Fruitomics: The Importance of Combining Sensory and Chemical Analyses in Assessing Cold Storage Responses of Six Peach (Prunus persica L. Batsch) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Analysis of Carotenoids
2.3. Extraction and Determination of Total Phenol Content
2.4. Extraction and Analysis of Vitamin C
2.5. Total Sugar Analysis
2.6. Qualitative Analysis
2.7. Sensory Evaluation
2.8. Statistical Approaches
3. Results
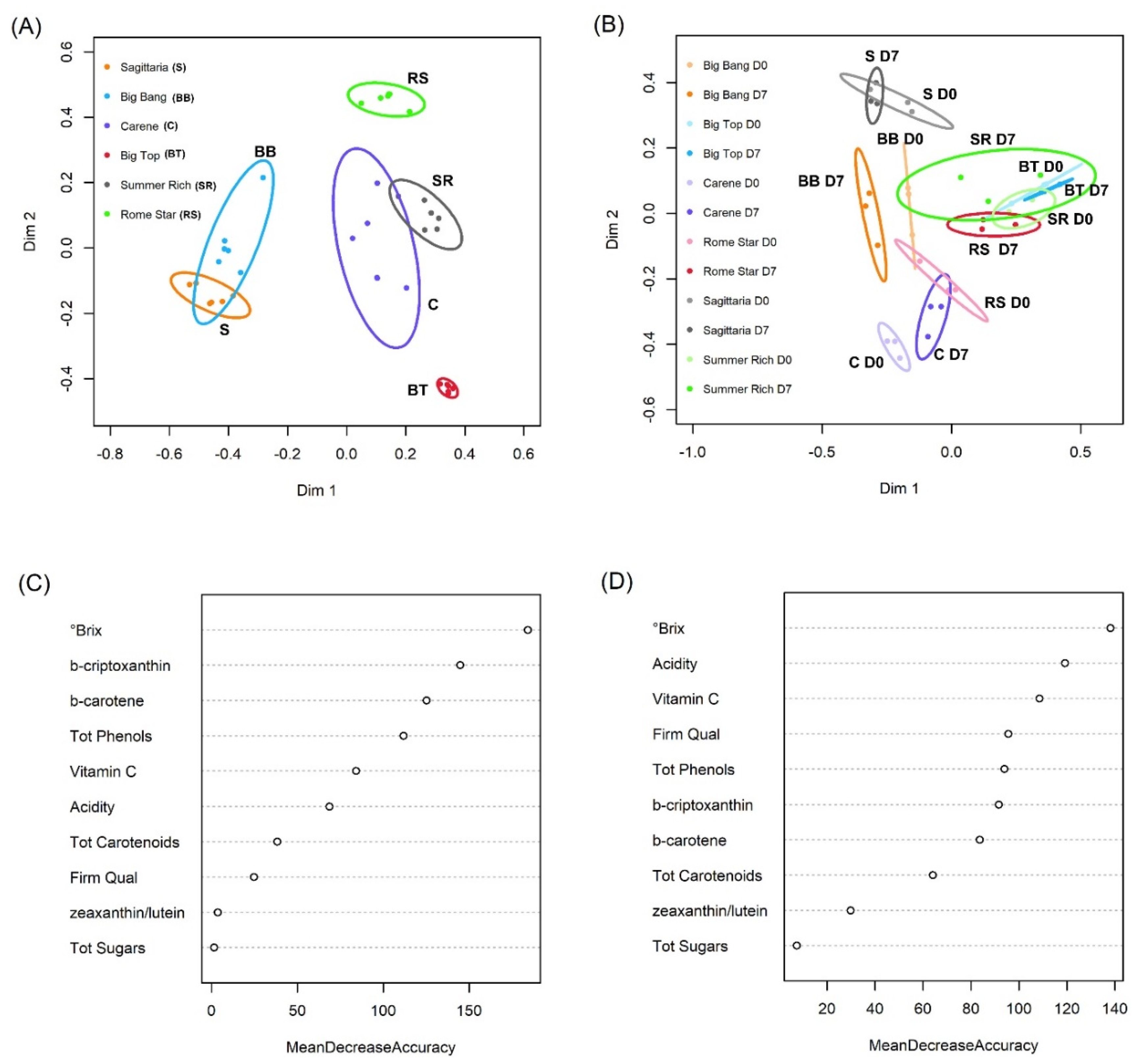
3.1. Intrinsic Quality Characters among Cultivars before and after Cold Storage
3.2. Sensory Descriptors among Cultivars before and after Storage
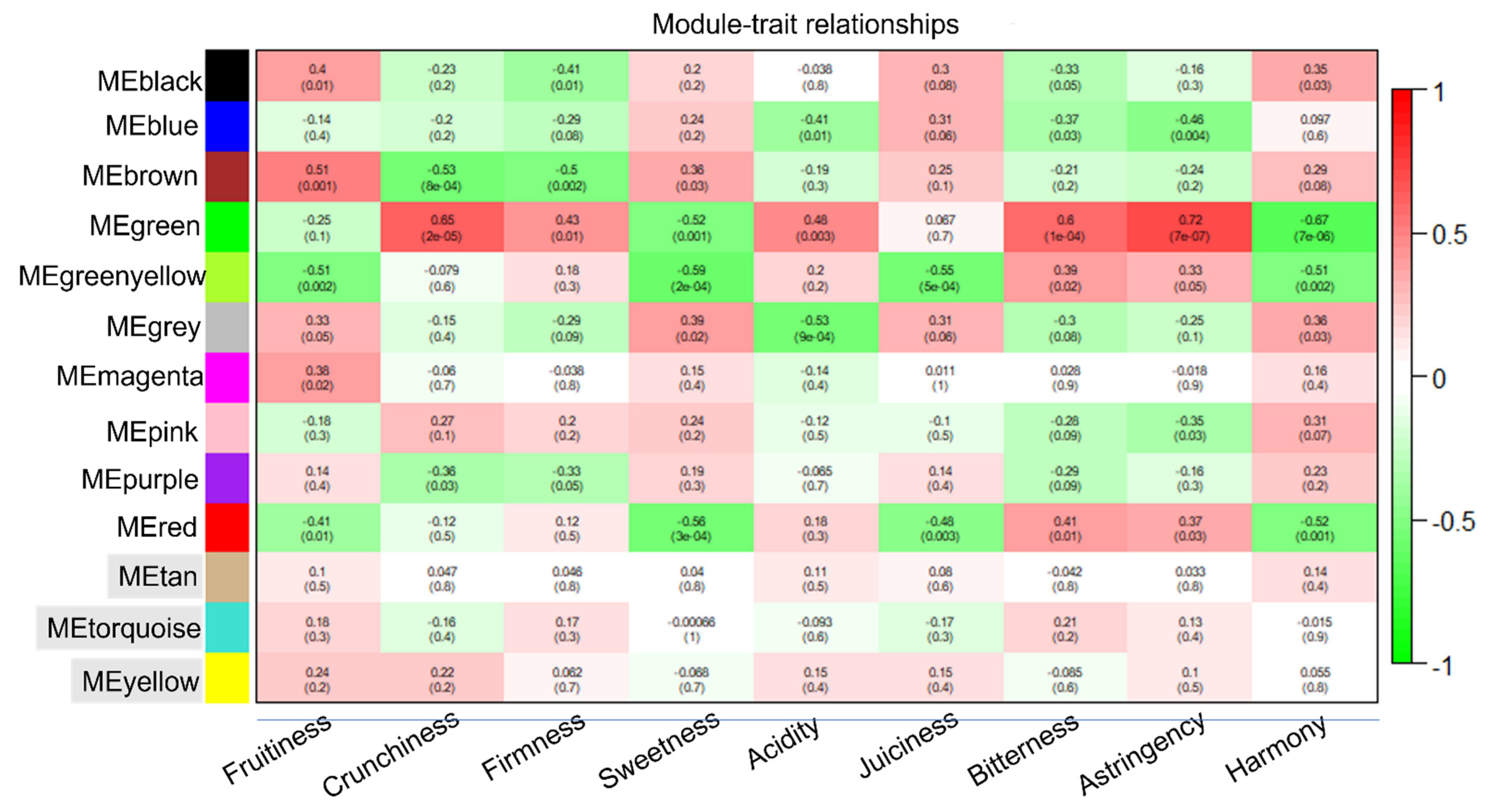
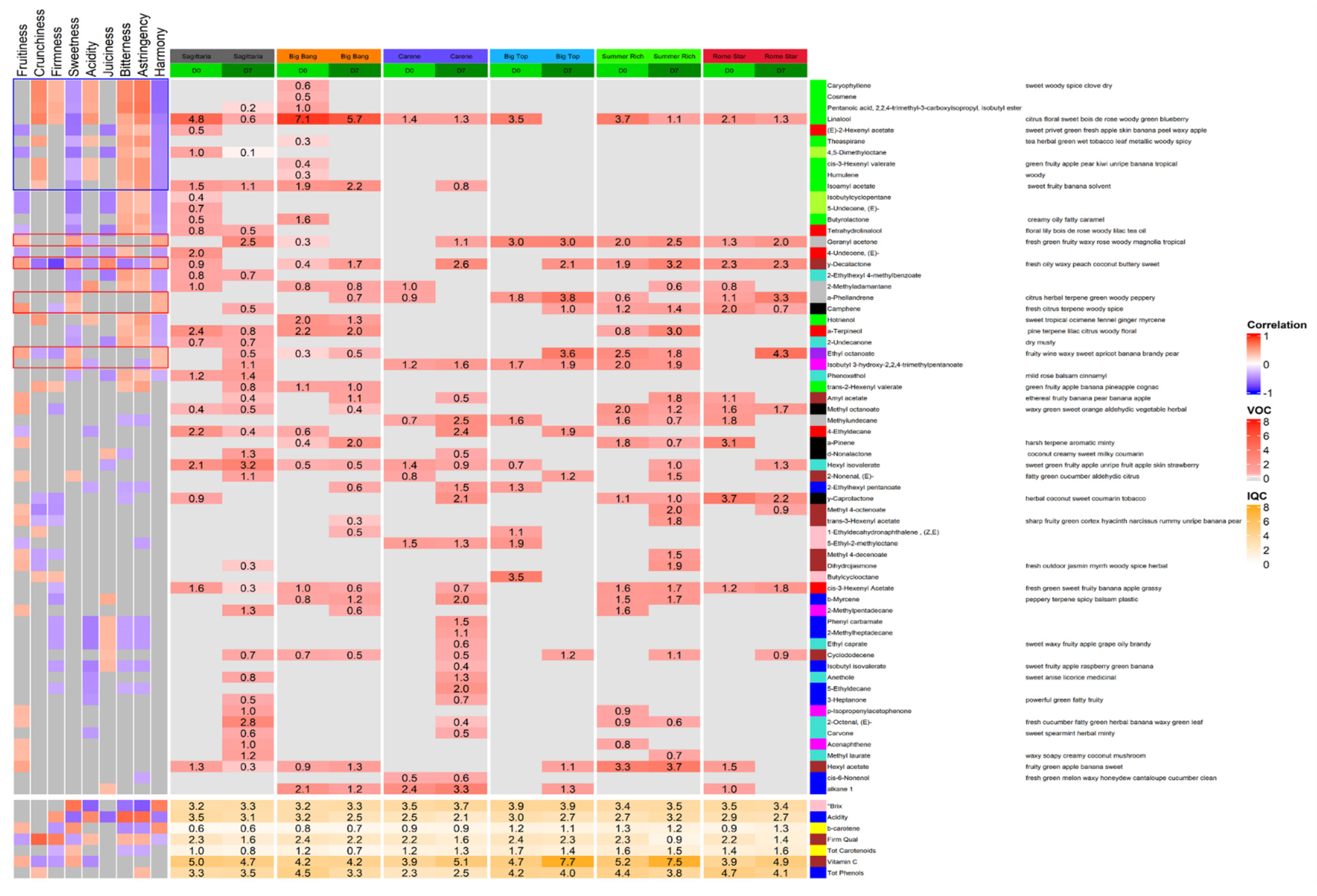
3.3. VOCs and Intrinsic Quality Characters in Relation to Sensory Parameters
3.4. VOCs and Intrinsic Quality Characters in Relation to Cultivar and Cold Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT (United Nations Food and Agriculture Organization Statistics Division). Productions of Peaches and Nectarines in 2019; Crops/Regions/World/Production Quality (from Pick List). Available online: https://www.fao.org/common-pages/search/en/?q=pick%20list (accessed on 7 July 2022).
- Dirlewanger, E.; Cosson, P.; Boudehri, K.; Renaud, C.; Capdeville, G.; Tauzin, Y.; Laigret, F.; Moing, A. Development of a second-generation genetic linkage map for peach [Prunus persica (L.) Batsch] and characterization of morphological traits affecting flower and fruit. Tree Genet. Genomes 2006, 3, 1–13. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vicente, A.R.; Martínez-García, P.J.; Crisosto, C.H. Peach and nectarine. In Postharvest Physiological Disorders in Fruits and Vegetables; de Freitas, S.T., Pareek, S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 293–304. [Google Scholar]
- Xi, W.; Zheng, Q.; Lu, J.; Quan, J. Comparative analysis of three types of peaches: Identification of the key individual characteristic flavor compounds by integrating consumers’ acceptability with flavor quality. Hortic. Plant J. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Sánchez, G.; Venegas-Calerón, M.; Salas, J.J.; Monforte, A.; Badenes, M.L.; Granell, A. An integrative “omics” approach identifies new candidate genes to impact aroma volatiles in peach fruit. BMC Genom. 2013, 14, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Tanou, G.; Minas, I.S.; Scossa, F.; Belghazi, M.; Xanthopoulou, A.; Ganopoulos, I.; Madesis, P.; Fernie, A.; Molassiotis, A. Exploring priming responses involved in peach fruit acclimation to cold stress. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shinya, P.; Contador, L.; Frett, T.; Infante, R. Effect of prolonged cold storage on the sensory quality of peach and nectarine. Postharvest Biol. Technol. 2014, 95, 7–12. [Google Scholar] [CrossRef]
- Cano-Salazar, J.; López, M.; Crisosto, C.; Echeverría, G. Volatile compound emissions and sensory attributes of ‘Big Top’ nectarine and ‘Early Rich’ peach fruit in response to a pre-storage treatment before cold storage and subsequent shelf-life. Postharvest Biol. Technol. 2013, 76, 152–162. [Google Scholar] [CrossRef]
- Farina, V.; Lo Bianco, R.; Mazzaglia, A. Evaluation of late-maturing peach and nectarine fruit quality by chemical, physical, and sensory determinations. Agriculture 2019, 9, 189. [Google Scholar] [CrossRef]
- Brizzolara, S.; Hertog, M.; Tosetti, R.; Nicolai, B.; Tonutti, P. Metabolic responses to low temperature of three peach fruit cultivars differently sensitive to cold storage. Front. Plant Sci. 2018, 28, 706. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M. Relationship between ripe soluble solids con- centration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharvest Biol. Technol. 2005, 38, 239–246. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of western red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef]
- Colarič, B.M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agr. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Sortino, G.; Allegra, A.; Farina, V.; Inglese, P. Postharvest quality and sensory attributes of ‘Pesca di Bivona’ peaches (Prunus persica L.) during storage. Bulg. J. Agric. Sci 2017, 23, 939–946. [Google Scholar]
- Echeverría, G.; Cantin, C.O.; Catalan, A.; López, L.; Lara, I.; Graell, J. The impact of maturity, storage temperature and storage duration on sensory quality and consumer satisfaction of ‘Big Top®’ nectarines. Sci. Hortic. 2015, 190, 179–186. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, Q.; Fu, Z.; Li, X.; Matetić, M.; Brkic Bakaric, M.; Jemrić, T.A. Comprehensive Peach Fruit Quality Evaluation Method for Grading and Consumption. Appl. Sci. 2020, 10, 1348. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Yousef, G.; Guzman, I.; Chebrolu, K.; Werner, D.; Parker, M.; Gasic, K.; Perkins, P. Variation of Carotenoids and Polyphenolics in Peach (Prunus persica L.) and Implications on Breeding for Modified Phytochemical Profiles. J. Am. Soc. Hortic. Sci. 2014, 139, 676–687. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G.E.; Goulas, V.; Manganaris, G.; Ziogas, V.; Manganaris, A. The appraisal of qualitative parameters and antioxidant contents during postharvest peach fruit ripening underlines the genotype significance. Postharvest Biol. Technol. 2016, 115, 142–150. [Google Scholar] [CrossRef]
- Serra, S.; Anthony, B.; Masia, A.; Giovannini, D.; Musacchi, S. Determination of biochemical composition in peach (Prunus persica L. Batsch) accessions characterized by different flesh color and textural typologies. Foods 2020, 9, 1452. [Google Scholar] [CrossRef]
- Dalla Valle, Z.; Mignani, I.; Spinardi, A.; Galvano, F.; Ciappellano, S. The antioxidant profile of three different peaches cultivars (Prunus persica) and their short-term effect on antioxidant status in human. Eur. Food Res. Technol. 2007, 225, 167–172. [Google Scholar] [CrossRef]
- Caprioli, I.; Lafuente, M.T.; Rodrigo, M.; Mencarelli, F. Influence of postharvest treatments on quality, carotenoids, and abscisic acid content of stored “Spring Belle” peach (Prunus persica) Fruit. J. Agr. Food Chem. 2009, 57, 7056–7063. [Google Scholar] [CrossRef]
- Aubert, C.; Chalot, G.; Sébastien, L.; Ronjon, A.; Cottet, V. Relationship between fruit density and quality parameters, levels of sugars, organic acids, bioactive compounds and volatiles of two nectarine cultivars, at harvest and after ripening. Food Chem. 2019, 297, 124954. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, P.E.; Vanoli, M.; Lovati, F.; Spinelli, L.; Torricelli, A.; Rizzolo, A.; Lurie, S. Maturity assessment at harvest and prediction of softening in a late maturing nectarine cultivar after cold storage. Postharvest Biol. Technol. 2011, 62, 275–281. [Google Scholar] [CrossRef]
- Delgado, C.; Crisosto, G.M.; Heymann, H.; Crisosto, C.H. Determining the primary drivers of liking to predict consumers’ acceptance of fresh nectarines and peaches. J. Food Sci. 2013, 78, S605–S614. [Google Scholar] [CrossRef]
- Scalisi, A.; Pelliccia, D.; O’Connell, M. Maturity Prediction in Yellow Peach (Prunus persica L.) Cultivars Using a Fluorescence Spectrometer. Sensors 2020, 20, 6555. [Google Scholar] [CrossRef]
- Muto, A.; Müller, C.T.; Bruno, L.; McGregor, L.; Ferrante, A.; Chiappetta, A.A.C.; Bitonti, M.B.; Rogers, H.J.; Spadafora, N.D. Fruit volatilome profiling through GC × GC-ToF-MS and gene expression analyses reveal differences amongst peach cultivars in their response to cold storage. Sci. Rep. 2020, 10, 18333. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Mérillon, J.M.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic contents and bioactive potential of peach fruit extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lobo, M.G.; Gonzalez, M. Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chem. 2006, 96, 654–664. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 7 July 2022).
- RStudio Team. RStudio: Integrated Development for R; RStudio: PBC, Boston, MA, USA, 2020; Available online: http://www.rstudio.com (accessed on 2 March 2020).
- Wang, Y.; Naumann, U.; Eddelbuettel, D.; Wilshire, J.; Warton, D. mvabund: Statistical Methods for Analysing Multivariate Abundance Data. R package Version 4.1.12. 2021. Available online: https://cran.r-project.org/web/packages/mvabund/, (accessed on 22 July 2022).
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Langfelder, P.; Horvath, S. Fast R functions for robust correlations and hierarchical clustering. J. Stat. Sofw. 2012, 46, i11. [Google Scholar]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef]
- Yu, A.N.; Yang, Y.N.; Yang, Y.; Zheng, F.P.; Sun, B.G. Free and bound volatile compounds in the Rubus coreanus fruits of different ripening stages. J. Food Biochem. 2019, 43, e12964. [Google Scholar] [CrossRef] [PubMed]
- Podestá, R.; Pagliosa, C.; Vieira, M.; Provesi, J.; Amante, E.; Zeni, A.L.; Raitz, I.; Rebelo, R. Identification of volatile compounds in thinning discards from plum trees (Prunus salicina Lindl.) cultivar Harry Pickstone. Food Sci.Technol. 2021, 31, 710–713. [Google Scholar] [CrossRef][Green Version]
- Robertson, G.W.; Griffiths, D.W.; Woodford, J.A.T.; Birch, A.N.E. Changes in the chemical composition of volatiles released by the flowers and fruits of the red raspberry (Rubus idaeus) cultivar glen prosen. Phytochemistry 1995, 38, 1175–1179. [Google Scholar] [CrossRef]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, W.; Cao, J.; Li, Y. Effect of chilling temperatures on physiological properties, phenolic metabolism and antioxidant level accompanying pulp browning of peach during cold storage. Sci. Hortic. 2019, 255, 175–182. [Google Scholar] [CrossRef]

| Cultivar | Day | Acidity | β Carotene | β Cryptoxanthin | Soluble Solids Content (SSC) | Firmness Quality | Total Carotenoids | Total Phenols | Total Sugars | Vitamin C | Zeaxanthin/ Lutein |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % of Malic Acid g/L | mg/Kg FW | mg/Kg FW | °Brix | Kg/cm−2 | mg/Kg FW | mg/100 g FW | mg/g FW | mg/Kg FW | mg/Kg FW | ||
| Sagittaria | D0 | 12.24 ± 0.27 | 0.36 ± 0.08 | 0.14 ± 0.04 | 10.3 ± 0.12 | 5.47 ± 0.68 | 1.09 ± 0.36 | 10.87 ± 1.09 | 32.4 ± 12.36 | 24.54 ± 1.71 | 0.59 ± 0.27 |
| D7 | 9.47 ± 1.04 | 0.42 ± 0.01 | 0.10 ± 0.03 | 10.8 ± 0.00 | 2.72 ± 0.71 | 0.73 ± 0.20 | 12.13 ± 0.63 | 37.68 ± 14.37 | 21.88 ± 2.33 | 0.35 ± 0.12 | |
| Big Bang | D0 | 9.96 ± 0.15 | 0.59 ± 0.03 | 0.070 ± 0.004 | 10.0 ± 0.00 | 5.77 ± 0.12 | 1.38 ± 0.29 | 20.22 ± 0.32 | 12.22 ± 8.04 | 17.35 ± 0.55 | 0.72 ± 0.26 |
| D7 | 6.48 ± 0.19 | 0.50 ± 0.17 | 0.02 ± 0.03 | 11.0 ± 0.00 | 4.82 ± 0.13 | 0.52 ± 0.21 | 11.18 ± 1.47 | 24.66 ± 7.08 | 17.66 ± 0.36 | 0.31 ± 0.53 | |
| Carene | D0 | 6.30 ± 0.12 | 0.76 ± 0.05 | 0.080 ± 0.003 | 11.9 ± 0.12 | 4.66 ± 0.37 | 1.38 ± 0.20 | 5.33 ± 0.08 | 19.31 ± 6.18 | 15.55 ± 1.70 | 0.54 ± 0.17 |
| D7 | 4.27 ± 0.64 | 0.77 ± 0.10 | 0.10 ± 0.01 | 14.0 ± 0.00 | 2.62 ± 0.47 | 1.70 ± 0.18 | 6.07 ± 0.62 | 15.47 ± 9.52 | 25.54 ± 0.48 | 0.83 ± 0.12 | |
| Big Top | D0 | 9.20 ± 0.27 | 1.53 ± 0.57 | 0.15 ± 0.05 | 15.0 ± 0.00 | 5.79 ± 0.46 | 2.84 ± 0.71 | 17.71 ± 1.71 | 30.56 ± 16.01 | 22.04 ± 1.06 | 1.16 ± 0.23 |
| D7 | 7.24 ± 0.12 | 1.20 ± 0.27 | 0.14 ± 0.04 | 14.8 ± 0.29 | 5.07 ± 0.07 | 1.89 ± 0.28 | 16.39 ± 1.49 | 35.59 ± 4.86 | 59.41 ± 2.75 | 0.55 ± 0.08 | |
| Summer Rich | D0 | 7.44 ± 0.70 | 1.75 ± 0.29 | 0.13 ± 0.03 | 11.8 ± 0.29 | 5.17 ± 0.41 | 2.66 ± 0.62 | 19.14 ± 2.88 | 32.68 ± 7.88 | 27.16 ± 2.12 | 0.78 ± 0.33 |
| D7 | 10.27 ± 0.39 | 1.48 ± 0.40 | 0.10 ± 0.04 | 12.0 ± 0.00 | 0.86 ± 0.27 | 2.45 ± 1.07 | 14.12 ± 0.31 | 25.18 ± 16.83 | 56.23 ± 5.38 | 0.87 ± 0.75 | |
| Rome Star | D0 | 8.24 ± 0.00 | 0.89 ± 0.07 | 0.06 ± 0.01 | 12.3 ± 0.00 | 5.01 ± 0.3 | 1.85 ± 0.46 | 21.76 ± 2.40 | 25.13 ± 27.83 | 15.59 ± 1.04 | 0.90 ± 0.41 |
| D7 | 7.15 ± 0.19 | 1.73 ± 0.11 | 0.04 ± 0.02 | 11.3 ± 0.12 | 2.12 ± 0.77 | 2.60 ± 0.33 | 16.49 ± 1.82 | 23.08 ± 4.46 | 23.87 ± 2.05 | 0.82 ± 0.23 |
| Acidity | β Carotene | β Cryptoxanthin | °Brix | Firmness Quality | Total Carotenoids | Total Phenols | Total Sugars | Vitamin C | Zeaxanthin/ Lutein | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LR | p | LR | p | LR | p | LR | p | LR | p | LR | p | LR | p | LR | p | LR | p | LR | p | |
| Cultivar | 10.16 | 0.002 | 22.18 | 0.002 | 7.96 | 0.002 | 46.5 | 0.002 | 2.67 | 0.003 | 11.16 | 0.002 | 23.23 | 0.002 | 2.25 | 0.14 | 6.37 | 0.002 | 1.27 | 0.26 |
| Storage | 13.56 | 0.013 | 0.21 | 0.842 | 4.93 | 0.145 | 5.75 | 0.126 | 54.04 | 0.002 | 2.38 | 0.316 | 12.11 | 0.016 | 0.28 | 0.842 | 28.24 | 0.002 | 2.85 | 0.297 |
| Acidity | Astringency | Bitterness | Crunchiness | Firmness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LR | p | LR | p | LR | p | LR | p | LR | p | |
| Cultivar | 8.99 | 0.002 | 19.74 | 0.002 | 9.24 | 0.002 | 11.2 | 0.002 | 6.84 | 0.002 |
| Storage | 93.14 | 0.002 | 24.4 | 0.002 | 33.78 | 0.002 | 64.64 | 0.002 | 58.29 | 0.002 |
| Cultivar-storage | 12.96 | 0.002 | 14.96 | 0.002 | 6.15 | 0.003 | 2.08 | 0.112 | 4.67 | 0.006 |
| Fruitiness | Harmony | Juiciness | Sweetness | |||||||
| LR | p | LR | p | LR | p | LR | p | |||
| Cultivar | 4.91 | 0.002 | 3.78 | 0.003 | 4.75 | 0.002 | 12.69 | 0.002 | ||
| Storage | 36.22 | 0.002 | 31.01 | 0.002 | 27.17 | 0.002 | 81.56 | 0.002 | ||
| Cultivar-storage | 4.28 | 0.009 | 4.97 | 0.006 | 2.26 | 0.112 | 4.71 | 0.006 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muto, A.; Christofides, S.R.; Sirangelo, T.M.; Bartella, L.; Muller, C.; Di Donna, L.; Muzzalupo, I.; Bruno, L.; Ferrante, A.; Chiappetta, A.A.C.; et al. Fruitomics: The Importance of Combining Sensory and Chemical Analyses in Assessing Cold Storage Responses of Six Peach (Prunus persica L. Batsch) Cultivars. Foods 2022, 11, 2554. https://doi.org/10.3390/foods11172554
Muto A, Christofides SR, Sirangelo TM, Bartella L, Muller C, Di Donna L, Muzzalupo I, Bruno L, Ferrante A, Chiappetta AAC, et al. Fruitomics: The Importance of Combining Sensory and Chemical Analyses in Assessing Cold Storage Responses of Six Peach (Prunus persica L. Batsch) Cultivars. Foods. 2022; 11(17):2554. https://doi.org/10.3390/foods11172554
Chicago/Turabian StyleMuto, Antonella, Sarah R. Christofides, Tiziana Maria Sirangelo, Lucia Bartella, Carsten Muller, Leonardo Di Donna, Innocenzo Muzzalupo, Leonardo Bruno, Antonio Ferrante, Adriana A. C. Chiappetta, and et al. 2022. "Fruitomics: The Importance of Combining Sensory and Chemical Analyses in Assessing Cold Storage Responses of Six Peach (Prunus persica L. Batsch) Cultivars" Foods 11, no. 17: 2554. https://doi.org/10.3390/foods11172554
APA StyleMuto, A., Christofides, S. R., Sirangelo, T. M., Bartella, L., Muller, C., Di Donna, L., Muzzalupo, I., Bruno, L., Ferrante, A., Chiappetta, A. A. C., Bitonti, M. B., Rogers, H. J., & Spadafora, N. D. (2022). Fruitomics: The Importance of Combining Sensory and Chemical Analyses in Assessing Cold Storage Responses of Six Peach (Prunus persica L. Batsch) Cultivars. Foods, 11(17), 2554. https://doi.org/10.3390/foods11172554










