | Fagaceae | Ericaceae | Rosaceae | Salicaceae | Asteraceae | Fabaceae | Malus/Pyrus (Apple–Dandelion) | Asteraceae, T-Form (Apple–Dandelion) | Robinia Pseudoacacia (Acacia) | Castanea (Chestnut) |
| Chestnut | 90–98 a,b,c | 0–4 a | 0 a,c | 0 a | 0 a | 0 a | 0 a,b | 0 a | 0 a | 90–98 a,b,c |
| Rhododendron | 0–64 a | 31–90 a,b,c | 0–5 b,d | 0 b | 0 b | 0 b | 0 c,d | 0 b | 0 b | 0–64 a |
| Acacia | 14–65 b | 0–31 b | 15–38 a,b | 0 c | 0 c | 17–20 a,b | 0–19 a,b,c | 0 c | 17–19 a,b,c | 0–65 b |
| Apple–dandelion | 0–49 c | 0 c | 12–38 c,d | 0–19 d | 0–23 a,b,c | 0–11 a,b | 12–22 b,d | 2–23 a,b,c | 0 c | 0–49 c |
| Sample | Floral Type | Glu | Fru | Fru/Glu | Sucr | Meli | Lacto | Lactu | Koji | Tura | Pala | Nige | Mele | Raffi | Isomal | Erlo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M36 | Multifloral | 19.1 | 43.4 | 2.27 | 0.02 | 0.054 | 4.12 | 1.85 | 0.63 | 3.41 | 4.01 | 0.89 | 0.09 | 0.03 | 0.36 | 0.08 |
| M37 § | Multifloral | 18.6 | 40.7 | 2.19 | 0.08 | 0.093 | 2.83 | 0.95 | 0.45 | 2.00 | 0.83 | 0.65 | 2.69 | 0.59 | 0.20 | 0.91 |
| M38 § | Multifloral | 17.1 | 39.9 | 2.33 | 0.11 | 0.068 | 2.65 | 1.01 | 0.77 | 2.35 | 1.21 | 0.73 | 2.11 | 0.78 | 0.14 | 1.31 |
| M39 § | Multifloral | 17.2 | 37.7 | 2.19 | 0.29 | 0.077 | 2.10 | 0.84 | 0.48 | 2.23 | 0.78 | 0.63 | 1.74 | 1.13 | 0.08 | 3.53 |
| M41 § | Multifloral | 15.3 | 36.1 | 2.36 | 0.23 | 0.065 | 2.28 | 0.82 | 0.50 | 1.78 | 0.69 | 0.53 | 3.47 | 1.21 | 0.13 | 2.26 |
| M42 | Multifloral | 15.9 | 38.5 | 2.42 | 0.24 | 0.108 | 2.77 | 0.82 | 0.68 | 2.40 | 0.96 | 0.62 | 3.38 | 1.10 | 0.17 | 2.79 |
| M44 § | Multifloral | 18.6 | 44.3 | 2.38 | 0.10 | 0.048 | 3.59 | 1.00 | 1.21 | 2.38 | 1.96 | 0.82 | 2.36 | 0.61 | 0.16 | 1.60 |
| M3-18 § | Multifloral | 17.4 | 43.5 | 2.50 | 0.20 | 0.036 | 2.31 | 0.81 | 1.21 | 1.34 | 0.31 | 0.86 | 6.31 | 0.38 | 0.09 | 1.72 |
| M5-18 § | Multifloral | 13.6 | 37.6 | 2.77 | 0.13 | 0.038 | 1.61 | 0.59 | 0.55 | 0.96 | 0.29 | 0.52 | 10.96 | 0.58 | 0.08 | 1.34 |
| M6-18 § | Multifloral | 12.9 | 35.6 | 2.76 | 0.11 | 0.084 | 2.23 | 0.78 | 0.73 | 0.83 | 0.39 | 0.59 | 15.89 | 0.79 | 0.10 | 0.68 |
| M7-18 § | Multifloral | 17.9 | 43.5 | 2.44 | 1.09 | 0.034 | 1.65 | 0.56 | 0.64 | 1.13 | 0.23 | 0.55 | 0.23 | 0.35 | 0.09 | 2.52 |
| M9-18 § | Multifloral | 16.2 | 44.2 | 2.72 | 0.15 | 0.017 | 1.45 | 0.61 | 0.55 | 1.20 | 0.35 | 0.56 | 0.60 | 0.03 | 0.08 | 1.14 |
| M16-18 § | Multifloral | 19.3 | 42.9 | 2.23 | 0.43 | 0.038 | 2.22 | 0.71 | 0.76 | 1.70 | 0.63 | 0.58 | 0.74 | 0.68 | 0.10 | 2.96 |
| M19-18 § | Multifloral | 17.7 | 40.4 | 2.28 | 0.37 | 0.050 | 1.76 | 0.53 | 0.57 | 0.87 | 0.28 | 0.47 | 0.31 | 0.50 | 0.07 | 2.80 |
| M20-18 § | Multifloral | 19.5 | 44.9 | 2.30 | 0.64 | 0.032 | 1.82 | 0.71 | 0.68 | 1.97 | 0.52 | 0.60 | 0.34 | 0.26 | 0.10 | 3.57 |
| M21-18 | Multifloral | 20.6 | 45.1 | 2.19 | 0.60 | 0.048 | 2.02 | 0.75 | 0.52 | 1.70 | 0.56 | 0.58 | 0.64 | 0.48 | 0.11 | 3.64 |
| M22-18 § | Multifloral | 19.0 | 51.2 | 2.69 | 0.60 | 0.036 | 1.74 | 0.58 | 0.64 | 1.77 | 0.67 | 0.53 | 0.07 | 0.01 | 0.08 | 1.96 |
| M23-18 § | Multifloral | 20.1 | 45.6 | 2.26 | 0.12 | 0.032 | 2.11 | 0.67 | 0.73 | 1.56 | 0.35 | 0.65 | 0.92 | 0.05 | 0.09 | 0.83 |
| M46-C § | Multifloral | 18.0 | 51.5 | 2.86 | 4.03 | 0.031 | 1.26 | 0.43 | 0.41 | 1.32 | 0.44 | 0.41 | 0.24 | 0.02 | 0.08 | 2.03 |
| M46-P | Multifloral | 18.3 | 67.1 | 3.67 | 4.65 | 0.020 | 1.28 | 0.45 | 0.43 | 1.31 | 0.34 | 0.45 | 0.31 | 0.02 | 0.04 | 2.10 |
| M46-D | Multifloral | 18.4 | 62.3 | 3.38 | 3.77 | 0.023 | 1.20 | 0.43 | 0.00 | 1.19 | 0.00 | 0.44 | 0.34 | 0.03 | 0.04 | 1.91 |
| M47-C § | Multifloral | 19.6 | 48.4 | 2.47 | 2.30 | 0.028 | 1.67 | 0.53 | 0.65 | 1.86 | 0.88 | 0.58 | 0.33 | 0.04 | 0.08 | 1.72 |
| M47-P | Multifloral | 18.6 | 59.2 | 3.19 | 2.04 | 0.027 | 1.55 | 0.51 | 0.51 | 1.51 | 0.82 | 0.55 | 0.29 | 0.04 | 0.05 | 1.77 |
| M47-D | Multifloral | 19.3 | 60.8 | 3.15 | 2.05 | 0.025 | 1.68 | 0.55 | 0.45 | 1.61 | 0.37 | 0.56 | 0.33 | 0.04 | 0.05 | 1.60 |
| Mean (SD) | 17.8 c (1.9) | 46.0 a (8.6) | 2.58 a,b (0.41) | 1.01 a (1.38) | 0.046 a (0.024) | 2.08 a (0.71) | 0.73 a (0.29) | 0.61 a (0.24) | 1.68 a (0.59) | 0.74 a (0.80) | 0.60 d (0.12) | 2.28 d (3.81) | 0.41 d,f (0.39) | 0.11 a (0.07) | 1.95 e (0.94) | |
| A1-18 § | Acacia | 16.8 | 44.8 | 2.67 | 0.29 | 0.031 | 1.89 | 0.65 | 0.63 | 1.27 | 0.35 | 0.61 | 0.41 | 0.13 | 0.09 | 1.74 |
| A11-18 § | Acacia | 17.3 | 46.5 | 2.68 | 0.13 | 0.021 | 1.56 | 0.57 | 0.61 | 0.88 | 0.36 | 0.54 | 0.10 | 0.02 | 0.07 | 1.17 |
| A28-18 § | Acacia | 19.7 | 48.7 | 2.47 | 0.33 | 0.028 | 1.50 | 0.69 | 0.77 | 1.56 | 0.61 | 0.56 | 0.44 | 0.04 | 0.05 | 2.19 |
| Mean (SD) | 18.0 a (1.6) | 46.7 b (2.0) | 2.61 c,d (0.12) | 0.25 b (0.11) | 0.027 b (0.005) | 1.65 b (0.21) | 0.64 b (0.06) | 0.67 b (0.09) | 1.24 b (0.34) | 0.44 b (0.15) | 0.57 a (0.04) | 0.32 a (0.19) | 0.06 a (0.06) | 0.07 b (0.02) | 1.70 a (0.51) | |
| AD25-18 § | Apple–dandelion | 22.8 | 50.6 | 2.22 | 0.09 | 0.041 | 2.87 | 1.02 | 0.94 | 2.67 | 1.81 | 0.79 | 0.54 | 0.05 | 0.13 | 0.79 |
| AD43 § | Apple–dandelion | 21.9 | 47.0 | 2.14 | 2.90 | 0.032 | 2.33 | 0.74 | 0.72 | 1.99 | 0.85 | 0.47 | 0.20 | 0.02 | 0.12 | 1.00 |
| AD45 § | Apple–dandelion | 21.8 | 47.2 | 2.16 | 0.12 | 0.027 | 1.78 | 0.63 | 0.56 | 1.94 | 1.04 | 0.53 | 0.41 | 0.05 | 0.13 | 1.10 |
| Mean (SD) | 22.2 a,b,c,d (0.5) | 48.3 c (2.0) | 2.18 a,c,d,e (0.04) | 1.04 c (1.62) | 0.033 c (0.007) | 2.33 c (0.55) | 0.80 c (0.20) | 0.74 c (0.19) | 2.20 c (0.40) | 1.23 c (0.51) | 0.60 b (0.17) | 0.38 b (0.17) | 0.04 b (0.02) | 0.13 c (0.01) | 0.96 b,f (0.16) | |
| R2-18 § | Rhododendron | 17.2 | 42.9 | 2.48 | 0.29 | 0.021 | 2.14 | 0.73 | 0.97 | 1.31 | 0.34 | 0.80 | 2.09 | 0.14 | 0.08 | 2.34 |
| R4-18 § | Rhododendron | 18.4 | 41.7 | 2.27 | 1.19 | 0.022 | 1.76 | 0.76 | 0.81 | 1.61 | 0.14 | 0.67 | 0.18 | 0.06 | 0.08 | 4.40 |
| R14-18 § | Rhododendron | 19.4 | 42.7 | 2.20 | 0.84 | 0.031 | 1.84 | 0.72 | 0.64 | 1.91 | 0.41 | 0.68 | 0.18 | 0.12 | 0.09 | 4.16 |
| R17-18 § | Rhododendron | 19.7 | 44.7 | 2.27 | 5.54 | 0.017 | 1.56 | 0.83 | 0.48 | 2.51 | 0.39 | 0.54 | 0.21 | 0.04 | 0.00 | 6.57 |
| R18-18 § | Rhododendron | 18.7 | 42.2 | 2.26 | 3.45 | 0.020 | 1.37 | 0.65 | 0.47 | 1.46 | 0.15 | 0.53 | 0.09 | 0.03 | 0.07 | 5.40 |
| R24-18 § | Rhododendron | 19.9 | 45.8 | 2.31 | 0.85 | 0.036 | 1.81 | 0.57 | 0.50 | 1.50 | 0.25 | 0.49 | 0.10 | 0.03 | 0.11 | 3.87 |
| R27-18 § | Rhododendron | 19.1 | 43.5 | 2.28 | 2.66 | 0.010 | 1.63 | 0.88 | 0.59 | 1.89 | 0.00 | 0.65 | 0.29 | 0.02 | 0.07 | 6.50 |
| Mean (SD) | 18.9 d (0.9) | 43.4 d (1.4) | 2.30 b,d,e,f (0.09) | 2.12 d (1.88) | 0.023 d (0.009) | 1.73 d (0.24) | 0.73 d (0.11) | 0.64 d (0.19) | 1.74 d (0.40) | 0.24 d (0.15) | 0.62 e (0.11) | 0.45 e (0.73) | 0.06 e,f (0.05) | 0.07 d (0.03) | 4.75 a,b,c,d,e (1.52) | |
| HD15-18 § | Honeydew | 18.1 | 39.2 | 2.16 | 0.25 | 0.049 | 2.22 | 0.66 | 0.55 | 1.26 | 0.62 | 0.57 | 0.87 | 0.89 | 0.08 | 2.42 |
| HD26-18 § | Honeydew | 14.9 | 35.7 | 2.40 | 0.95 | 0.033 | 2.88 | 0.70 | 0.72 | 1.49 | 0.73 | 0.65 | 10.21 | 1.34 | 0.10 | 3.75 |
| HD29-18 § | Honeydew | 14.3 | 36.1 | 2.53 | 0.29 | 0.044 | 2.73 | 0.88 | 0.80 | 1.68 | 0.60 | 0.78 | 15.52 | 1.29 | 0.11 | 1.85 |
| Mean (SD) | 15.5 a,d (1.8) | 37.5 e (1.8) | 2.44 g (0.21) | 0.41 e (0.36) | 0.043 e (0.007) | 2.41 e (0.50) | 0.74 e (0.10) | 0.68 e (0.11) | 1.62 e (0.34) | 0.72 e (0.15) | 0.66 c (0.09) | 8.82 a,b,c,d,e (6.06) | 1.02 a,b,c,d,e (0.37) | 0.09 e (0.02) | 2.31 d,f,g (1.07) | |
| C40 | Chestnut | 15.3 | 36.7 | 2.41 | 0.25 | 0.088 | 2.90 | 0.95 | 0.68 | 2.74 | 1.12 | 0.76 | 1.24 | 1.04 | 0.10 | 2.85 |
| C8-18 | Chestnut | 15.0 | 42.3 | 2.81 | 0.05 | 0.056 | 3.87 | 1.36 | 0.97 | 2.65 | 0.96 | 1.05 | 0.23 | 0.02 | 0.21 | 0.10 |
| C12-18 | Chestnut | 16.1 | 42.6 | 2.65 | 0.10 | 0.055 | 2.71 | 0.79 | 0.76 | 1.62 | 0.65 | 0.68 | 0.71 | 0.19 | 0.12 | 0.71 |
| C13-18 | Chestnut | 18.8 | 47.8 | 2.53 | 0.21 | 0.042 | 2.64 | 0.94 | 1.17 | 2.37 | 0.77 | 0.99 | 1.51 | 0.12 | 0.10 | 1.61 |
| C52-C | Chestnut | 17.8 | 47.4 | 2.66 | 0.28 | 0.032 | 1.98 | 0.70 | 0.72 | 1.68 | 0.60 | 0.68 | 0.51 | 0.13 | 0.09 | 0.75 |
| C52-P | Chestnut | 18.2 | 63.4 | 3.48 | 0.24 | 0.033 | 1.90 | 0.69 | 0.55 | 1.71 | 0.55 | 0.65 | 0.52 | 0.13 | 0.05 | 0.75 |
| C52-D | Chestnut | 20.4 | 69.7 | 3.41 | 0.28 | 0.038 | 2.23 | 0.79 | 0.78 | 1.85 | 0.84 | 0.81 | 0.58 | 0.16 | 0.06 | 0.91 |
| Mean (SD) | 17.4 b (2.0) | 50.0 f (12.0) | 2.85 e,f,g (0.43) | 0.20 f (0.09) | 0.049 f (0.019) | 2.60 f (0.67) | 0.89 f (0.23) | 0.80 f (0.20) | 2.09 f (0.48) | 0.78 f (0.21) | 0.80 b,c,d,e (0.16) | 0.76 c (0.45) | 0.26 c (0.35) | 0.11 f (0.05) | 1.10 c,g (0.89) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, R.; Scalabrin, E.; Malagnini, V.; Strojnik, L.; Ogrinc, N.; Capodaglio, G. Characterization of Botanical Origin of Italian Honey by Carbohydrate Composition and Volatile Organic Compounds (VOCs). Foods 2022, 11, 2441. https://doi.org/10.3390/foods11162441
Tedesco R, Scalabrin E, Malagnini V, Strojnik L, Ogrinc N, Capodaglio G. Characterization of Botanical Origin of Italian Honey by Carbohydrate Composition and Volatile Organic Compounds (VOCs). Foods. 2022; 11(16):2441. https://doi.org/10.3390/foods11162441
Chicago/Turabian StyleTedesco, Raffaello, Elisa Scalabrin, Valeria Malagnini, Lidija Strojnik, Nives Ogrinc, and Gabriele Capodaglio. 2022. "Characterization of Botanical Origin of Italian Honey by Carbohydrate Composition and Volatile Organic Compounds (VOCs)" Foods 11, no. 16: 2441. https://doi.org/10.3390/foods11162441
APA StyleTedesco, R., Scalabrin, E., Malagnini, V., Strojnik, L., Ogrinc, N., & Capodaglio, G. (2022). Characterization of Botanical Origin of Italian Honey by Carbohydrate Composition and Volatile Organic Compounds (VOCs). Foods, 11(16), 2441. https://doi.org/10.3390/foods11162441








