Comparison of Volatile Compounds Contributing to Flavor of Wild Lowbush (Vaccinium augustifolium) and Cultivated Highbush (Vaccinium corymbosum) Blueberry Fruit Using Gas Chromatography-Olfactometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blueberry Samples
2.2. Gas Chromatography-Olfactometry (GC-O)
2.3. 2Dimensional Gas Chromatography-Time of Flight-Mass Spectrometry
2.4. Statistical Analysis
3. Results and Discussion
3.1. Aroma-Active Volatiles of Wild Lowbush Blueberries
3.2. Aroma-Active Volatiles of Cultivated Highbush Blueberries
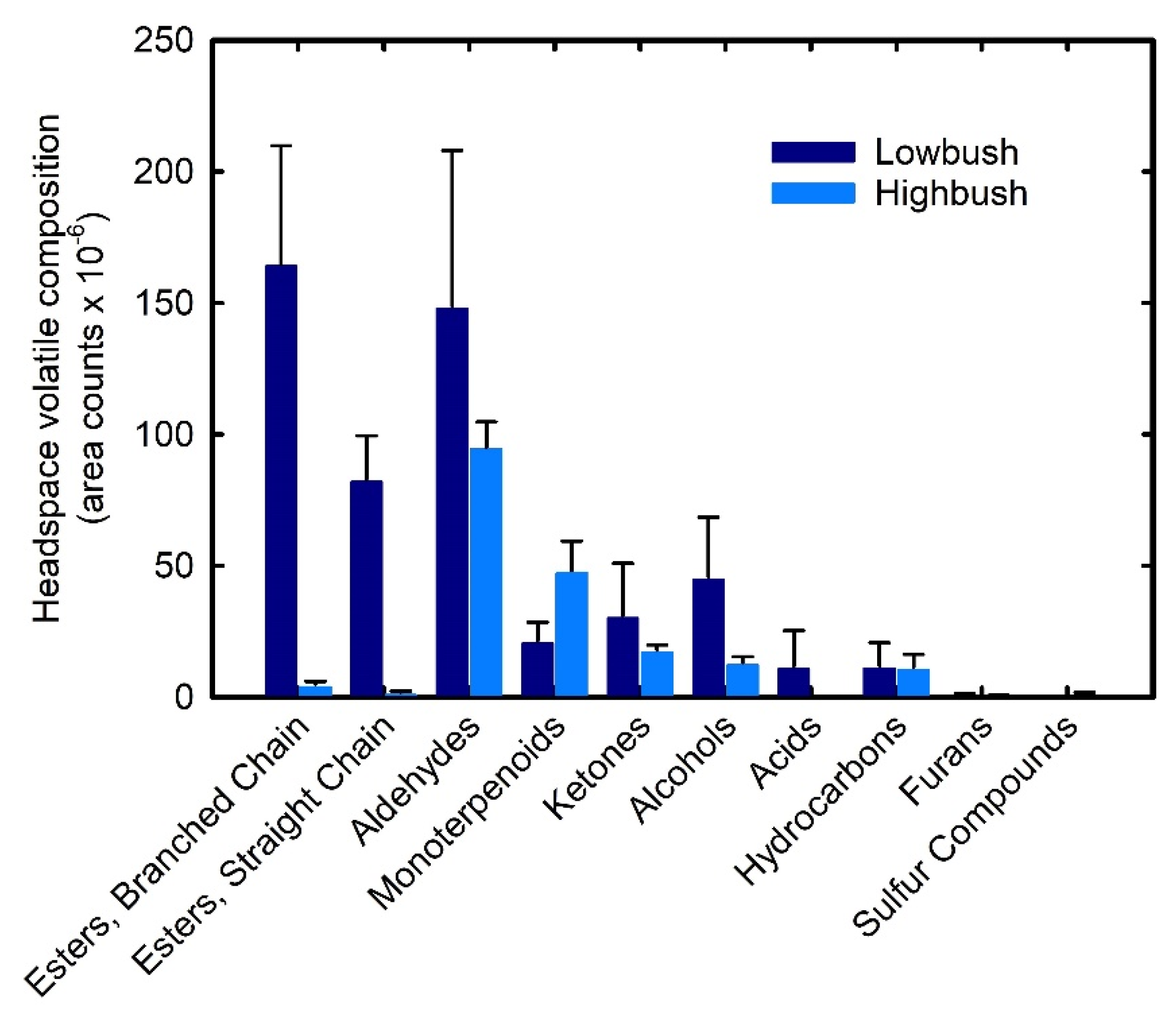
3.3. Comparison of Aroma-Active Compounds in Wild Lowbush and Cultivated Highbush Blueberries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forney, C.F.; Kalt, W. Blueberry and cranberry. In Health-Promoting Properties of Fruit and Vegetables; Terry, L.A., Ed.; CABI: Oxfordshire, UK, 2011; pp. 51–73. [Google Scholar] [CrossRef]
- Brazelton, C.; Fain, C.; Ogg, M. Global State of the Blueberry Industry Report 2021; International Blueberry Organization: Santiago, Chile, 2022; p. 84. [Google Scholar]
- WBANA. Growing Wild Blueberries. Available online: https://www.wildblueberryassociation.ca/the-better-blueberry/the-story-of-wild/growing-wild-blueberries/ (accessed on 9 October 2020).
- Lobos, G.A.; Hancock, J.F. Breeding blueberries for a changing global environment: A review. Front. Plant. Sci. 2015, 6, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalt, W.; Ryan, D.A.J.; Duy, J.C.; Prior, R.L.; Ehlenfeldt, M.K.; Vander Kloet, S.P. Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of highbush and lowbush blueberries (vaccinium section cyanococcus spp.). J. Agric. Food Chem. 2001, 49, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- AgMRC. Blueberries. Available online: https://www.agmrc.org/commodities-products/fruits/blueberries (accessed on 3 March 2022).
- Gilbert, J.L.; Olmstead, J.W.; Colquhoun, T.A.; Levin, L.A.; Clark, D.G.; Moskowitz, H.R. Consumer-assisted selection of blueberry fruit quality traits. HortScience 2014, 49, 864–873. [Google Scholar] [CrossRef]
- Forney, C.F.; Kalt, W.; Jordan, M.A.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E. Blueberry and cranberry fruit composition during development. J. Berry Res. 2012, 2, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Muñoz, P.D. A review of the fruit volatiles found in blueberry and other vaccinium species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar] [CrossRef]
- Du, X.; Rouseff, R. Aroma active volatiles in four southern highbush blueberry cultivars determined by gas chromatography-olfactometry (gc-o) and gas chromatography-mass spectrometry (gc-ms). J. Agric. Food Chem. 2014, 62, 4537–4543. [Google Scholar] [CrossRef]
- Forney, C.F.; Qiu, S.; Jordan, M.A.; Munro Pennell, K.; Fillmore, S. Impact of species, growing location and cultivar on flavor chemistry of blueberry fruit. Acta Hortic. 2022, in press.
- Farneti, B.; Khomenko, I.; Grisenti, M.; Ajelli, M.; Betta, E.; Algarra, A.A.; Cappellin, L.; Aprea, E.; Gasperi, F.; Biasioli, F.; et al. Exploring blueberry aroma complexity by chromatographic and direct-injection spectrometric techniques. Front. Plant. Sci. 2017, 8, 617. [Google Scholar] [CrossRef]
- Forney, C.F. Horticultural and other factors affecting aroma volatile composition of small fruit. HortTechnology 2001, 11, 529–538. [Google Scholar] [CrossRef]
- Du, X.; Plotto, A.; Song, M.; Olmstead, J.; Rouseff, R. Volatile composition of four southern highbush blueberry cultivars and effect of growing location and harvest date. J. Agric. Food Chem. 2011, 59, 8347–8357. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Stein-Chisholm, R.E.; Boykin, D.L. Qualitative analysis of volatiles in rabbiteye blueberry cultivars at various maturities using rapid solid-phase microextraction. J. Am. Soc. Hortic. Sci. 2014, 139, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Horvat, R.J.; Schlotzhauer, W.S.; Chortyk, O.T.; Nottingham, S.F.; Payne, J.A. Comparison of volatile compounds from rabbiteye blueberry (vaccinium ashei) and deerberry (v. Stamineum) during maturation. J. Essent. Oil Res. 1996, 8, 645–648. [Google Scholar] [CrossRef]
- Parllment, T.H.; Kolor, M.G. Identification of the major volatile components of blueberry. J. Food Sci. 1975, 40, 762–763. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Guthart, M.J.; Gezan, S.A.; De Carvalho, M.P.; Schwieterman, M.L.; Colquhoun, T.A.; Bartoshuk, L.M.; Sims, C.A.; Clark, D.G.; Olmstead, J.W. Identifying breeding priorities for blueberry flavor using biochemical, sensory, and genotype by environment analyses. PLoS ONE 2015, 10, e0138494. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Peng, B.; Yuan, F. Volatile composition of eight blueberry cultivars and their relationship with sensory attributes. Flavor Fragr. J. 2020, 35, 443–453. [Google Scholar] [CrossRef]
- Qian, Y.L.; Zhang, D.; An, Y.; Zhou, Q.; Qian, M.C. Characterization of aroma-active compounds in northern highbush blueberries “bluecrop” (vaccinium corymbosum “bluecrop”) and “elliott” (vaccinium corymbosum “elliott”) by gas chromatography-olfactometry dilution analysis and odor activity value. J. Agric. Food Chem. 2021, 69, 5691–5701. [Google Scholar] [CrossRef]
- Cai, X.; Mai, R.Z.; Zou, J.J.; Zhang, H.Y.; Zeng, X.L.; Zheng, R.R.; Wang, C.Y. Analysis of aroma-active compounds in three sweet osmanthus (osmanthus fragrans) cultivars by gc-olfactometry and gc-ms. J. Zhejiang Univ. Sci B 2014, 15, 638–648. [Google Scholar] [CrossRef] [Green Version]
- Campo, E.; Ferreira, V.; Escudero, A.; Cacho, J. Prediction of the wine sensory properties related to grape variety from dynamic-headspace gas chromatography-olfactometry data. J. Agric. Food Chem. 2005, 53, 5682–5690. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, H.; Niu, Y.; Zhu, J. Characterization of the aroma-active compounds in banana (musa aaa red green) and their contributions to the enhancement of sweetness perception. J. Agric. Food Chem. 2021, 69, 15301–15313. [Google Scholar] [CrossRef]
- De-La-fuente-blanco, A.; Ferreira, V. Gas chromatography olfactometry (gc-o) for the (semi)quantitative screening of wine aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Wang, S. Effect of mechanical vibration on postharvest quality and volatile compounds of blueberry fruit. Food Chem. 2021, 349, 129216. [Google Scholar] [CrossRef] [PubMed]
- Lugemwa, F.N.; Lwande, W.; Bentley, M.D.; Mendel, M.J.; Alford, A.R. Volatiles of wild blueberry, vaccinium angustifolium: Possible attractants for the blueberry maggot fruit fly, rhagoletis mendax. J. Agric. Food Chem. 1989, 37, 232–233. [Google Scholar] [CrossRef]
- Forney, C.F.; Kalt, W.; Vander Kloet, S.P. Comparison of berry composition of selected vaccinium species (ericaceae) with gaylussacia dumosa. Botany 2012, 90, 355–363. [Google Scholar] [CrossRef]
- Du, X.; Olmstead, J.; Rouseff, R. Comparison of fast gas chomatography-surface acoustic wave (fgc-saw) detection and gc-ms for characterizing blueberry cultivars and maturity. J. Agric. Food Chem. 2012, 60, 5099–5106. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Gerbrandt, E.M.; Castellarin, S.D. Optimization and validation of a spme-gc/ms method for the determination of volatile compounds, including enantiomeric analysis, in northern highbush blueberries (vaccinium corymbosum l.). Food Chem. 2022, 368, 130812. [Google Scholar] [CrossRef]
- Cometto-Muñiz, J.E.; Cain, W.S.; Abraham, M.H.; Kumarsingh, R. Sensory properties of selected terpenes. Thresholds for odor, nasal pungency, nasal localization, and eye irritation. Ann. N. Y. Acad. Sci. 1998, 855, 648–651. [Google Scholar] [CrossRef] [Green Version]
- Elsharif, S.A.; Buettner, A. Structure-odor relationship study of c-6 unsaturated acyclic monoterpene alcohols: A comparative approach. In Flavour Sci; Siegmund, B., Leitner, E., Eds.; Verlag der Technischen Universität Graz: Graz, Austria, 2018. [Google Scholar] [CrossRef]
- Hirvi, T.; Honkanen, E. The aroma of blueberries. J. Sci. Food. Agric. 1983, 34, 992–996. [Google Scholar] [CrossRef]
- Qian, Y.P.L.; Zhou, Q.; Magana, A.A.; Qian, M.C. Comparative study of volatile composition of major northern highbush blueberry (vaccinium corymbosum) varieties. J. Food Compos. Anal. 2022, 110, 104538. [Google Scholar] [CrossRef]
- Di Cesare, L.F.; Nani, R.; Proietti, M.; Giombelli, R. Volatile composition of the fruit and juice of some blueberry cultivars grown in italy. Ind. Aliment. 1999, 38, 277–282. [Google Scholar]
- Farneti, B.; Emanuelli, F.; Khomenko, I.; Ajelli, M.; Biasioli, F.; Giongo, L. Development of a novel phenotypic roadmap to improve blueberry quality and storability. Front. Plant. Sci. 2020, 11, 1140. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Sater, H.; Lyrene, P.; Amadeu, R.R.; Sims, C.A.; Tieman, D.M.; Munoz, P.R. Terpene volatiles mediates the chemical basis of blueberry aroma and consumer acceptability. Food Res. Int. 2022, 158, 111468. [Google Scholar] [CrossRef]
- Horvat, R.J.; Senter, S.D. Comparison of the volatile constituents from rabbiteye blueberries (vaccinium ashei) during ripening. J. Food Sci. 1985, 50, 429–431. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. Gc-o-ms technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Rubico, S.M.; McDaniel, M.R. Sensory evaluation of acids by free-choice profiling. Chem. Senses 1992, 17, 273–289. [Google Scholar] [CrossRef]


| Compound | RI | MF Value (%) | Aroma Descriptors 2 | ID Basis 3 | ||||
|---|---|---|---|---|---|---|---|---|
| NS | PE | NB | QC | Ave | ||||
| Ethyl 2-methylbutanoate/3-Hexanone | 1052 | 70.6 | 56.6 | 79.2 | 74.0 | 70.1 | Fruity (30) 4, Sweet (30), Blueberry (17), Floral (10), Citrus (4) | RI, Std, AD, MS/ RI, Std, AD, MS |
| Methyl 2-methylbutanoate | 1012 | 72.1 | 59.6 | 73.0 | 67.1 | 68.0 | Sweet (32), Fruity (21), Blueberry (15), Floral (12), Caramel (8) | RI, Std, AD, MS |
| Methyl 3-methylbutanoate | 1022 | 64.5 | 60.3 | 49.3 | 66.1 | 60.1 | Fruity (15), Sweet (14), Rancid-cheesy (12), Pungent, sharp (10), Blueberry (8) | RI, Std, AD, MS |
| Ethyl 3-methylbutanoate | 1066 | 48.1 | 43.7 | 58.4 | 54.2 | 51.1 | Fruity (19), Sweet (18), Blueberry (11), Floral (9), Herb-like (3), Pungent-sharp (3), Rancid-cheesy (3) | RI, Std, AD, MS |
| Ethyl propanoate | 951 | 53.7 | 47.3 | 49.9 | 55.1 | 51.5 | Citrus (13), Green-grassy (11), Sweet (8), Chemical (6), Fruity (6), Floral (6) | RI, Std, AD, MS |
| (E)-2-Hexen-1-ol | 1401 | 57.5 | 39.5 | 44.7 | 61.1 | 50.7 | Rancid-cheesy (19), Sulfury (13), Earthy-musty (9), Pungent-sharp (8), Roasted-nutty (8) | RI, Std, AD, MS |
| (Z)-3-Hexen-1-ol | 1378 | 52.5 | 46.9 | 52.3 | 47.3 | 49.8 | Green-grassy (18), Rancid-cheesy (10), Earthy-musty (9), Herb-like (8), Sweet (5) | RI, Std, AD, MS |
| 1-Octen-3-one/Methyl 2-pentenoate 5 | 1303 | 64.5 | 30.1 | 51.6 | 49.9 | 49.0 | Earthy-musty (18), Rancid-cheesy (9), Herb-like (9), Pungent-sharp (8), Fruity (5) | RI, Std, AD, MS/ RI, AD, MS |
| 2-Dodecanone | 1719 | 48.4 | 48.4 | 42.9 | 54.2 | 48.5 | Floral (16), Fruity (10), Herb-like (10), Citrus (10), Sweet (9) | RI, Std, AD, MS |
| Ethyl 2-methylpropanoate | 970 | 51.4 | 29.4 | 49.5 | 60.2 | 47.6 | Sweet (23), Fruity (15), Floral (8), Citrus (6), Blueberry (4) | RI, Std, AD, MS |
| Linalool | 1531 | 51.4 | 36.9 | 46.5 | 47.3 | 45.5 | Floral (22), Sweet (19), Fruity (11), Herb-like (6), Blueberry (6) | RI, Std, AD, MS |
| Geraniol | 1827 | 63.2 | 0.0 | 45.0 | 65.4 | 43.4 | Floral (18), Sweet (10), Citrus (6), Fruity (5), Blueberry (5) | RI, Std, AD, MS |
| 2-Ethyl-1-hexanol | 1504 | 44.2 | 23.1 | 35.8 | 59.9 | 40.7 | Sweet (11), Floral (10), Herb-like (10), Citrus (9), Green-grassy (8) | RI, Std, AD, MS |
| Unknown 786 | 786 | 40.0 | 29.7 | 39.0 | 46.2 | 38.7 | Rancid-cheesy (18), Earthy-musty (8), Pungent-sharp (4), Blueberry (3), Floral (3), Herb-like (3) | |
| (E)-2-Hexenal | 1226 | 20.7 | 51.4 | 21.9 | 48.4 | 35.6 | Floral (10), Sweet (7), Fruity (5), Green-grassy (4), Herb-like (4), Roasted-nutty (4) | RI, Std, AD, MS |
| 1-Penten-3-one | 1031 | 25.3 | 37.9 | 27.1 | 46.2 | 34.1 | Rancid-cheesy (9), Earthy-musty (5), Floral (3), Green-grass (3), Herb-like (3), Pungent-sharp (3) | RI, Std, AD, MS |
| Hexanal | 1087 | 32.7 | 31.0 | 20.7 | 48.1 | 33.1 | Green-grassy (20), Herb-like (6), Blueberry (2), Earthy-musty (2), Roasted-nutty (2), Fruity (2) | RI, Std, AD, MS |
| 1-Pentanol | 1245 | 32.7 | 30.6 | 21.9 | 47.1 | 33.1 | Citrus (4), Roasted-nutty (3), Fruity (3), Rancid-cheesy (3), Pungent-sharp (3), Floral (3) | RI, Std, AD, MS |
| Methyl 3-methyl-2-butenoate | 1175 | 38.6 | 24.2 | 25.3 | 37.9 | 31.5 | Sweet (12), Fruity (11), Herb-like (5), Blueberry (3), Floral (3), Caramel (3) | RI, Std, AD, MS |
| 1-Hexanol | 1345 | 33.5 | 23.1 | 28.3 | 37.4 | 30.6 | Fruity (11), Sweet (10), Floral (4), Blueberry (4), Carmel (2) | RI, Std, AD, MS |
| (Z)-3-Hexenal | 1149 | 25.8 | 37.4 | 27.1 | 30.6 | 30.2 | Green-grassy (13), Herb-like (6), Rancid-cheesy (4), Fruity (3), Floral (3) | RI, Std, AD, MS |
| 3-Heptanone | 1152 | 23.1 | 0.0 | 49.4 | 35.8 | 27.1 | Green-grassy (14), Earthy-musty (7), Floral (4), Herb-like (3), Fruity (2), Roasted-nutty (2) | RI, Std, AD, MS |
| (E)-2-Nonenal | 1541 | 42.2 | 31.0 | 0.0 | 34.6 | 26.9 | Earthy-musty (8), Roasted-nutty (6), Rancid-cheesy (5), Sweet (4), Fruity (3) | RI, Std, AD, MS |
| Compound | RI | RI-Ref 2 | Similarity 3 | Volatile Composition (Area Counts) | F Prob 4 | % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NS | PE | NB | QC | Grand Mean | SEM | ||||||
| Acids | |||||||||||
| Octanoic acid | 2059 | 2051 | 902.2 | 4429 | -- 5 | 6898 | 8099 | 4857 | 5916 | ns | 0.93 |
| 2-Ethylhexanoic acid | 1946 | 1934 | 926.0 | 3587 | -- | 2396 | 4468 | 2613 | 2962 | ns | 0.50 |
| Heptanoic acid | 1952 | 1953 | 914.5 | 2383 | -- | 2442 | 3380 | 2051 | 2390 | ns | 0.39 |
| R-4-Methylhexanoic acid | 1928 | 1925 | 832.0 | 1096 | -- | 981 | 1512 | 897 | 1042 | ns | 0.17 |
| 5-Methylhexanoic acid | 1907 | 1914 6 | 910.3 | 825 | -- | 734 | 1204 | 691 | 812 | ns | 0.13 |
| 3-Methylhexanoic acid | 1886 | 1869 6 | 894.0 | 563 | -- | 651 | 962 | 544 | 657 | ns | 0.10 |
| Total | 12,884 | 0.000 | 14,102 | 19,625 | 11,653 | 13,779 | ns | 2.24 | |||
| Alcohols | |||||||||||
| 2-Ethyl-1-hexanol | 1486 | 1491 | 934.0 | 84,188 | 961 | 12,129 | 10,615 | 26,973 | 24,867 | ns | 5.17 |
| (E)-2-Hexen-1-ol | 1404 | 1402 | 935.0 | 3385 | 4479 | 4458 | 9004 | 5332 | 1241 | 0.068 | 1.02 |
| 1-Hexanol | 1349 | 1370 | 902.6 | 2966 | 1915 | 1900 | 7792 | 3643 | 973 | 0.015 | 0.70 |
| Ethanol | 929 | 929 | 944.0 | 2140 | 390 | 1335 | 9875 | 3435 | 2702 | ns | 0.66 |
| 1-Pentanol | 1245 | 1241 | 895.0 | 1715 | 2169 | -- | 3920 | 1951 | 1591 | ns | 0.37 |
| 3-Methyl-1-butanol | 1202 | 1204 | 913.0 | 326 | 351 | 1716 | 4004 | 1599 | 651 | 0.022 | 0.31 |
| (Z)-3-Hexen-1-ol | 1382 | 1381 | 932.0 | 906 | 817 | 698 | 3227 | 1412 | 411 | 0.013 | 0.27 |
| 2-Hexyn-1-ol | 1205 | 1207 | 885.5 | 1355 | 1477 | 827 | 267 | 981 | 721 | ns | 0.19 |
| 1-Heptanol | 1451 | 1463 | 893.0 | 781 | -- | 182 | 195 | 289 | 256 | ns | 0.06 |
| Total | 97,762 | 12,559 | 23,244 | 48,899 | 45,616 | 33,412 | ns | 8.75 | |||
| Aldehydes | |||||||||||
| (E)-2-Hexenal | 1223 | 1216 | 940.0 | 27,315 | 101,405 | 43,858 | 38,607 | 52,796 | 15,783 | 0.058 | 10.13 |
| Hexanal | 1080 | 1087 | 911.0 | 53,158 | 40,319 | 27,297 | 24,565 | 36,335 | 14,383 | ns | 6.97 |
| (Z)-3-Hexenal | 1144 | 1142 | 870.0 | 10,064 | 34,454 | 30,491 | 19,731 | 23,685 | 13,809 | ns | 4.54 |
| Heptanal | 1186 | 1182 | 892.5 | 43,871 | 891 | 6925 | 4602 | 14,072 | 14,755 | ns | 2.70 |
| 2-Ethylhexanal | 1188 | 1210 | 935.0 | 32,448 | -- | 887 | 789 | 8531 | 11,435 | ns | 1.64 |
| Pentanal | 982 | 981 | 897.0 | 9001 | 2580 | 3061 | 6614 | 5314 | 2160 | ns | 1.02 |
| (E,E)- 2,4-Hexadienal | 1405 | 1414 | 924.9 | 1043 | 2449 | 1979 | 1472 | 1736 | 746 | ns | 0.33 |
| Octanal | 1291 | 1288 | 920.0 | 3339 | 524 | 686 | 487 | 1259 | 1065 | ns | 0.24 |
| Nonanal | 1396 | 1392 | 883.0 | 1281 | 996 | 1223 | 1123 | 1156 | 477 | ns | 0.22 |
| (E)-2-Heptenal | 1328 | 1319 | 915.0 | 1552 | 1038 | 505 | 965 | 1015 | 504 | ns | 0.19 |
| 3-Methylbutanal | 918 | 912 | 868.0 | 1286 | 610 | -- | 775 | 668 | 381 | ns | 0.13 |
| 3-Methylpentanal | 1034 | 1032 7 | 829.6 | 2212 | -- | 408 | -- | 655 | 595 | ns | 0.13 |
| 2-Pentenal | 1133 | 1073 6 | 860.6 | 656 | 382 | 148 | 499 | 421 | 396 | ns | 0.08 |
| 2-Methylpentanal | 996 | na 8 | 862.6 | 1135 | -- | 243 | -- | 345 | 356 | ns | 0.07 |
| 4-Methylhexanal | 1158 | na | 815.0 | 1233 | -- | -- | -- | 308 | 478 | ns | 0.06 |
| Methacrolein | 888 | 886.3 | 913.0 | 965 | 216 | -- | -- | 295 | 323 | ns | 0.06 |
| Unknown 1165 | 1165 | NA | 845.0 | 1041 | -- | -- | -- | 260 | 521 | ns | 0.05 |
| Total | 191,601 | 185,863 | 117,712 | 100,229 | 148,851 | 78,165 | ns | 28.55 | |||
| Amines | |||||||||||
| Dimethylamine | 882 | na | 893.5 | -- | -- | 1497 | 1334 | 708 | 1083 | ns | 0.14 |
| Total | -- | -- | 1497 | 1334 | 708 | 1083 | ns | 0.14 | |||
| Esters-Branched Chain | |||||||||||
| Methyl 3-methylbutanoate | 1017 | 1018 | 943.5 | 76,446 | 102,763 | 125,131 | 95,571 | 99,978 | 28,679 | ns | 19.18 |
| Ethyl 3-methylbutanoate | 1063 | 1068 | 948.3 | 13,835 | 13,575 | 28,727 | 83,042 | 34,795 | 15,906 | 0.061 | 6.67 |
| Methyl 2-methylbutanoate | 1012 | 1048 | 901.9 | 10,061 | 9057 | 24,997 | 13,592 | 14,427 | 4852 | ns | 2.77 |
| Ethyl 2-methylbutanoate | 1046 | 1052 | 939.8 | 3362 | 2575 | 6763 | 12,207 | 6227 | 3146 | ns | 1.19 |
| Methyl 3-methyl-2-butenoate | 1170 | 1170 | 937.0 | 1806 | 2358 | 3925 | 1022 | 2278 | 746 | ns | 0.44 |
| Methyl 3-methyl-3-butenoate | 1118 | na | 926.0 | 1855 | 1961 | 2593 | 407 | 1704 | 306 | 0.012 | 0.33 |
| Methyl 2-methylpropanoate | 921 | 919 | 889.8 | 600 | 778 | 3579 | 969 | 1481 | 366 | 0.004 | 0.28 |
| 3-Methylbutyl acetate | 1120 | 1124 | 884.7 | 326 | 392 | 4359 | 99 | 1294 | 556 | 0.004 | 0.25 |
| Methyl 3-hydroxy-3-methylbutanoate | 1375 | 1374 | 884.3 | 523 | 2636 | 1245 | 271 | 1169 | 1269 | ns | 0.22 |
| Ethyl 2-hydroxy-3-methylbutanoate | 1426 | 1422 | 922.0 | 904 | -- | 1661 | 514 | 770 | 403 | ns | 0.15 |
| Ethyl 2-methylpropanoate | 961 | 960 | 918.0 | 127 | -- | 1473 | 1170 | 693 | 375 | 0.074 | 0.13 |
| Total | 109,844 | 136,094 | 204,453 | 208,864 | 164,814 | 56,604 | ns | 31.62 | |||
| Esters-Straight Chain | |||||||||||
| Ethyl Acetate | 888 | 879 | 953.0 | 65,690 | 10,775 | 89,966 | 67,634 | 58,516 | 13,283 | 0.026 | 11.23 |
| Methyl acetate | 825 | 815 | 973.7 | 15,204 | 3424 | 29,942 | 22,563 | 17,783 | 5228 | 0.052 | 3.41 |
| Methyl butanoate | 986 | 983 | 942.0 | 1382 | 1173 | 2380 | 2994 | 1982 | 1007 | ns | 0.38 |
| Ethyl butanoate | 1032 | 1032 | 894.5 | -- | -- | 919 | 3931 | 1212 | 1564 | ns | 0.23 |
| (E)-Hexenyl acetate | 1333 | 1334 | 888.6 | 212 | 857 | 935 | 1477 | 870 | 620 | ns | 0.17 |
| Ethyl propanoate | 954 | 952 | 874.6 | 289 | 0 | 1338 | 1034 | 665 | 617 | ns | 0.13 |
| Methyl propanoate | 907 | 906 | 937.5 | 401 | 422 | 1561 | 221 | 651 | 264 | 0.039 | 0.12 |
| Pentyl acetate | 1120 | 1175 | 875.5 | -- | -- | -- | 2511 | 628 | 891 | ns | 0.12 |
| Methyl 2-pentenoate | 1305 | na | 729.4 | 688 | 698 | 855 | -- | 560 | 569 | ns | 0.11 |
| Total | 83,866 | 17,349 | 127,897 | 102,364 | 82,869 | 24,042 | 0.016 | 15.90 | |||
| Furans | |||||||||||
| 2-Ethylfuran | 954 | 949 | 875.0 | 558 | 511 | 529 | 411 | 502 | 421 | ns | 0.10 |
| 2-Ethyl-5-methyltetrahydrofuran | 939 | na | 777.6 | 228 | 151 | -- | 856 | 309 | 428 | ns | 0.06 |
| Total | 786 | 663 | 529 | 1267 | 811 | 849 | ns | 0.16 | |||
| Hydrocarbons | |||||||||||
| Toluene | 1041 | 1042 | 912.5 | 9754 | 1147 | -- | 2228 | 3282 | 2965 | ns | 0.63 |
| (Z)-1-Ethyl-2-methylcyclopropane | 1061 | 1062 | 752.5 | -- | -- | 7323 | 409 | 1933 | 3701 | ns | 0.37 |
| Benzene | 946 | 943 | 965.0 | 7207 | -- | 206 | 0 | 1853 | 1872 | 0.084 | 0.36 |
| Hexane | 600 | 600 | 909.0 | 1085 | 1138 | 1729 | 783 | 1183 | 380 | ns | 0.23 |
| m-Xylene | 1144 | 1144 | 953.6 | 1002 | 2253 | -- | -- | 814 | 322 | 0.007 | 0.16 |
| Ethylbenzene | 1130 | 1141 | 916.4 | 2037 | 826 | -- | -- | 716 | 534 | ns | 0.14 |
| 4-Methyl-1,3-pentadiene | 782 | 796 6 | 946.8 | 227 | 470 | 876 | 757 | 583 | 260 | ns | 0.11 |
| o-Xylene | 1190 | 1188 | 934 | 984 | 975 | -- | -- | 490 | 511 | ns | 0.09 |
| 2-Octene | 841 | 858 | 861.8 | 1217 | -- | -- | 220 | 359 | 396 | ns | 0.07 |
| Indene | 1495 | 1471 | 927.0 | 1117 | -- | -- | -- | 279 | 282 | 0.073 | 0.05 |
| 4,4-Dimethyl-1,2-pentadiene | 954 | na | 721.7 | 556 | 357 | -- | 108 | 255 | 370 | ns | 0.05 |
| 3-Propoxy-1-propene | 1007 | na | 865.0 | 938 | -- | -- | -- | 235 | 469 | ns | 0.04 |
| Total | 26,124 | 7165 | 10,134 | 4505 | 11,982 | 12,063 | ns | 2.30 | |||
| Ketones | |||||||||||
| 2-Heptanone | 1182 | 1184 | 922.5 | 19,191 | 8673 | 6086 | 4419 | 9592 | 6738 | ns | 1.84 |
| 2-Nonanone | 1389 | 1390 | 909.75 | 3152 | 6738 | 2002 | 7649 | 4885 | 2166 | ns | 0.94 |
| 3-Heptanone | 1152 | 1151 | 865 | 9414 | 231 | 2065 | 616 | 3081 | 2745 | ns | 0.59 |
| 4-Heptanone | 1124 | 1142 | 912.75 | 6389 | -- | 1833 | 176 | 2100 | 1874 | ns | 0.40 |
| 5-Methyl-3-methylene-2-hexanone | 1254 | na | 811.0 | 8056 | -- | -- | -- | 2014 | 3120 | ns | 0.39 |
| 6-Methyl-5-hepten-2-one | 1339 | 1340 | 879.7 | 1633 | 1768 | 1165 | 2348 | 1729 | 409 | ns | 0.33 |
| 2-Methyl-2-hepten-4-one | 1214 | NA | 808.5 | 5568 | -- | -- | -- | 1392 | 2260 | ns | 0.27 |
| 3-Hexanone | 1049 | 1047 | 898 | 4281 | -- | 226 | -- | 1127 | 1325 | ns | 0.22 |
| 2-Butanone | 904 | 903 | 843.7 | 4053 | 159 | -- | -- | 1053 | 1070 | 0.09 | 0.20 |
| 3-Methyl-3-buten-2-one | 996 | 996 | 922.8 | 616 | 1265 | -- | 1229 | 778 | 726 | ns | 0.15 |
| 1-Penten-3-one | 1022 | 1030 | 881.75 | 664 | 826 | 186 | 1011 | 672 | 520 | ns | 0.13 |
| 2-Methylcyclopentanone | 1198 | 1187 | 841.0 | 2125 | -- | -- | -- | 531 | 703 | ns | 0.10 |
| 5-Hexen-2-one | 1130 | 1137 7 | 939.0 | 1673 | -- | -- | -- | 418 | 564 | ns | 0.08 |
| Acetone | 819 | 817 | 958.5 | 1110 | 190 | -- | 256 | 389 | 319 | ns | 0.07 |
| 2-Octanone | 1286 | 1290 | 915.0 | 1499 | -- | -- | -- | 375 | 483 | ns | 0.07 |
| 1-Octen-3-one | 1304 | 1301 | 842.5 | 1446 | -- | -- | -- | 361 | 531 | ns | 0.07 |
| 3-Methyl-2-butanone | 932 | 939 | 826.0 | 1359 | -- | -- | -- | 340 | 528 | ns | 0.07 |
| 3-Hexen-2-one | 1218 | 1212 | 903.0 | -- | -- | -- | 117 | 29 | 58 | ns | 0.01 |
| Total | 72,230 | 19,851 | 13,564 | 17,822 | 30,866 | 26,137 | ns | 5.92 | |||
| Monoterpenoids | |||||||||||
| Linalool | 1542 | 1540 | 897.4 | 10,974 | 8268 | 12,813 | 13,949 | 11,501 | 3640 | ns | 2.21 |
| (2R,5S)-2-Methyl-5-(prop-1-en-2-yl)-2-vinyltetrahydrofuran | 1243 | 1226 | 887 | 2217 | 2380 | 4403 | 3021 | 3005 | 1601 | ns | 0.58 |
| α-Terpineol | 1698 | 1690 | 922.5 | 1589 | 924 | 1739 | 2598 | 1713 | 482 | ns | 0.33 |
| (2R,5R)-2-Methyl-5-(prop-1-en-2-yl)-2-vinyltetrahydrofuran | 1211 | 1237 | 778.3 | 1415 | 925 | 814 | 1660 | 1204 | 932 | ns | 0.23 |
| α-Myrcene | 1162 | 1164 | 894.4 | 956 | 973 | 1158 | 1378 | 1116 | 497 | ns | 0.21 |
| 2-(1-Hexyn-1-yl)-3-(methoxymethyl)oxirane | 1325 | na | 735.3 | 504 | 962 | 1802 | 597 | 967 | 622 | ns | 0.19 |
| Limonene | 1202 | 1198 | 908.8 | 543 | 580 | 983 | 1394 | 875 | 359 | ns | 0.17 |
| Eucalyptol | 1209 | 1225 | 880 | -- | 421 | 2375 | -- | 699 | 298 | 0.004 | 0.13 |
| β-Ocimene | 1252 | 1248 | 898 | 1077 | 428 | 702 | 582 | 697 | 325 | ns | 0.13 |
| Terpinolene | 1286 | 1281 | 906.6 | 651 | 402 | 851 | 788 | 673 | 377 | ns | 0.13 |
| p-Cymenene | 1444 | 1439 | 933.1 | 754 | 478 | 774 | 648 | 663 | 276 | ns | 0.13 |
| Total | 20,680 | 16,742 | 28,414 | 26,617 | 23,113 | 9409 | ns | 4.43 | |||
| Grand Total | 615,776 | 396,284 | 541,546 | 531,525 | 521,283 | 255,543 | ns | 100.0 | |||
| Compound | RI | MF Value (%) | Aroma Descriptors 2 | ID Basis 3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Duke | Brigitta | Jersey | Liberty | Aurora | Ave | ||||
| Geraniol | 1827 | 68.0 | 69.7 | 75.7 | 74.0 | 66.1 | 70.7 | Floral (38) 4, Citrus (18), Fruity (18), Sweet (15), Blueberry (11) | RI, Std, AD, MS |
| (Z)-3-Hexen-1-ol | 1378 | 70.6 | 65.3 | 61.0 | 64.5 | 72.1 | 66.7 | Green-grassy (20), Earthy-musty (18), Herb-like (16), Rancid-cheesy (14), Floral (13) | RI, Std, AD, MS |
| 1-Octen-3-one | 1303 | 60.2 | 58.5 | 66.3 | 61.1 | 64.5 | 62.1 | Earthy-musty (19), Mushroom (13), Pungent-sharp (11), Herb-like (10), Rancid-cheesy (9) | RI, Std, AD, MS |
| α-Terpineol | 1720 | 51.4 | 64.8 | 46.2 | 58.4 | 65.7 | 57.3 | Floral (20), Herb-like (16), Green-grassy (14), Citrus (11), Fruity (9) | RI, Std, AD, MS |
| Linalool | 1531 | 65.3 | 46.5 | 66.9 | 44.7 | 55.1 | 55.7 | Floral (19), Fruity (18), Sweet (18),Herb-like (9), Citrus (8) | RI, Std, AD, MS |
| 2-Ethyl-1-hexanol | 1495 | 46.5 | 59.3 | 69.7 | 52.5 | 31.5 | 51.9 | Floral (18), Sweet (11), Fruity (8), Citrus (8), Green-grassy (7), Herb-like (7) | RI, Std, AD, MS |
| 2-Undecanone | 1592 | 37.7 | 44.2 | 33.7 | 55.9 | 44.7 | 43.3 | Green-grassy (12), Floral (11), Herb-like (9), Earthy-musty (5), Rancid-cheesy (4), Pungent-sharp (4), Blueberry (4), Roasted-nutty (4) | RI, Std, AD, MS |
| (E)-2-Hexenal/ɑ-Ocimene | 1228 | 34.8 | 45.6 | 62.0 | 72.1 | 42.9 | Sweet (16), Floral (14), Fruity (12), Citrus (5), Green-grassy (5) | RI, Std, AD, MS/ RI, Std, AD, MS | |
| Ethyl 3-methylbutanoate | 1067 | 37.0 | 50.4 | 26.8 | 60.2 | 42.4 | Sweet (15), Blueberry (14), Fruity (14), Floral (6) | RI, Std, AD, MS | |
| Hexanal | 1088 | 40.0 | 60.8 | 49.3 | 62.0 | 42.4 | Green-grassy (28), Floral (8), Herb-like (6), Fruity (4), Sweet (2) | RI, Std, AD, MS | |
| (E)-2-Hexen-1-ol/2,6-Dimethyl-2,6-octadiene 5 | 1401 | 58.2 | 54.8 | 42.2 | 34.8 | 38.0 | Rancid-cheesy (16), Roasted-nutty (9), Earthy-musty (7), Sulfury (5), Blueberry (3), Herb-like (3) | RI, Std, AD, MS/ RI, MS | |
| Ethyl 2-methylbutanoate | 1051 | 33.5 | 30.6 | 61.1 | 62.7 | 37.6 | Sweet (16), Fruity (15), Blueberry (8), Floral (7), Citrus (2), Herb-like (2) | RI, Std, AD, MS | |
| 1-Pentanol | 1244 | 40.4 | 52.5 | 46.2 | 45.4 | 36.9 | Herb-like (7), Rancid-cheesy, (7) Earthy-musty (5), Citrus (5), Pungent-sharp (4), Floral (4), Roasted-nutty (4), Sweet (4) | RI, Std, AD, MS | |
| Nonanal | 1395 | 33.5 | 59.3 | 43.2 | 45.6 | 36.3 | Herb-like (5), Fruity (2), Sweet (2), Floral (2), Citrus (2), Pungent-sharp (2) | RI, Std, AD, MS | |
| Methyl 2-methylbutanoate | 1014 | 24.2 | 48.1 | 69.7 | 37.7 | 35.9 | Fruity (22), Sweet (17), Blueberry (8), Floral (6), Citrus (3), Carmel (3) | RI, Std, AD, MS | |
| (Z)-3-Hexenal | 1151 | 39.0 | 48.3 | 42.1 | 48.1 | 35.5 | Green-grassy (22), Floral (9), Herb-like (7), Fruity (6), Citrus (4) | RI, Std, AD, MS | |
| 2-Nonanone | 1384 | 56.6 | 17.9 | 48.2 | 39.8 | 32.5 | Sweet (13), Fruity (12), Floral (10), Rancid-cheesy (6), Blueberry (5), Earthy-musty (5) | RI, Std, AD, MS | |
| (2R,5S)-2-Methyl-5-(prop-1-en-2-yl)-2-vinyltetrahydrofuran 5/β-Phellandrene 5/Eucalyptol | 1212 | 42.9 | 42.9 | 52.3 | 24.2 | 32.5 | Floral (11), Sweet (7), Fruity (6), Citrus (6), Herb-like (4), Green-grassy (4), Rancid-cheesy (4) | RI, Std, MS/ RI, Std, AD, MS/ RI, Std, AD, MS | |
| 1-Penten-3-one/Ethyl butanoate | 1031 | 21.9 | 24.0 | 39.8 | 67.9 | 30.7 | Roasted-nutty (7), Herb-like (5), Earthy-musty (5), Fruity (4), Floral (3), Green-grassy (3), Rancid-cheesy (3) | RI, Std, AD, MS /RI, Std, AD, MS | |
| (E)-2-Nonenal | 1540 | 29.2 | 44.7 | 52.5 | 25.3 | Earthy-musty (7), Rancid-cheesy (6), Roasted-nutty (5), Forest soil (4), Blueberry (3), Floral (3), Chemical (3), Herb-like (3) | RI, Std, AD, MS | ||
| 2,6,6-Trimethyl-2-vinyltetrahydropyran 5 | 1111 | 29.2 | 40.0 | 35.8 | 18.9 | 24.8 | Fruity (5), Earthy-musty (5), Herb-like (4), Rancid-cheesy (3), Roasted-nutty (3), Green-grassy (3), Citrus (3) | RI, AD, MS | |
| 2-Heptanone | 1180 | 31.0 | 33.5 | 49.4 | 22.8 | Sweet (6), Floral (6), Green-grassy (5), Fruity (3), Earthy-musty (3), Roasted-nutty (3) | RI, Std, AD, MS | ||
| (E,E)-2,4-Heptadienal 5/(E)-Linalool oxide | 1468 | 24.5 | 53.7 | 29.5 | 21.5 | Roasted-nutty (9), Rancid-cheesy (8), Earthy-musty (2), Blueberry (2) | RI, AD, MS/ RI, Std, AD, MS | ||
| Methyl 3-methylbutanoate | 1021 | 43.8 | 56.6 | 20.1 | Sweet (6), Fruity (5), Rancid-cheesy (4), Pungent-sharp (3), Blueberry (2), Caramel (2), Earthy-musty (2) | RI, Std, AD, MS | |||
| Unknown 787 | 787 | 55.9 | 34.4 | 18.1 | Rancid-cheesy (9), Earthy-musty (7), Pungent-sharp (5), Sweet (2) | ||||
| (E)-2-Heptenal | 1330 | 33.5 | 50.4 | 16.8 | Floral (4), Fruity (4), Citrus (4), Sweet (3), Roasted-nutty (3) | RI, Std, AD, MS | |||
| Decanal | 1491 | 24.5 | 24.5 | 31.6 | 16.1 | Earthy-musty (5), Rancid-cheesy (3), Herb-like (3), Fruity (2), Floral (2), Green-grassy (2), Sweet (2) | RI, Std, AD, MS | ||
| 2,2-Dimethyl propanoic acid 5 | 1572 | 25.3 | 24.5 | 10.0 | Citrus (1), Blueberry (1), Carmel (1), Pungent-sharp (1), Roasted-nutty (1), Animal (1), cooked chicken (1), Acidic-vinegar (1), Earthy-musty (1), Herb-like (1) | RI, Std, MS | |||
| D-Carvone | 1752 | 25.3 | 24.2 | 9.9 | Floral (4), Fruity (3), Roasted-nutty (3), Herb-like (2), Green-grassy (2), Sweet (1) Acidic-vinegar (1), Earthy-musty (1), Rancid-cheesy (1) | RI, Std, AD, MS | |||
| Acetophenone | 1643 | 45.6 | 9.1 | Floral (2), Green-grassy (2), Blueberry (1), Fruity (1), Earthy-musty (2), Roasted-nutty (1), Chemical (1), Burnt Toast (1), Burning grass (1), Rancid-cheesy (1) | RI, Std, AD, MS | ||||
| 2-Dodecanone | 1711 | 44.7 | 8.9 | Floral (4), Roasted-nutty (3), Herb-like (3), Green-grassy (1), Pungent-sharp (1), Rancid-cheesy (1) | RI, Std, AD, MS | ||||
| 2-Methyl -1,4-pentadiene 5 | 1100 | 36.9 | 7.4 | Roasted-nutty (2), Fruity (2), Sweet (1), Floral (1), Herb-like (1), Pungent-sharp (1), Ground coffee (1), Green-grassy (1), Chemical (1) | RI, MS | ||||
| (E)-Isopiperitenol 5 | 1769 | 29.5 | 7.3 | 7.4 | Sweet (3), Blueberry (2), Citrus (1), Fruity (1), Floral (1), Blackberry (1), Herb-like (1), Roasted-nutty (1) | RI, AD, MS | |||
| Geranyl acetone 5 | 1863 | 34.8 | 7.0 | Fruity (3), Sweet (2), Roasted-nutty (2), Floral (1), Citrus (1), Blueberry (1), Earthy-musty (1), Horse (1) | RI, AD, MS | ||||
| 2,3,6-Trimethyl-1,5-heptadiene 5 | 1407 | 34.4 | 6.9 | Floral (4), Sweet (2), Fruity (2), Earthy-musty (2), Citrus (1), Blueberry (1) | RI, MS | ||||
| ɑ-Phellandrene 1 | 1170 | 32.7 | 6.5 | Rancid-cheesy (3), Fruity (3), Sweet (2), Blueberry (1), Floral (1), Earthy-musty (1), Herb-like (1), Roasted-nutty (1) | RI, Std, AD, MS | ||||
| Nerol | 1797 | 29.5 | 5.9 | Sweet (3), Floral (3), Citrus (2), Herb-like (2), Dill (1), Green-grassy (1), Acidic-vinegar (1) | RI, Std, AD, MS | ||||
| Anethofuran 5 | 1509 | 29.2 | 5.8 | Roasted, nutty (3), Floral (2), Rose (1), Sweet (1), Fruity (1), Earthy, musty (1), Camp Fire (1), Herb-like (1), Rancid, cheesy (1) | RI, AD, MS | ||||
| (E)-3-Hexen-1-ol | 1355 | 28.0 | 5.6 | Sweet (2), Citrus (2), Foral (2), Rancid-cheesy (2), Fruity (1), Blueberry (1) | RI, Std, AD, MS | ||||
| Octanal | 1290 | 26.7 | 5.3 | Rancid-cheesy (3), Citrus (2), Roasted-nutty (1), Sweet(1), Floral (1), Earthy-musty (1) | RI, Std, AD, MS | ||||
| Limonene | 1203 | 25.8 | 5.2 | Earthy-musty (2), Sweet (2), Green-grassy (1), Floral (1) | RI, Std, AD, MS | ||||
| 5-Methylhexanoic acid | 1906 | 25.3 | 5.1 | Floral (2), Fruity (1), Rancid-cheesy (1), Earthy-musty (1), Sulfury (1), Chemical cleaner (1) | RI, Std, AD, MS | ||||
| Compound | RI | RI-Ref 2 | Sim 3 | Volatile Composition (Area Counts) | F Prob 4 | % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duke | Brigitta | Jersey | Liberty | Aurora | Mean | SEM | ||||||
| Acids | ||||||||||||
| 2-Ethylhexenoic acid | 1935 | 1952 | 933 | -- 5 | 227 | 306 | -- | 442 | 195 | 191 | ns | 0.10 |
| Total | -- | 227 | 306 | -- | 442 | 195 | 191 | ns | 0.10 | |||
| Alcohols | ||||||||||||
| (E)-2-Hexen-1-ol | 1404 | 1402 | 933 | 4509 | 2895 | 1493 | 4395 | 4530 | 3564 | 646 | 0.012 | 1.82 |
| (E)-2-Hexen-4-yn-1-ol | 1221 | na 6 | 856 | 1854 | 1604 | 685 | 2651 | 4001 | 2159 | 718 | 0.046 | 1.10 |
| 1-Hexanol | 1347 | 1373 | 923 | 2141 | 1058 | 1531 | 2447 | 2324 | 1900 | 249 | 0.004 | 0.97 |
| 2-Ethyl-1-hexanol | 1487 | 1492 | 924 | 1885 | 1827 | 1468 | 538 | 3080 | 1760 | 1034 | ns | 0.90 |
| 1-Pentanol | 1245 | 1242 | 896 | 636 | 1514 | 1298 | 1414 | 634 | 1099 | 221 | 0.022 | 0.56 |
| 2-Hexyn-1-ol | 1205 | 1207 | 886 | 993 | 960 | 643 | 1418 | 1174 | 1038 | 349 | ns | 0.53 |
| (Z)-3-Hexen-1-ol | 1383 | 1381 | 941 | 1177 | 149 | 1195 | 835 | 540 | 779 | 143 | <0.001 | 0.40 |
| 2-Butanol | 1016 | 1020 | 843 | 221 | 188 | 329 | 638 | 449 | 365 | 69 | 0.001 | 0.19 |
| 1-Octen-3-ol | 1446 | 1443 | 868 | 141 | 288 | 215 | 242 | 266 | 230 | 53 | ns | 0.12 |
| Cyclobutanol | 1043 | NA | 789 | 131 | 198 | 172 | 131 | 284 | 183 | 51 | ns | 0.09 |
| Total | 13,689 | 10,680 | 9028 | 14,709 | 17,282 | 13,185 | 2155 | ns | 6.69 | |||
| Aldehydes | ||||||||||||
| (E)-2-Hexenal | 1222 | 1216 | 941 | 49,150 | 49,217 | 22,884 | 69,553 | 73,969 | 52,955 | 6302 | <0.001 | 27.09 |
| Hexanal | 1083 | 1087 | 905 | 29,102 | 14,807 | 14,784 | 21,079 | 18,948 | 19,744 | 2270 | 0.002 | 10.10 |
| (Z)-3-Hexenal | 1148 | 1146 7 | 887 | 31,965 | 1581 | 8590 | 9550 | 7589 | 11855 | 3119 | <0.001 | 6.06 |
| Pentanal | 979 | 981 | 899 | 1800 | 7104 | 1710 | 5148 | 4975 | 4148 | 1065 | 0.008 | 2.12 |
| (E,E)-2,4-Hexadienal | 1405 | 1401 | 926 | 2022 | 469 | 636 | 1162 | 1068 | 1071 | 201 | <0.001 | 0.55 |
| (E)-3-Hexenal | 1137 | 1138 | 866 | 1302 | 745 | 732 | 886 | 878 | 909 | 232 | ns | 0.46 |
| Heptanal | 1186 | 1184 | 909 | 274 | 1161 | 425 | 697 | 1338 | 779 | 231 | 0.018 | 0.40 |
| Nonanal | 1396 | 1389 | 897 | 990 | 621 | 298 | 331 | 233 | 494 | 276 | ns | 0.25 |
| Methacrolein | 881 | 890 | 844 | 113 | 610 | 187 | 333 | 589 | 366 | 110 | 0.013 | 0.19 |
| 4-Pentenal | 1133 | 1129 | 852 | 88 | 345 | 143 | 287 | 755 | 323 | 59 | <0.001 | 0.17 |
| (E)-2-Heptenal | 1329 | 1323 | 922 | 319 | 388 | 202 | 212 | 318 | 288 | 109 | ns | 0.15 |
| Octanal | 1291 | 1284 | 919 | 249 | 196 | -- | 75 | 244 | 153 | 90 | ns | 0.08 |
| (E)-2-Octenal | 918 | 913 | 835 | 30 | 176 | -- | 82 | 263 | 110 | 64 | 0.053 | 0.06 |
| 3-Methylbutanal | 1435 | 1436 | 909 | 81 | 81 | 130 | 35 | 116 | 89 | 53 | ns | 0.05 |
| Total | 117,485 | 77,501 | 50,721 | 109,430 | 111,282 | 95,184 | 9472 | <0.001 | 47.72 | |||
| Esters-Branched Chain | ||||||||||||
| Methyl 3-methylbutanoate | 1018 | 1016 | 933 | 1803 | 5783 | 12664 | -- | 1332 | 4316 | 826 | <0.001 | 2.21 |
| Methyl 2-methylbutanoate | 1010 | 1008 | 896 | -- | 563 | 1012 | -- | -- | 315 | 68 | <0.001 | 0.16 |
| Ethyl 3-methylbutanoate | 1065 | 1066 | 942 | 7 | 177 | 398 | -- | -- | 116 | 48 | <0.001 | 0.06 |
| Total | 1809 | 6523 | 14,073 | -- | 1332 | 4895 | 1062 | <0.001 | 2.43 | |||
| Esters-Straight Chain | ||||||||||||
| Ethyl acetate | 894 | 893 | 866 | 331 | 74 | 3093 | -- | -- | 699 | 133 | <0.001 | 0.36 |
| Methyl acetate | 858 | 856 | 857 | -- | 1639 | 1288 | -- | 409 | 667 | 140 | <0.001 | 0.34 |
| (Z)-2-Hexen-1-ol acetate | 1334 | 1329 | 881 | 1397 | 337 | 80 | 665 | 539 | 604 | 116 | <0.001 | 0.31 |
| Total | 1727 | 2051 | 4461 | 665 | 948 | 1887 | 320 | <0.001 | 1.01 | |||
| Furans | ||||||||||||
| 2-Ethylfuran | 951 | 950 | 918 | 364 | 89 | 220 | 393 | 481 | 309 | 93 | 0.06 | 0.16 |
| Tetrahydrofuran | 880 | 862 | 850 | 46 | -- | 242 | 422 | 185 | 179 | 20 | <0.001 | 0.09 |
| Total | 410 | 89 | 462 | 816 | 666 | 535 | 122 | 0.001 | 0.25 | |||
| Hydrocarbons | ||||||||||||
| Hexane | 594 | 600 | 852 | 10127 | 7083 | 8141 | 10429 | 8238 | 8804 | 4146 | ns | 4.50 |
| Ethylcyclobutane | 809 | 692 7 | 886 | 2079 | 1936 | 1980 | 1994 | 1986 | 1995 | 712 | ns | 1.02 |
| (Z,Z)-2,4-Hexadiene | 738 | na | 933 | 686 | 51 | 45 | 260 | 383 | 285 | 107 | 0.003 | 0.15 |
| Toluene | 1048 | 1053 | 916 | 133 | 121 | 605 | -- | -- | 172 | 40 | <0.001 | 0.09 |
| (E,Z)-2,4-Hexadiene | 754 | na | 937 | 23 | 205 | 62 | 286 | 157 | 147 | 73 | ns | 0.08 |
| Total | 13,048 | 9395 | 10,834 | 12,969 | 10,765 | 11,463 | 4888 | ns | 5.83 | |||
| Ketones | ||||||||||||
| 2-Butanone | 905 | 903 | 916 | 21,885 | 5006 | 16,128 | 14,576 | 8948 | 13,309 | 854 | <0.001 | 6.81 |
| 6-Methyl-5-hepten-2-one | 1338 | 1340 | 884 | 3923 | 2772 | 657 | 2336 | 1820 | 2302 | 458 | 0.002 | 1.18 |
| 3-Ethylidene-1-methoxy-5-hexen-2-one | 1325 | na | 731 | 5929 | 119 | 43 | 1568 | -- | 1532 | 51 | <0.001 | 0.78 |
| Acetone | 852 | 836 | 953 | 354 | 406 | 377 | 554 | 348 | 408 | 48 | 0.043 | 0.21 |
| 1-Penten-3-one | 1022 | 1032 | 878 | 164 | 484 | 134 | 332 | 506 | 324 | 98 | 0.04 | 0.17 |
| 2-Heptanone | 1182 | 1180 | 927 | 843 | 44 | 26 | 346 | 195 | 291 | 143 | 0.005 | 0.15 |
| 3-Methyl-3-buten-2-one | 995 | 997 8 | 935 | 155 | 368 | 161 | 61 | 679 | 285 | 109 | 0.006 | 0.15 |
| 1-Octen-3-one | 1304 | 1301 | 842.5 | 35 | 255 | -- | 58 | 219 | 113 | 71 | 0.066 | 0.06 |
| Total | 33,287 | 9454 | 17,526 | 19,832 | 12,715 | 17,923 | 1963 | <0.001 | 9.50 | |||
| Monoterpenoids | ||||||||||||
| Linalool | 1542 | 1540 | 902 | 48,358 | 8419 | 7395 | 15,063 | 9012 | 17,649 | 1408 | <0.001 | 9.03 |
| Linalool acetate | 1543 | 1548 | 866 | 31,870 | -- | -- | 34 | 30 | 6387 | 23 | <0.001 | 3.27 |
| α-Terpineol | 1698 | 1690 | 933 | 11,103 | 1428 | 2097 | 3456 | 2993 | 4215 | 1696 | 0.005 | 2.16 |
| (2R,5R)-2-Methyl-5-(prop-1-en-2-yl)-2-vinyltetrahydrofuran | 1243 | 1233 | 905 | 12,803 | 216 | 105 | 2565 | 96 | 3157 | 94 | <0.001 | 1.61 |
| Limonene | 1211 | 1226 | 892 | 11,375 | 437 | 543 | 2500 | 917 | 3154 | 1118 | <0.001 | 1.61 |
| β-Myrcene | 1201 | 1226 | 885 | 8391 | 533 | 540 | 1228 | 707 | 2280 | 1119 | <0.001 | 1.17 |
| (2R,5S)-2-Methyl-5-(prop-1-en-2-yl)-2-vinyltetrahydrofuran | 1162 | 1164 | 886 | 9476 | -- | -- | 624 | -- | 2020 | 75 | <0.001 | 1.03 |
| α-Ocimene | 1234 | 1238 | 900 | 6264 | 334 | 233 | 798 | 383 | 1602 | 889 | <0.001 | 0.82 |
| Terpinolene | 1210 | 1204 | 850 | 6576 | 18 | 46 | 644 | 158 | 1489 | 1024 | <0.001 | 0.76 |
| Eucalyptol | 1285 | 1280 | 898 | -- | 4650 | 250 | 2417 | 38 | 1471 | 245 | <0.001 | 0.75 |
| 1,3,8-p-Menthatriene | 1234 | 1242 | 912 | 5427 | -- | -- | 1175 | -- | 1320 | 239 | <0.001 | 0.68 |
| Dehydro-p-cymene | 1616 | 1627 7 | 888 | 4424 | -- | 42 | 456 | -- | 984 | 67 | <0.001 | 0.50 |
| β-Ocimene | 1443 | 1440 | 915 | 3370 | 175 | 121 | 468 | 279 | 883 | 481 | <0.001 | 0.45 |
| o-Cymene | 1276 | 1284 | 936 | 2316 | -- | -- | 258 | 0 | 515 | 5 | <0.001 | 0.26 |
| Geraniol | 1844 | 1851 | 855 | 1614 | 44 | 78 | 314 | 336 | 477 | 247 | 0.001 | 0.24 |
| α-Terpinene | 1182 | 1178 | 858 | 1988 | -- | -- | 193 | 0 | 436 | 306 | <0.001 | 0.22 |
| 2,6,6-Trimethyl-2-vinyltetrahydropyran | 1111 | 1112 | 827 | 906 | -- | -- | 57 | 1094 | 411 | 189 | <0.001 | 0.21 |
| (E)-Geranylacetone | 1849 | 1857 | 820 | 364 | 900 | -- | 549 | 191 | 401 | 77 | <0.001 | 0.21 |
| (E)-Dihydrocarvone | 1616 | 1627 7 | 888 | 1594 | -- | -- | -- | -- | 319 | 0 | ns | 0.16 |
| Unknown 1611 | 1611 | 1035 | -- | -- | 381 | -- | 283 | 72 | <0.001 | 0.14 | ||
| 2,6-Dimethyl-2,6-octadiene | 1405 | na | 797 | 65 | 494 | -- | 270 | 396 | 245 | 119 | 0.041 | 0.13 |
| γ-Terpinene | 1248 | 1241 | 864 | 1125 | -- | -- | 25 | -- | 230 | 191 | 0.002 | 0.12 |
| α-Phellandrene | 1167 | 1172 | 890 | 1125 | -- | -- | -- | -- | 225 | 0 | ns | 0.12 |
| P-Cymene-8-ol | 1845 | 1839 | 859 | 705 | -- | -- | -- | -- | 141 | 0 | ns | 0.07 |
| Z-Linalool oxide | 1519 | 1513 | 873 | 91 | -- | -- | 442 | -- | 107 | 13 | <0.001 | 0.05 |
| β-Phellandrene | 1212 | 1212 | 869 | 39 | -- | -- | 130 | 25 | 39 | 19 | <0.001 | 0.02 |
| Total | 172,402 | 17,647 | 11,448 | 34,047 | 16,655 | 48,273 | 11,115 | <0.001 | 25.80 | |||
| Sulfur compounds | ||||||||||||
| Dimethyl trisulfide | 1391 | 1390 | 903 | 3759 | -- | -- | -- | -- | 752 | 49 | <0.001 | 0.38 |
| Dimethyl disulfide | 1079 | 1069 | 944 | 2578 | -- | -- | -- | -- | 516 | 394 | <0.001 | 0.26 |
| Total | 6337 | -- | -- | -- | -- | 1027 | 747 | <0.001 | 0.65 | |||
| Grand Total | 360,259 | 133,672 | 118,910 | 192,484 | 172,128 | 206,647 | 26,301 | <0.001 | 100 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forney, C.F.; Qiu, S.; Jordan, M.A.; McCarthy, D.; Fillmore, S. Comparison of Volatile Compounds Contributing to Flavor of Wild Lowbush (Vaccinium augustifolium) and Cultivated Highbush (Vaccinium corymbosum) Blueberry Fruit Using Gas Chromatography-Olfactometry. Foods 2022, 11, 2516. https://doi.org/10.3390/foods11162516
Forney CF, Qiu S, Jordan MA, McCarthy D, Fillmore S. Comparison of Volatile Compounds Contributing to Flavor of Wild Lowbush (Vaccinium augustifolium) and Cultivated Highbush (Vaccinium corymbosum) Blueberry Fruit Using Gas Chromatography-Olfactometry. Foods. 2022; 11(16):2516. https://doi.org/10.3390/foods11162516
Chicago/Turabian StyleForney, Charles F., Songshan Qiu, Michael A. Jordan, Dylan McCarthy, and Sherry Fillmore. 2022. "Comparison of Volatile Compounds Contributing to Flavor of Wild Lowbush (Vaccinium augustifolium) and Cultivated Highbush (Vaccinium corymbosum) Blueberry Fruit Using Gas Chromatography-Olfactometry" Foods 11, no. 16: 2516. https://doi.org/10.3390/foods11162516
APA StyleForney, C. F., Qiu, S., Jordan, M. A., McCarthy, D., & Fillmore, S. (2022). Comparison of Volatile Compounds Contributing to Flavor of Wild Lowbush (Vaccinium augustifolium) and Cultivated Highbush (Vaccinium corymbosum) Blueberry Fruit Using Gas Chromatography-Olfactometry. Foods, 11(16), 2516. https://doi.org/10.3390/foods11162516






