Si-Wu Water Extracts Protect against Colonic Mucus Barrier Damage by Regulating Muc2 Mucin Expression in Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Composition and Preparation of SWE
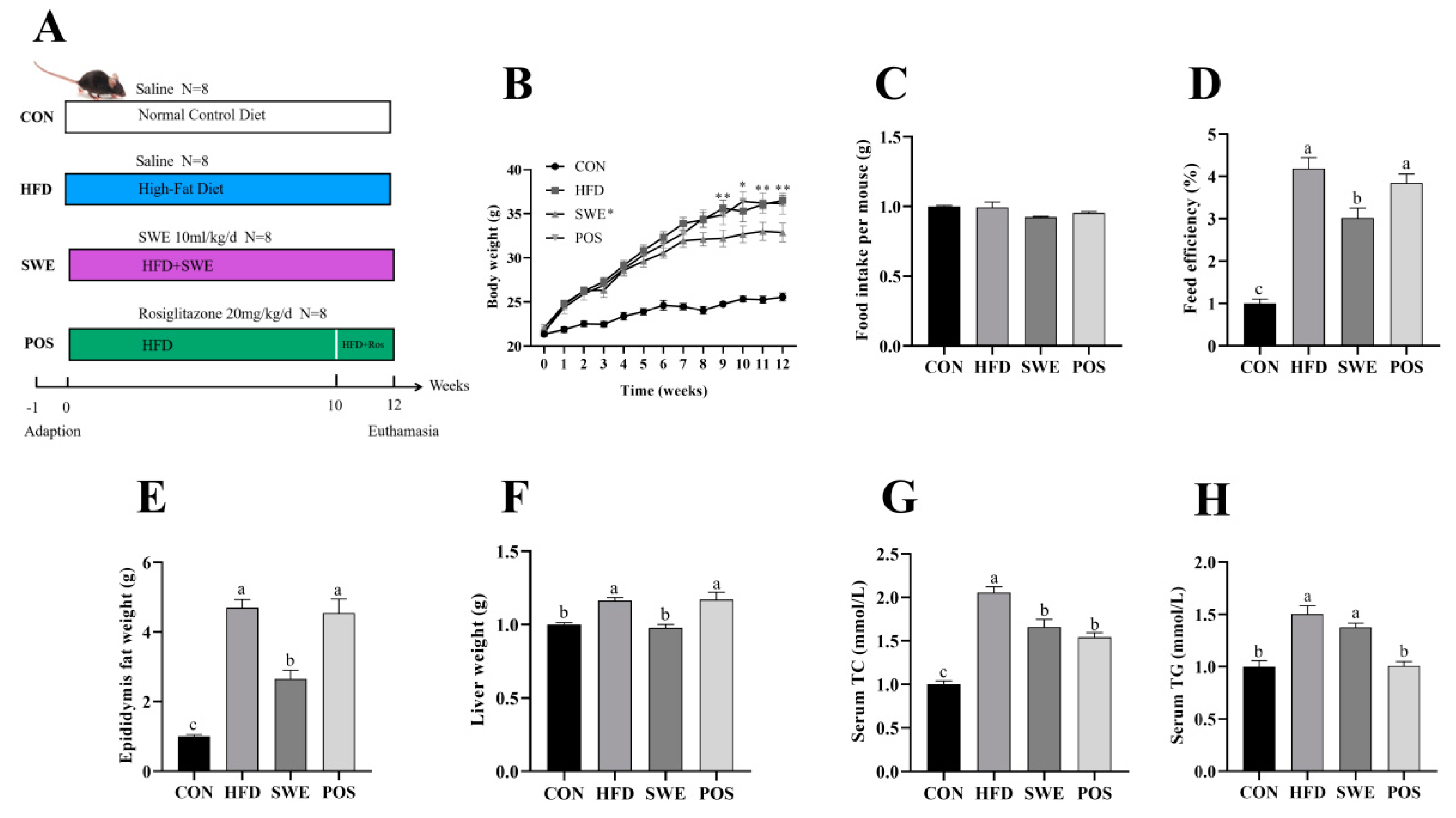
2.3. Animals and Experimental Protocols
2.4. Determination of the Total Phenolic and Flavonoid Contents
2.5. Inflammatory Cytokines and Serum Endotoxin Detection
2.6. Histologic and Immunohistochemical Analyses
2.7. Protein Quantitative Analysis
2.8. Statistical Analysis
3. Results
3.1. Determination of Total Phenolic and Flavonoid Contents
3.2. SWE Reduced the Signs of Obesity after High-Fat Diet Intervention
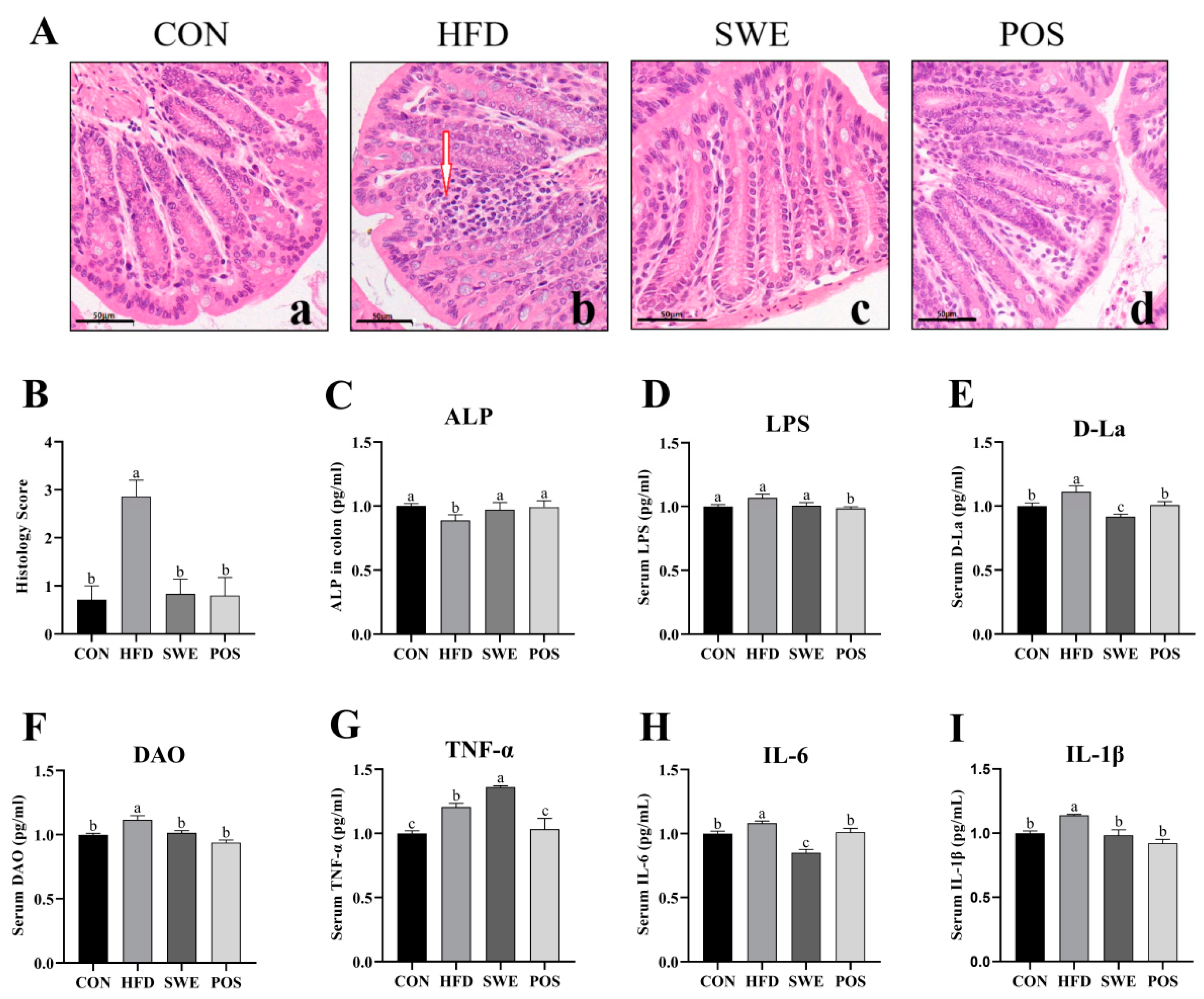
3.3. Effects of SWE on Colonic Mucosal Morphology, Intestinal Permeability, and Inflammation
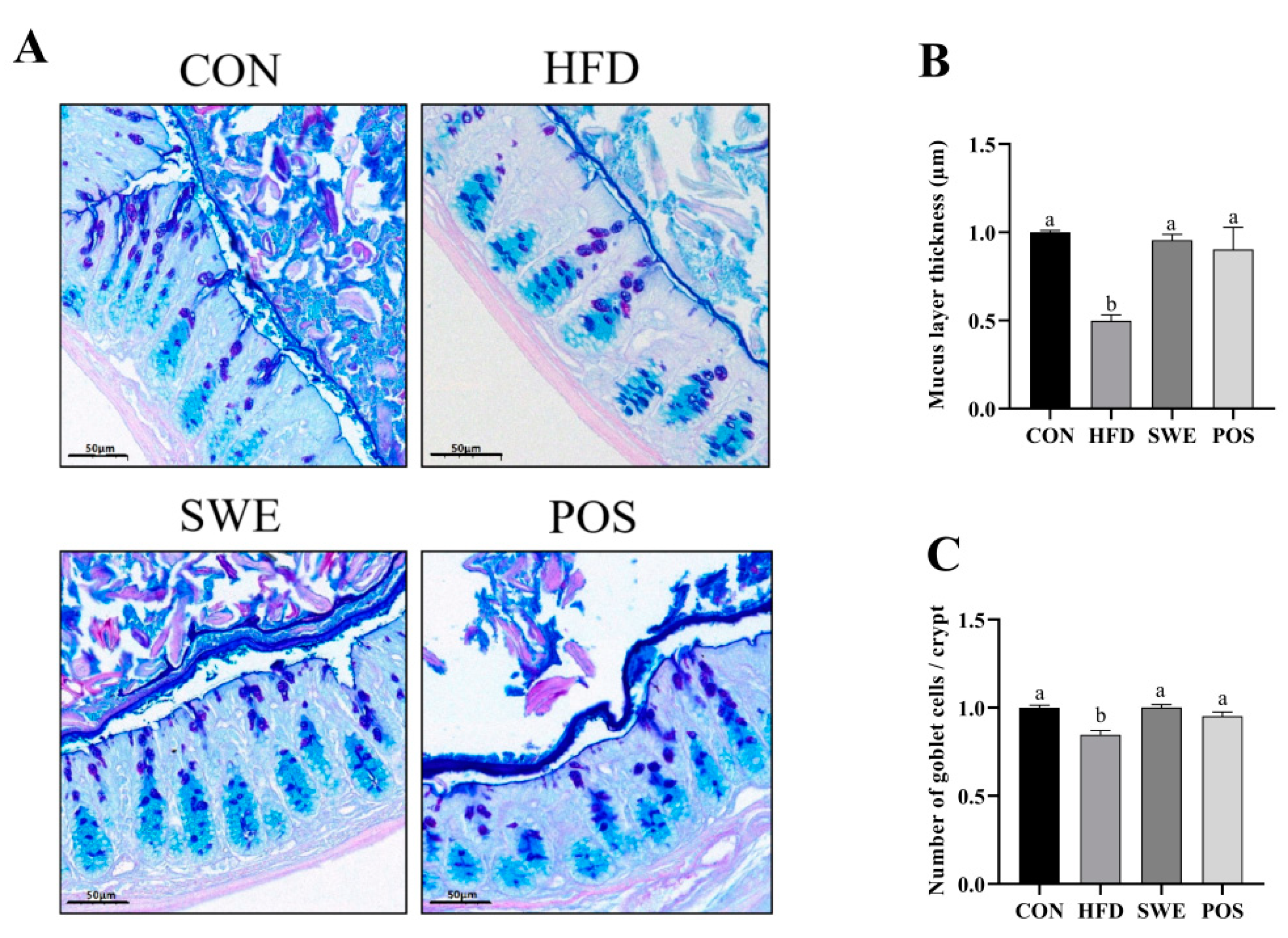
3.4. Effects of SWE on Colonic Mucus Layer Thickness and the Number of Goblet Cells in HFD-Fed Mice
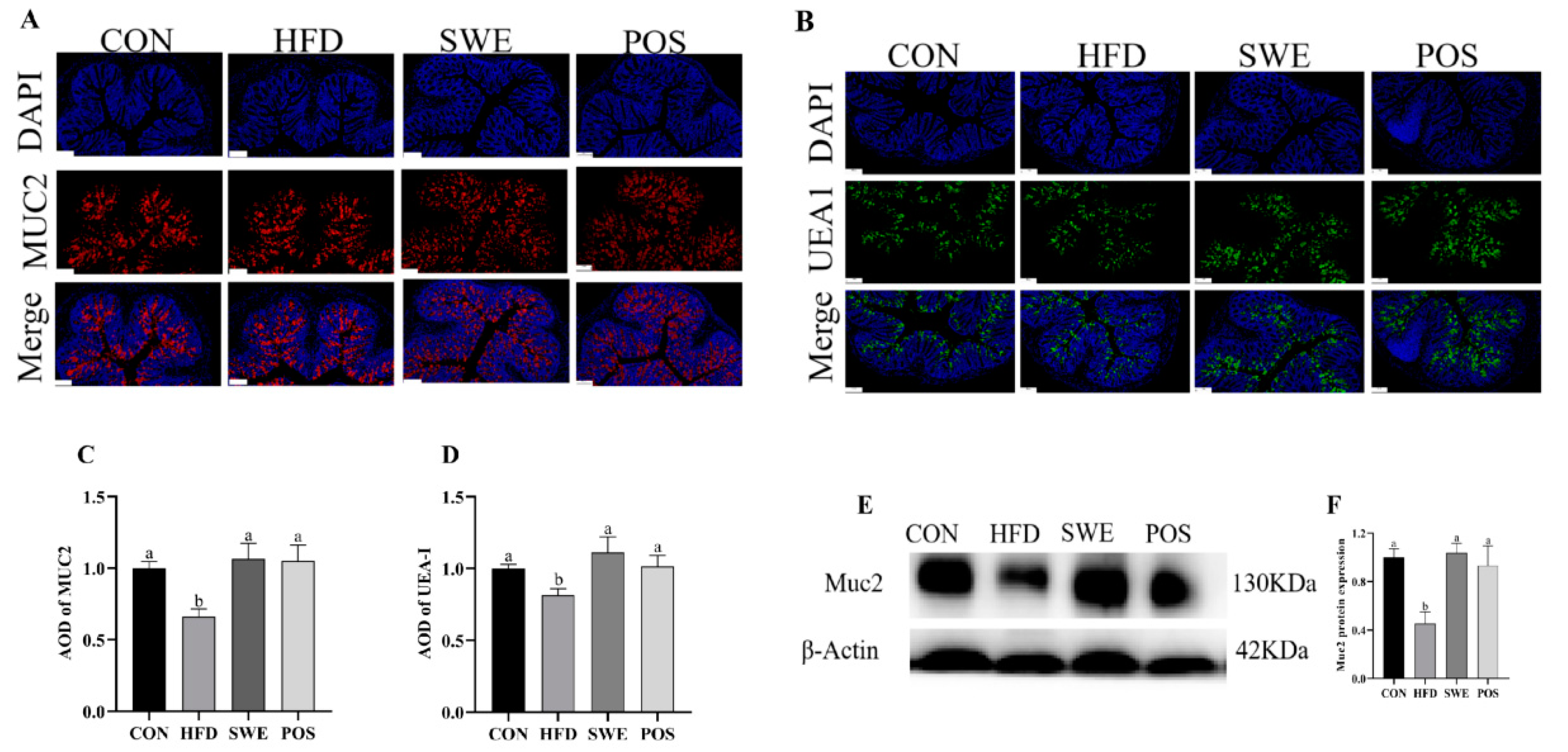
3.5. Effects of SWE on the Production and Distribution of Colonic Mucin
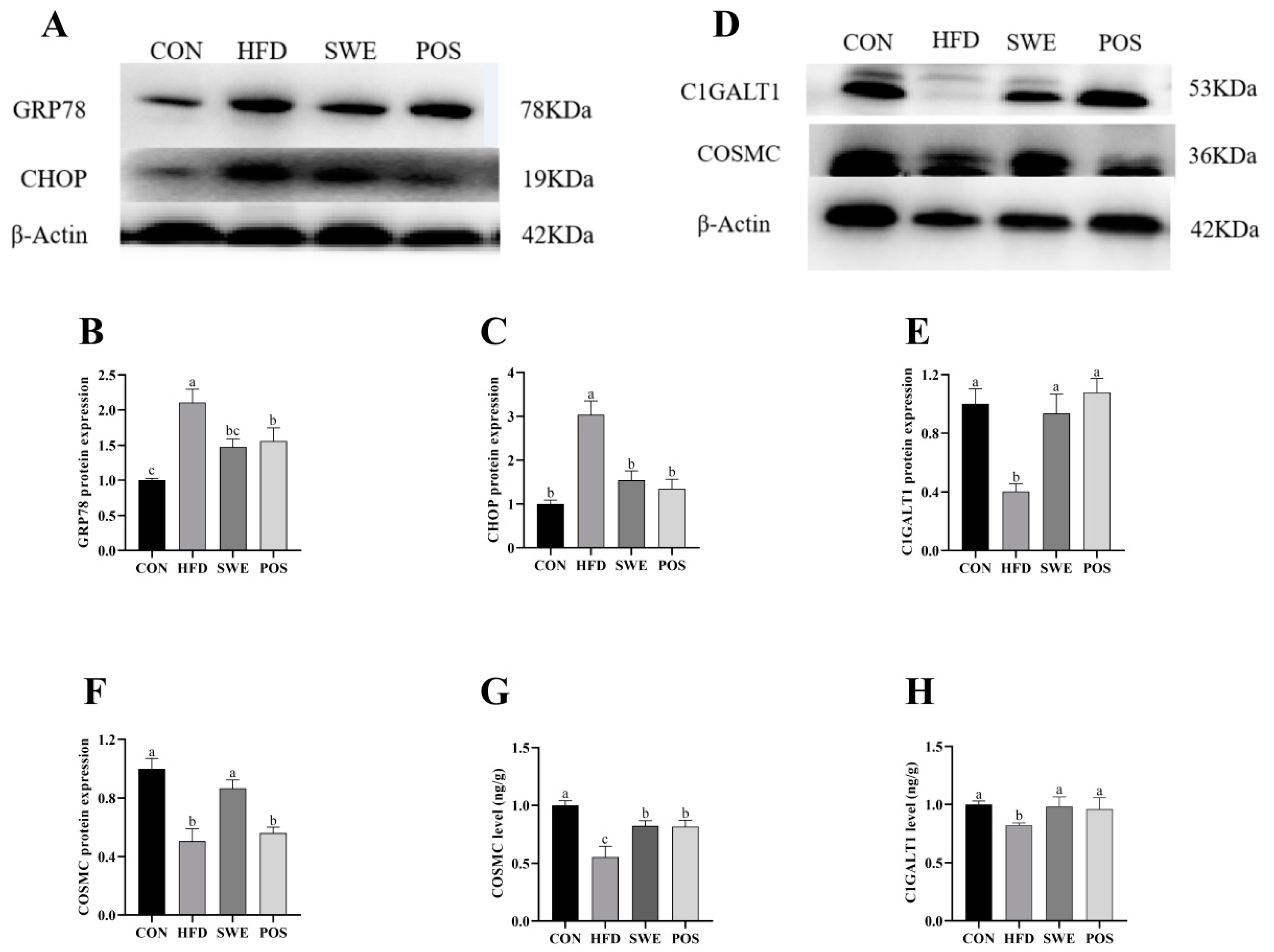
3.6. Effects of SWE on Colonic Mucin Procession
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nagao, M.; Asai, A.; Sugihara, H.; Oikawa, S. Fat intake and the development of type 2 diabetes. Endocr. J. 2015, 62, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.-J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Lassenius, M.I.; Pietiläinen, K.H.; Kaartinen, K.; Pussinen, P.J.; Syrjänen, J.; Forsblom, C.; Pörsti, I.; Rissanen, A.; Kaprio, J.; Mustonen, J.; et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 2011, 34, 1809–1815. [Google Scholar] [CrossRef]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 2010, 5, e12238. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Lang, T.; Hansson, G.C.; Samuelsson, T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 16209–16214. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.D.; Li, L.; Li, N.; Tian, X.Q.; Huang, P.L. Suppression of mucin 2 enhances the proliferation and invasion of LS174T human colorectal cancer cells. Cell Biol. Int. 2011, 35, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Handa, O.; Naito, Y.; Takayama, S.; Suyama, Y.; Ushiroda, C.; Majima, A.; Hirai, Y.; Mizushima, K.; Okayama, T.; et al. High-Fat Diet Causes Constipation in Mice via Decreasing Colonic Mucus. Dig. Dis. Sci. 2020, 65, 2246–2253. [Google Scholar] [CrossRef]
- Liu, H.Y.; Walden, T.B.; Ahl, D.; Nyman, M.; Bertilsson, S.; Phillipson, M.; Holm, L. High-Fat Diet Enriched with Bilberry Modifies Colonic Mucus Dynamics and Restores Marked Alterations of Gut Microbiome in Rats. Mol. Nutr. Food Res. 2019, 63, 13. [Google Scholar] [CrossRef]
- Ma, Z.; Huo, C.; Zhou, S.; Liang, Q.; Wang, Y.; Tan, H.; Xiao, C.; Gao, Y. Metabonomic study on siwu tang in radiation-induced blood deficient mice. Zhongguo Zhong Yao Za Zhi 2012, 37, 1289–1295. [Google Scholar]
- Jing, S.; Li, Z.; Yujun, H.; Kun, Z.; Liping, W.; Yongsheng, F.; Zhijun, X. To Unveil the Molecular Mechanisms of Qi and Blood through Systems Biology-Based Investigation into Si-Jun-Zi-Tang and Si-Wu-Tang formulae. J. Sci. Rep. 2016, 6, 1–10. [Google Scholar]
- Zhang, H.; Shen, P.; Cheng, Y. Identification and determination of the major constituents in traditional Chinese medicine Si-Wu-Tang by HPLC coupled with DAD and ESI–MS. J. Pharm. Biomed. Anal. 2004, 34, 705–713. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wo, S.K.; Wang, L.; Lau, C.B.; Lee, V.H.; Chow, M.S.; Zuo, Z. Simultaneous quantification of active components in the herbs and products of Si-Wu-Tang by high performance liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2009, 50, 232–244. [Google Scholar] [CrossRef]
- Yan, B.; Shen, M.; Fang, J.; Wei, D.; Qin, L. Advancement in the chemical analysis of Paeoniae Radix (Shaoyao). J. Pharm. Biomed. Anal. 2018, 160, 276–288. [Google Scholar] [CrossRef]
- Chiu, H.F.; Wu, Y.H.; Shen, Y.C.; Wang, S.J.; Venkatakrishnan, K.; Wang, C.K. Antioxidant and physiological effects of Si-Wu-Tang on skin and liver: A randomized, double-blind, placebo-controlled clinical trial. Chin. Med. 2016, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, J.; Li, S.; Wang, W.; Cai, X.; Huang, D.; Gong, L.; Li, X. Composition of The Essential Oil from Danggui-zhiqiao Herb-Pair and Its Analgesic Activity and Effect on Hemorheology in Rats with Blood Stasis Syndrome. Pharmacogn. Mag. 2016, 12, 271–275. [Google Scholar] [CrossRef]
- Du, L.D.; Ren, Y.; Wu, G.T.; Niu, T.H.; Wang, Z.W.; Shao, J. Effect of Angelica sinensis radix on colonic morphology and mucus secretion of experimental XuexuBianmi model mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2018, 34, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Š., Jr.; Pekárová, T.; Varga, J.; Tóth, Š.; Tomečková, V.; Gál, P.; Veselá, J.; Guzy, J. Intravenous administration of tetramethylpyrazine reduces intestinal ischemia-reperfusion injury in rats. Am. J. Chin. Med. 2013, 41, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Yu, L.; Cho, C.H. Protective effect of polysaccharides from Angelica sinensis on ulcerative colitis in rats. Inflammopharmacology 2008, 16, 162–167. [Google Scholar] [CrossRef]
- Ling, X.; Wang, X.; Li, J.; Zhang, T.; Zhou, X.; Zhou, Y.; Kang, J. Shu-Di-Huang and Gan-Cao Herb Pair Restored the Differentiation Potentials of Mesenchymal Stem Progenitors in Treating Osteoporosis via Downregulation of NF-κB Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 7795527. [Google Scholar] [CrossRef]
- Chen, R.; Wu, P.; Cai, Z.; Fang, Y.; Zhou, H.; Lasanajak, Y.; Tang, L.; Ye, L.; Hou, C.; Zhao, J. Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J. Nutr. Biochem. 2019, 65, 101–114. [Google Scholar] [CrossRef]
- Yang, J.Y.; Du, K.; Yang, L.; Wang, Z.T.; Wang, R.; Shi, Y.H. Pharmacological effects of Huangqin Decoction prepared from roots of multi-originated peony on ulcerative colitis in mice:a comparative study. Zhongguo Zhong Yao Za Zhi 2021, 46, 6395–6402. [Google Scholar] [CrossRef]
- Lee, C.-J.; Kapelemera, A.M.; Tsai, Y.-Z.; Lee, C.-T.; Xu, M.-Y.; Wang, C.-C. Evaluating the Therapeutic Efficacy of Si-Wu-Tang Decoction and Concentrated Extract in Follicular Maldevelopment-Related Menstrual Disorders Through Pharmacokinetic/Pharmacodynamic Studies. Front. Pharm. 2020, 11, 1245. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, R.; Cheng, L.; Yang, J.; Zhu, L. Peroxisome proliferator-activated receptor gamma activation promotes intestinal barrier function by improving mucus and tight junctions in a mouse colitis model. Dig. Liver Dis. 2018, 50, 1195–1204. [Google Scholar] [CrossRef]
- Gao, M.; Ma, Y.; Alsaggar, M.; Liu, D. Dual Outcomes of Rosiglitazone Treatment on Fatty Liver. AAPS J. 2016, 18, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Yang, W.-J.; Li, D.-P.; Li, J.-K.; Li, M.-H.; Chen, Y.-L.; Zhang, P.-Z. Synergistic Antioxidant Activities of Eight Traditional Chinese Herb Pairs. Biol. Pharm. Bull. 2009, 32, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.H.; Zhang, H.H.; Su, Y.M.; Liu, X.; Bai, J.S.; Lang, W.Y.; Shi, Q.M.; Feng, M.S. Strictosamide alleviates the inflammation in an acute ulcerative colitis (UC) model. J. Physiol. Biochem. 2021, 77, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Murano, M.; Maemura, K.; Hirata, I.; Toshina, K.; Nishikawa, T.; Hamamoto, N.; Sasaki, S.; Saitoh, O.; Katsu, K. Therapeutic effect of intracolonically administered nuclear factor κB (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin. Exp. Immunol. 2000, 120, 51–58. [Google Scholar] [CrossRef]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37.e26. [Google Scholar] [CrossRef]
- Qiu, W.; Carson-Walter, E.B.; Kuan, S.F.; Zhang, L.; Yu, J. PUMA suppresses intestinal tumorigenesis in mice. Cancer Res. 2009, 69, 4999–5006. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhao, Y.; Fan, Z.Q.; Kang, L.P.; Qiao, L.R.; Zhang, J.; Gao, Y.; Ma, B.P. Qualitative analysis of chemical constituents in Si-Wu Decoction based on TCM component database. Yao Xue Xue Bao 2015, 50, 1309–1317. [Google Scholar]
- Duan, X.; Pan, L.; Bao, Q.; Peng, D. UPLC-Q-TOF-MS Study of the Mechanism of THSWD for Breast Cancer Treatment. Front. Pharm. 2020, 10, 1625. [Google Scholar] [CrossRef]
- Su, S.L.; Cui, W.X.; Zhou, W.; Duan, J.A.; Shang, E.X.; Tang, Y.P. Chemical fingerprinting and quantitative constituent analysis of Siwu decoction categorized formulae by UPLC-QTOF/MS/MS and HPLC-DAD. Chin. Med. 2013, 8, 15. [Google Scholar] [CrossRef]
- Wan, F.; Cai, X.Y.; Wang, M.Y.; Chen, L.; Zhong, R.Q.; Liu, L.; Yi, B.; Hou, F.J.; Zhang, H.F. Chlorogenic acid supplementation alleviates dextran sulfate sodium (DSS)-induced colitis via inhibiting inflammatory responses and oxidative stress, improving gut barrier integrity and Nrf-2/HO-1 pathway. J. Funct. Food. 2021, 87, 104808. [Google Scholar] [CrossRef]
- Shi, C.J.; Wen, X.S.; Gao, H.F.; Liu, Z.H.; Xu, X.K.; Li, L.F.; Shen, T.; Xian, C.J. Steamed root of Rehmannia glutinosa Libosch (Plantaginaceae) alleviates methotrexate-induced intestinal mucositis in rats. J. Ethnopharmacol 2016, 183, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.X.; Huang, X.L.; Chen, R.R.; Li, T.; Ye, H.J.; Xie, W.; Huang, Z.M.; Cao, G.Z. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation 2019, 42, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Reinke, D.; Kritas, S.; Polychronopoulos, P.; Skaltsounis, A.L.; Aligiannis, N.; Tran, C.D. Herbal substance, acteoside, alleviates intestinal mucositis in mice. Gastroenterol. Res. Pract. 2015, 2015, 327872. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.X.; He, J.; Wu, S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Miao, L.; Liu, Y.; Lin, X.; Guo, Y.; Yuan, R.; Tian, H. Synthesis and radioprotective effects of novel hybrid compounds containing edaravone analogue and 3-n-butylphthalide ring-opening derivatives. J. Cell. Mol. Med. 2021, 25, 5470–5485. [Google Scholar] [CrossRef]
- Lee, S.-E.; Oh, H.; Yang, J.-A.; Jo, S.-K.; Byun, M.-W.; Yee, S.-T.; Kim, S.-H. Radioprotective effects of two traditional Chinese medicine prescriptions: Si-wu-tang and si-jun-zi-tang. Am. J. Chin. Med. 1999, 27, 387–396. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Liang, Q.D.; Gao, Y.; Tan, H.L.; Guo, P.; Li, Y.F.; Zhou, Z.; Tan, W.; Ma, Z.C.; Ma, B.P.; Wang, S.Q. Effects of four Si-Wu-Tang’s constituents and their combination on irradiated mice. Biol. Pharm. Bull. 2006, 29, 1378–1382. [Google Scholar] [CrossRef]
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Jones, S.A. Directing transition from innate to acquired immunity: Defining a role for IL-6. J. Immunol. 2005, 175, 3463–3468. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Ahmad, Z.; Snider, A.J.; Thomas, R.; Kochumon, S.; Melhem, M.; Sindhu, S.; Obeid, L.M.; Al-Mulla, F.; Hannun, Y.A.; et al. Ceramide kinase regulates TNF-α-induced immune responses in human monocytic cells. Sci Rep. 2021, 11, 8259. [Google Scholar] [CrossRef] [PubMed]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Chen, W.M.; Zhang, T.Z.; Cao, H.J.; Zhou, J. Effects of cardiopulmonary bypass on tight junction protein expressions in intestinal mucosa of rats. World J. Gastroenterol. 2008, 14, 5868–5875. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef] [PubMed]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.B.; Ducroc, R.; Regnault, B.; Kun Tan, C.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943. [Google Scholar] [CrossRef] [PubMed]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef]
- Hanisch, F.G. O-glycosylation of the mucin type. Biol. Chem. 2001, 382, 143–149. [Google Scholar] [CrossRef]
- Levine, S.J.; Larivée, P.; Logun, C.; Angus, C.W.; Ognibene, F.P.; Shelhamer, J.H. Tumor necrosis factor-alpha induces mucin hypersecretion and MUC-2 gene expression by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1995, 12, 196–204. [Google Scholar] [CrossRef]
- Iwashita, J.; Sato, Y.; Sugaya, H.; Takahashi, N.; Sasaki, H.; Abe, T. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-α through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol. Cell Biol. 2003, 81, 275–282. [Google Scholar] [CrossRef]
- Larsson, J.M.; Karlsson, H.; Crespo, J.G.; Johansson, M.E.; Eklund, L.; Sjövall, H.; Hansson, G.C. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 2011, 17, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiong, X.; Ren, K.; Xu, B.; Cheng, M.; Sahu, C.; Wu, K.; Nie, Y.; Huang, Z.; Blumberg, R.S.; et al. Deficiency in intestinal epithelial O-GlcNAcylation predisposes to gut inflammation. EMBO Mol. Med. 2018, 10, e8736. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Bergstrom, K.; Fu, J.; Xie, B.; Chen, W.; Xia, L. Loss of intestinal O-glycans promotes spontaneous duodenal tumors. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G74–G83. [Google Scholar] [CrossRef]
- Chi, H.; Wang, D.; Chen, M.; Lin, J.; Zhang, S.; Yu, F.; Zhou, J.; Zheng, X.; Zou, Y. Shaoyao Decoction Inhibits Inflammation and Improves Intestinal Barrier Function in Mice with Dextran Sulfate Sodium-Induced Colitis. Front. Pharm. 2021, 12, 524287. [Google Scholar] [CrossRef]
- Niu, X.; Zhang, J.; Ling, C.; Bai, M.; Peng, Y.; Sun, S.; Li, Y.; Zhang, Z. Polysaccharide from Angelica sinensis protects H9c2 cells against oxidative injury and endoplasmic reticulum stress by activating the ATF6 pathway. J. Int. Med. Res. 2018, 46, 1717–1733. [Google Scholar] [CrossRef]
- Boltin, D.; Perets, T.T.; Vilkin, A.; Niv, Y. Mucin Function in Inflammatory Bowel Disease: An Update. J. Clin. Gastroenterol. 2013, 47, 106–111. [Google Scholar] [CrossRef]
- Ju, T.; Aryal, R.P.; Stowell, C.J.; Cummings, R.D. Regulation of protein O-glycosylation by the endoplasmic reticulum-localized molecular chaperone Cosmc. J. Cell Biol. 2008, 182, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Du, T.; Hu, X.; Dong, X.; Li, L.; Wang, Y.; Liu, J.; Liu, L.; Gu, T.; Wen, T. Cosmc overexpression enhances malignancies in human colon cancer. J. Cell. Mol. Med. 2020, 24, 362–370. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Z.; Yu, Y.; Han, P.; Zhang, L.; Hu, Z. Si-Wu Water Extracts Protect against Colonic Mucus Barrier Damage by Regulating Muc2 Mucin Expression in Mice Fed a High-Fat Diet. Foods 2022, 11, 2499. https://doi.org/10.3390/foods11162499
Ruan Z, Yu Y, Han P, Zhang L, Hu Z. Si-Wu Water Extracts Protect against Colonic Mucus Barrier Damage by Regulating Muc2 Mucin Expression in Mice Fed a High-Fat Diet. Foods. 2022; 11(16):2499. https://doi.org/10.3390/foods11162499
Chicago/Turabian StyleRuan, Zheng, Yujuan Yu, Peiheng Han, Li Zhang, and Zhongyi Hu. 2022. "Si-Wu Water Extracts Protect against Colonic Mucus Barrier Damage by Regulating Muc2 Mucin Expression in Mice Fed a High-Fat Diet" Foods 11, no. 16: 2499. https://doi.org/10.3390/foods11162499
APA StyleRuan, Z., Yu, Y., Han, P., Zhang, L., & Hu, Z. (2022). Si-Wu Water Extracts Protect against Colonic Mucus Barrier Damage by Regulating Muc2 Mucin Expression in Mice Fed a High-Fat Diet. Foods, 11(16), 2499. https://doi.org/10.3390/foods11162499





