Metabolomic Characteristics of Liver and Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Lactobacillus plantarum FRT10
Abstract
:1. Introduction
2. Materials and Methods
2.1. L. Plantarum FRT10 Culture
2.2. Animals and Experiment Design
2.3. Collection of Liver Tissue and Cecum Contents Samples
2.4. Hepatic Metabolomics Analysis
2.5. Cecum Contents Metabolomics Analysis
2.6. Data Processing and Statistical Analysis
3. Results
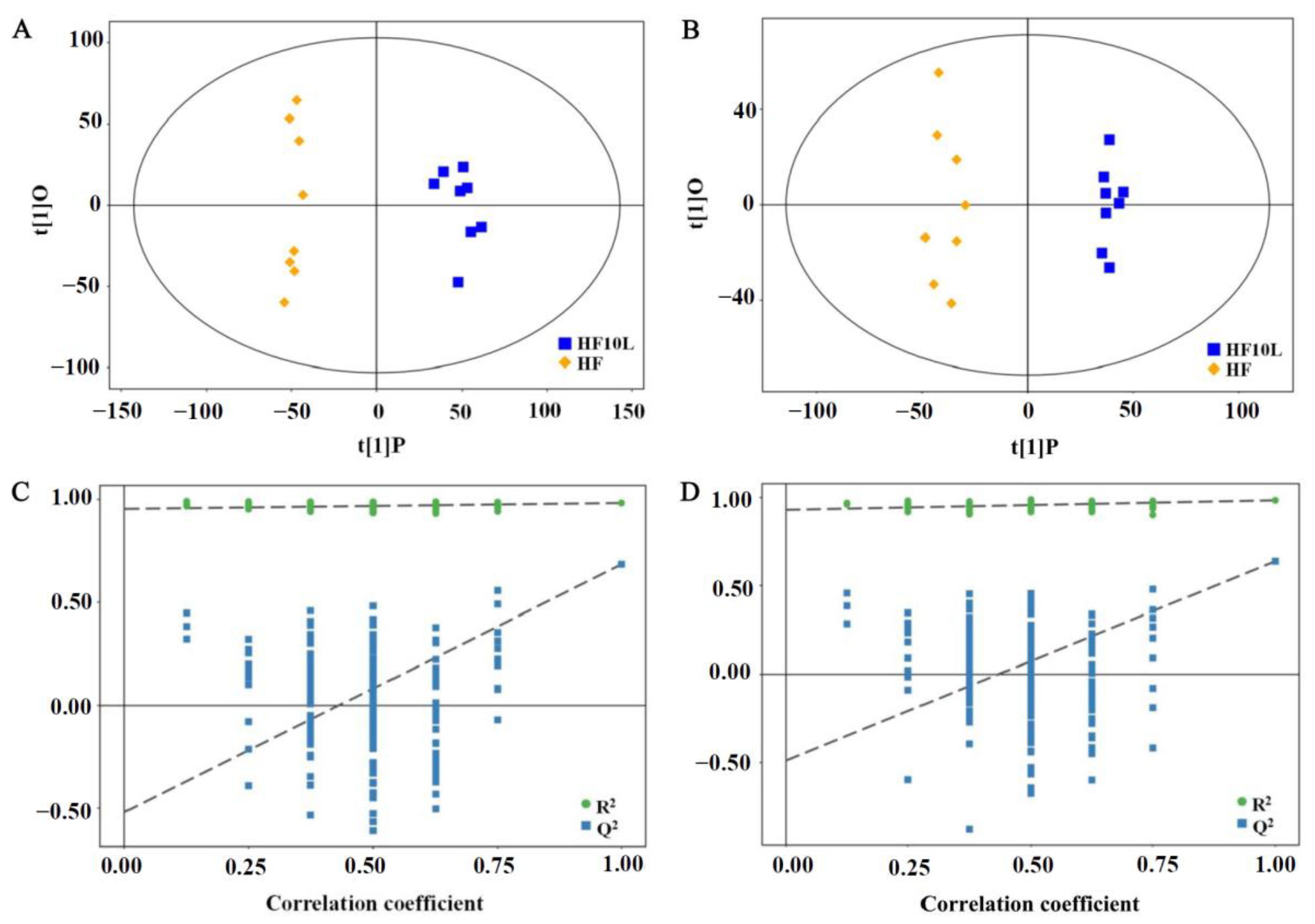
3.1. Multivariate Statistical Analysis of Liver Metabolites
3.2. Effect of L. Plantarum FRT10 on Liver Tissue Metabolomics
3.3. Multivariate Statistical Analysis of Cecal Contents
3.4. Effect of L. plantarum FRT10 on Cecal Contents Metabolomics
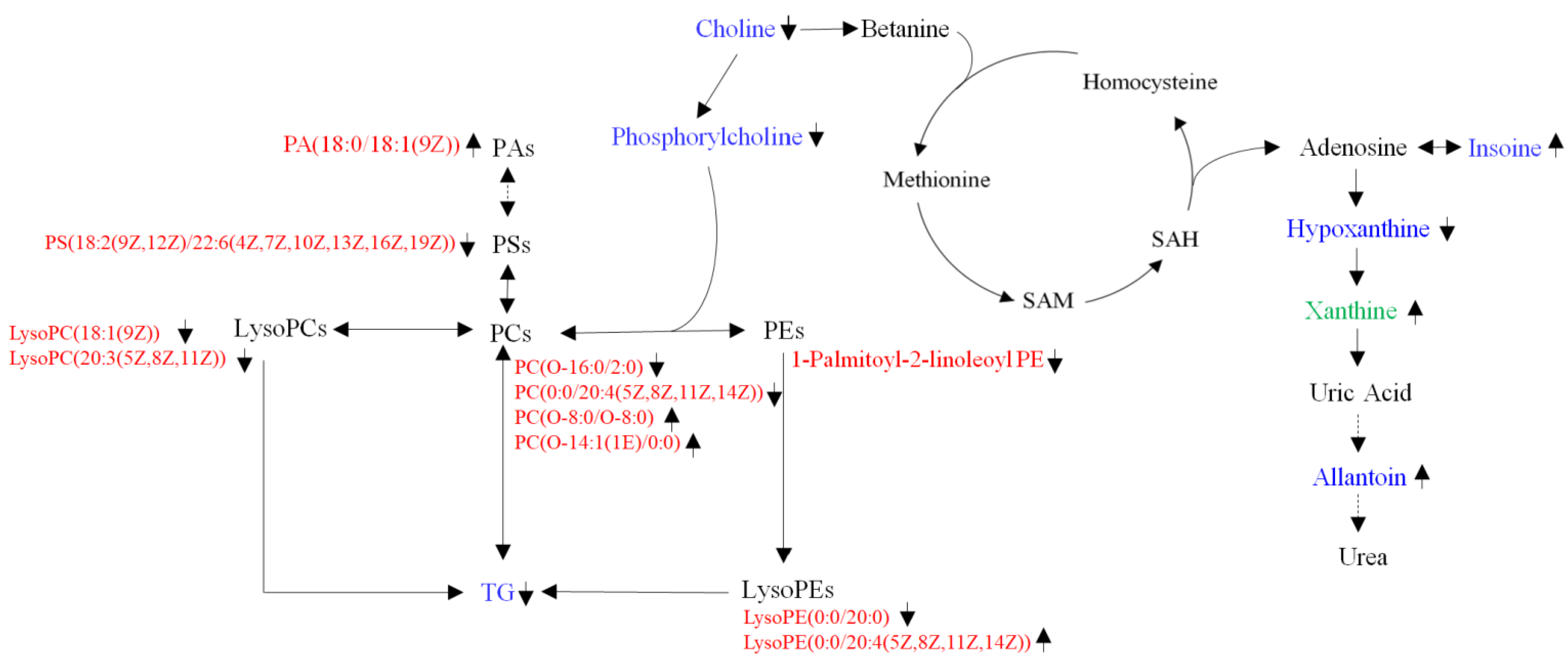
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, N.D.; Shivani, P.S.A.; Narayan, K.M.V. Obesity in low- and middle-income countries: Burden, drivers, and emerging challenges. Annu. Rev. Public Health 2017, 38, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Kussmann, M.; Raymond, F.; Affolter, M. OMICS-driven biomarker discovery in nutrition and health. J. Biotechnol. 2006, 124, 758–787. [Google Scholar] [CrossRef] [PubMed]
- Bashiardes, S.; Shapiro, H.; Rozin, S.; Shibolet, O.; Elinav, E. Non-alcoholic fatty liver and the gut microbiota. Mol. Metab. 2016, 5, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Junli, M.; Qihang, Z.; Houkai, L. Gut microbiota and nonalcoholic fatty liver disease: Insights on mechanisms and therapy. Nutrients 2017, 9, 1124. [Google Scholar]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Intestinal microbiota modulation in obesity-related non-alcoholic fatty liver disease. Front. Physiol. 2018, 9, 1813. [Google Scholar] [CrossRef]
- Szabo, G. Gut–liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef]
- Visschers, R.; Luyer, M.; Schaap, F.G.; Damink, S.; Soeters, P.B. The gut-liver axis. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 576–581. [Google Scholar] [CrossRef]
- Hou, Q.; Ye, L.; Liu, H.; Huang, L.; Yang, Q.; Turner, J.; Yu, Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018, 25, 1657–1670. [Google Scholar] [CrossRef]
- Yu, Q.; Yuan, L.; Deng, J.; Yang, Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front. Cell. Infect. Microbiol. 2015, 5, 26. [Google Scholar] [CrossRef]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef]
- Cai, H.; Wen, Z.; Li, X.; Meng, K.; Yang, P. Lactobacillus plantarum FRT10 alleviated high-fat diet–induced obesity in mice through regulating the PPARα signal pathway and gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 5959–5972. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, Y.; Song, L.; Huang, Y.; Chu, Q.; Zhu, S.; Lu, S.; Hou, L.; Li, Z.; Li, J.; et al. Effect of Lactobacillus plantarum HT121 on serum lipid profile, gut microbiota, and liver transcriptome and metabolomics in a high-cholesterol diet–induced hypercholesterolemia rat model. Nutrition 2020, 79–80, 110966. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, J.; Wang, Z.; Zhang, G.; Liu, L.; Huo, G.; Li, C. Lactobacillus plantarum KLDS1.0318 ameliorates impaired intestinal immunity and metabolic disorders in cyclophosphamide-treated mice. Front. Microbiol. 2019, 10, 731. [Google Scholar] [CrossRef]
- Long, X.; Zeng, X.; Tan, F.; Yi, R.; Pan, Y.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus plantarum KFY04 prevents obesity in mice through the PPAR pathway and alleviates oxidative damage and inflammation. Food Funct. 2020, 11, 5460–5472. [Google Scholar] [CrossRef]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Ryu, D.W.; Song, S.M.; Kim, Y.R.; Jung, H.H.; Kim, T.H.; Kim, Y. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci. Rep. 2020, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kwon, M.H.; Kim, H.K.; Lee, H.S.; Cho, J.S.; Lee, Y.I. Anti-obesity effect of Lactobacillus plantarum LB818 is associated with regulation of gut microbiota in high-fat diet-fed obese mice. J. Med. Food 2020, 23, 750–759. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Martinic, A.; Barouei, J.; Bendiks, Z.; Mishchuk, D.; Heeney, D.D.; Martin, R.; Marco, M.L.; Slupsky, C.M. Supplementation of Lactobacillus plantarum improves markers of metabolic dysfunction induced by a high fat diet. J. Proteome Res. 2018, 17, 2790–2802. [Google Scholar] [CrossRef]
- Yosep, J.; Park, S.; Chung, Y.; Kim, B.; Park, H.; Huang, E.; Jeong, D.; Jung, H.; Kim, B.; Hyun, C.; et al. Amelioration of obesity-related biomarkers by Lactobacillus sakei CJLS03 in a high-fat diet-induced obese murine model. Sci. Rep. 2019, 9, 6821. [Google Scholar]
- Cai, H.; Wen, Z.; Meng, K.; Yang, P. Metabolomic signatures for liver tissue and cecum contents in high-fat diet-induced obese mice based on UHPLC-Q-TOF/MS. Nutr. Metab. 2021, 18, 69. [Google Scholar] [CrossRef]
- Yin, X.; Peng, J.; Zhao, L.; Yu, Y.; Zhang, X.; Liu, P.; Feng, Q.; Hu, Y.; Pang, X. Structural changes of gut microbiota in a rat non-alcoholic fatty liver disease model treated with a Chinese herbal formula. Syst. Appl. Microbiol. 2013, 36, 188–196. [Google Scholar] [CrossRef]
- Usami, M.; Miyoshi, M.; Yamashita, H. Gut microbiota and host metabolism in liver cirrhosis. World J. Gastroenterol. 2015, 41, 11597–11608. [Google Scholar] [CrossRef]
- Park, S.; Ji, Y.; Jung, H.Y.; Park, H.; Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef]
- Huang, E.; Kim, S.; Park, H.; Park, S.; Ji, Y.; Todorov, S.D.; Lim, S.; Holzapfel, W.H. Modulation of the gut microbiome and obesity biomarkers by Lactobacillus plantarum KC28 in a diet-induced obesity murine model. Probiotics Antimicrob. Proteins 2021, 13, 677–697. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Blusztajn, J.K. Choline and Human Nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef]
- Zeisel, H.S. Choline: An important nutrient in brain development, liver function and carcinogenesis. J. Am. Coll. Nutr. 1992, 11, 473–481. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Dumas, M.; Barton, R.; Toye, A.; Cloarec, O.; Blancher, C.; Rothwell, A.; Fearnside, J.; Tatoud, R.; Blanc, V.; Lindon, J. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Pro. Natl. Acad. Sci. USA 2006, 103, 12511–12516. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Moffat, T.C.; Gable, K.; Ganesan, S.; Niewola-Staszkowska, K.; Johnston, A.; Nislow, C.; Giaever, G.; Harris, L.J.; Loewith, R. A signaling lipid associated with Alzheimer’s disease promotes mitochondrial dysfunction. Sci. Rep. 2016, 6, 19332. [Google Scholar] [CrossRef]
- Benno, Y.; Endo, K.; Mizutani, T.; Namba, Y.; Mitsuoka, T. Comparison of fecal microflora of elderly persons in rural and urban areas of Japan. Appl. Environ. Microbiol. 1989, 55, 1100–1105. [Google Scholar] [CrossRef]
- Brki, L.; Riederer, M.; Graier, W.F.; Malli, R.; Frank, S.A. Acyl chain-dependent effect of lysophosphatidylcholine on cyclooxygenase (COX)-2 expression in endothelial cells. Atherosclerosis 2012, 224, 348–354. [Google Scholar] [CrossRef]
- Zhao, Y.; Miao, H.; Cheng, X.; Wei, F. Lipidomics: Novel insight into the biochemical mechanism of lipid metabolism and dysregulation-associated disease. Chem. Biol. Interact. 2015, 240, 220–238. [Google Scholar] [CrossRef]
- Lutkewitte, A.J.; Finck, B.N. Regulation of signaling and metabolism by lipin-mediated phosphatidic acid phosphohydrolase activity. Biomolecules 2020, 10, 1386. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Wang, T.; Li, Y.; Li, Y.; Lu, X.; Wang, Y.; Zhang, H.; Liu, P.; Wu, J. Protective effect of herbal medicine Huangqi decoction against chronic cholestatic liver injury by inhibiting bile acid-stimulated inflammation in DDC-induced mice. Phytomedicine 2019, 62, 152948. [Google Scholar] [CrossRef]
- Luo, L.; Aubrecht, J.; Li, D.; Warner, R.L.; Johnson, K.J.; Kenny, J.; Colangelo, J.L.; Aspichueta, P. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS ONE 2018, 13, e0193824. [Google Scholar] [CrossRef]
- Liang, C.; Zhou, X.; Gong, P.M.; Niu, H.; Lyu, L.Z.; Wu, Y.F.; Han, X.; Zhang, L.W. Lactiplantibacillus plantarum H-87 prevents high-fat diet-induced obesity by regulating bile acid metabolism in C57BL/6J mice. Food Funct. 2021, 12, 4315–4324. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kido, J.; Matsumoto, S.; Shimizu, K.; Nakamura, K. Associations among amino acid, lipid, and glucose metabolic profiles in childhood obesity. BMC Pediatr. 2019, 19, 273. [Google Scholar] [CrossRef]
- Newsholme, P.; Procopio, J.; Lima, M.; Pithon-Curi, T.C.; Rui, C. Glutamine and glutamate—Their central role in cell metabolism and function. Cell Biochem. Func. 2003, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.H.; Noh, S.; Hur, H.J.; Sung, M.J.; Hwang, J.T.; Park, J.H.; Yang, H.J.; Kim, M.S.; Kwon, D.Y. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J. Proteome Res. 2011, 10, 722–731. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xu, W.; Li, H.; Lei, H.; Zhang, L.; Hao, F.; Duan, Y.; Yan, X.; Zhao, Y.; Wu, J. High-fat diet induces dynamic metabolic alterations in multiple biological matrices of rats. J. Proteome Res. 2013, 12, 3755–3768. [Google Scholar] [CrossRef]
- Kim, M.; Kim, I.Y.; Sung, H.R.; Nam, M.; Kim, Y.J.; Kyung, D.S.; Seong, J.K.; Hwang, G. Metabolic dysfunction following weight regain compared to initial weight gain in a high-fat diet-induced obese mouse model. J. Nutr. Biochem. 2019, 69, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hye, P.; Kab-Tae, P.; Edmond, P.; Seung, K.; Myung, C.; Liu, K.H.; Choong, L. Mass spectrometry-based metabolomic and lipidomic analyses of the effects of dietary platycodon grandiflorum on liver and serum of obese mice under a high-fat diet. Nutrients 2017, 9, 71. [Google Scholar]
- Zhang, L.; Wang, P.; Shi, M.; Fang, Z.; Ji, J.; Liao, X.; Hu, X.; Chen, F. The modulation of Luffa cylindrica (L.) Roem supplementation on gene expression and amino acid profiles in liver for alleviating hepatic steatosis via gut microbiota in high-fat diet-fed mice: Insight from hepatic transcriptome analysis. J. Nutr. Biochem. 2020, 80, 108365. [Google Scholar] [CrossRef]
- Manna, S.K.; Thompson, M.D.; Gonzalez, F.J. Application of mass spectrometry-based metabolomics in identification of early noninvasive biomarkers of alcohol-induced liver disease using mouse model. Adv. Exp. Med. Biol. 2014, 815, 217–238. [Google Scholar]
- Manna, S.K.; Patterson, A.D.; Yang, Q.; Krausz, K.W.; Idle, J.R.; Fornace, A.J.; Gonzalez, F.J. UPLC-MS-based urine metabolomics reveals indole-3-lactic acid and phenyllactic acid as conserved biomarkers for alcohol-induced liver disease in the Ppara-null mouse model. J. Proteome Res. 2011, 10, 4120–4133. [Google Scholar] [CrossRef]
- Weckwerth, W.; Miyamoto, K.; Iinuma, K.; Krause, M.; Glinski, M.; Storm, T.; Bonse, G.; Kleinkauf, H.; Zocher, R. Biosynthesis of PF1022A and related cyclooctadepsipeptides. J. Biol. Chem. 2000, 275, 17909–17915. [Google Scholar] [CrossRef]
- Urban, F.J.; Moore, B.S. Synthesis of optically active 2-benzyldihydrobenzopyrans for the hypoglycemic agent englitazone. J. Heterocycl. Chem. 1992, 29, 431–438. [Google Scholar] [CrossRef]
- Zhou, Q.; Gu, R.; Xue, B.; Li, P.; Gu, Q. Phenyl lactic acid alleviates Samonella Typhimurium-induced colitis via regulating microbiota composition, SCFA production and inflammatory responses. Food Funct. 2021, 12, 5591–5606. [Google Scholar] [CrossRef]
- Nishino, T.; Okamoto, K. Mechanistic insights into xanthine oxidoreductase from development studies of candidate drugs to treat hyperuricemia and gout. J. Biol. Inorg. Chem. 2015, 20, 195–207. [Google Scholar] [CrossRef]
- Saiki, S.; Sato, T.; Kohzuki, M.; Kamimoto, M.; Yosida, T. Changes in serum hypoxanthine levels by exercise in obese subjects. Metabolism 2001, 50, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Koyama, M.; Higashiura, Y.; Murase, T.; Miura, T. Differential regulation of hypoxanthine and xanthine by obesity in a general population. J. Diabetes Investig. 2020, 11, 878–887. [Google Scholar] [CrossRef]
- Nakamura, T.; Mai, N.; Murase, T.; Satoh, E.; Mizukami, H. Influence of xanthine oxidoreductase inhibitor, topiroxostat, on body weight of diabetic obese mice. Nutr. Diabetes 2021, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Wang, S.; Wang, Y.; Ke, S.; Wang, H. Gut microbiota-mediated xanthine metabolism is associated with resistance to high-fat diet-induced obesity. J. Nutr. Biochem. 2021, 88, 108533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, C.; Zhai, Z.; Deng, Z.Y.; Zheng, R. Uridine attenuates obesity, ameliorates hepatic lipid accumulation and modifies the gut microbiota composition in mice fed with a high-fat diet. Food Funct. 2021, 12, 1829–1840. [Google Scholar] [CrossRef]
- Setzer, B.; Lebrecht, D.; Walker, U.A. Pyrimidine nucleoside depletion sensitizes to the mitochondrial hepatotoxicity of the reverse transcriptase inhibitor stavudine. Am. J. Pathol. 2008, 172, 681–690. [Google Scholar] [CrossRef]
- Go, H.; Rahman, M.M.; Kim, G.; Na, C.; Song, C.; Kim, J.; Kim, S.; Kang, H. Antidiabetic effects of yam (Dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin-induced diabetes. Nutrients 2015, 7, 8532–8544. [Google Scholar] [CrossRef]
- Jm, A.; Xma, B.; Yi, L.A.; Cheng, Y.A.; Tong, Z.A.; Pw, A.; Ykpa, C.; Hwja, C. Effects of a rhizome aqueous extract of Dioscorea batatas and its bioactive compound, allantoin in high fat diet and streptozotocin-induced diabetic mice and the regulation of liver, pancreas and skeletal muscle dysfunction. J. Ethnopharmacol. 2020, 259, 112926. [Google Scholar]

| Metabolites | RT (s) | m/z | Fold Change | |
|---|---|---|---|---|
| HF10L vs. HF | HF10H vs. HF | |||
| Choline | 780.035 | 104.1058352 | 0.83 * | 1.55 * |
| Glycerophosphocholine | 862.561 | 258.1094388 | 0.61 + | 0.51 + |
| CDP-choline | 878.204 | 489.1135327 | - | 0.52 + |
| Phosphorylcholine | 717.05 | 242.0794821 | 0.28 * | 0.63 * |
| sn-Glycerol 3-phosphoethanolamine | 788.63 | 198.0517674 | - | 0.67 * |
| Taurocholic acid | 413.829 | 498.2875244 | 0.30 * | - |
| Cholic acid | 391.737 | 373.2730818 | 0.45 * | - |
| Phenyllactic acid | 521.458 | 149.0586964 | 1.18 * | 1.18 * |
| L-Phenylalanine | 542.1065 | 166.0855048 | 1.29 * | 1.27 * |
| Tyramine | 521.673 | 120.0799482 | 1.21 * | 1.21 * |
| L-Threonine | 704.9 | 120.0647667 | 1.23 * | 1.21 * |
| D-Ornithine | 1012.455 | 115.0853902 | 1.18 * | 1.24 * |
| L-Asparagine | 750.138 | 133.0599344 | 1.16 * | 1.12 + |
| D-Proline | 1012.13 | 116.0694156 | 1.17 * | 1.27 * |
| L-Pyroglutamic acid | 745.28 | 147.0754241 | 1.33 * | - |
| L-Histidine | 1015.6 | 156.0760477 | 1.22 + | 1.25 + |
| Thymine | 283.614 | 127.0491779 | 1.45 + | - |
| Thymidine | 180.4075 | 241.0827884 | 3.33 * | 2.66 * |
| Uracil | 149.349 | 111.0197557 | 1.56 * | 1.65 * |
| Allantoin | 367.132 | 159.0505486 | 1.23 * | - |
| Uridine | 321.659 | 245.0764623 | 0.55 * | 0.45 * |
| Inosine | 236.175 | 249.0626869 | 1.63 * | 1.86 * |
| Hypoxanthine | 412.355 | 137.0448623 | 0.16 + | 0.53 + |
| Xanthine | 428.656 | 151.0258253 | 4.90 * | 2.24 + |
| Xanthosine | 394.365 | 283.067998 | 2.24 * | 2.38 * |
| Deoxyinosine | 325.282 | 251.078228 | 2.94 * | - |
| Metabolites | RT (s) | m/z | Fold Change HF10L vs. HF |
|---|---|---|---|
| PC(O-16:0/2:0) | 330.811 | 524.3154739 | 0.65 |
| PC(0:0/20:4(5Z,8Z,11Z,14Z)) | 401.017 | 543.3885338 | 0.02 |
| PC(O-8:0/O-8:0) | 469.048 | 482.3602632 | 1.94 |
| PC(O-14:1(1E)/0:0) | 286.959 | 451.3413931 | 1.64 |
| LysoPC(18:1(9Z)) | 458.095 | 478.2949417 | 0.14 |
| LysoPC(20:3(5Z,8Z,11Z)) | 458.234 | 545.4042362 | 0.14 |
| LysoPE(0:0/20:0) | 477.56 | 510.3550402 | 0.14 |
| LysoPE(0:0/20:4(5Z,8Z,11Z,14Z)) | 392.452 | 501.3202624 | 2.69 |
| PS(18:2(9Z,12Z))/22:6(4Z,7Z,10Z,13Z,16Z,19Z) | 508.345 | 832.5033673 | 0.14 |
| PA(18:0/18:1(9Z)) | 558.79 | 700.557389 | 9.78 |
| 1-Palmitoyl-2-linoleoyl PE | 540.667 | 715.4910486 | 0.68 |
| 2-amino-3-(3,4-dihydroxyphenyl)propanoic acid | 216.208 | 197.1284116 | 0.61 |
| Cholic acid | 392.413 | 373.2732771 | 1.70 |
| Taurocholic acid | 302.382 | 514.2852392 | 0.42 |
| Xanthine | 62.5003 | 152.0540194 | 1.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Li, D.; Song, L.; Xu, X.; Han, Y.; Meng, K.; Wen, Z.; Yang, P. Metabolomic Characteristics of Liver and Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Lactobacillus plantarum FRT10. Foods 2022, 11, 2491. https://doi.org/10.3390/foods11162491
Cai H, Li D, Song L, Xu X, Han Y, Meng K, Wen Z, Yang P. Metabolomic Characteristics of Liver and Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Lactobacillus plantarum FRT10. Foods. 2022; 11(16):2491. https://doi.org/10.3390/foods11162491
Chicago/Turabian StyleCai, Hongying, Daojie Li, Liye Song, Xin Xu, Yunsheng Han, Kun Meng, Zhiguo Wen, and Peilong Yang. 2022. "Metabolomic Characteristics of Liver and Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Lactobacillus plantarum FRT10" Foods 11, no. 16: 2491. https://doi.org/10.3390/foods11162491
APA StyleCai, H., Li, D., Song, L., Xu, X., Han, Y., Meng, K., Wen, Z., & Yang, P. (2022). Metabolomic Characteristics of Liver and Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Lactobacillus plantarum FRT10. Foods, 11(16), 2491. https://doi.org/10.3390/foods11162491






