Detection of Nitrated, Oxygenated and Hydrogenated Polycyclic Aromatic Compounds in Smoked Fish and Meat Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Samples
2.3. The Smoking Experiments
2.4. Sample Treatment
2.5. GC-QTOFMS Analysis and Quantification
2.6. Method Validation
2.7. Non-Target Screening
3. Results and Discussion
3.1. Extraction Method and Instrumental Analysis Optimization
3.2. Validation Results
3.3. Smoked Samples
3.4. Non-Target Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, B.H. Analysis, Formation and Inhibition of Polycyclic Aromatic Hydrocarbons in Foods. An Overview. J. Food Drug Anal. 1997, 5, 25–42. [Google Scholar] [CrossRef]
- Dennis, M.J.; Massey, R.C.; McWeeny, D.J.; Knowles, M.E. Estimation of nitropolycyclic aromatic hydrocarbons in foods. Food Addit. Contam. 1984, 1, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, G.; Su, S.; Shen, H.; Huang, Y.; Li, T.; Li, W.; Zhang, Y.; Lu, Y.; Chen, H.; et al. Contamination and distribution of parent, nitrated, and oxygenated polycyclic aromatic hydrocarbons in smoked meat. Environ. Sci. Pollut. Res. 2014, 21, 11521–11530. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.; Jariyasopit, N.; Massey Simonich, S.L.; Tao, S.; Atkinson, R.; Arey, J. Formation of nitro-PAHs from the heterogeneous reaction of ambient particle-bound PAHs with N2O5/NO3/NO 2. Environ. Sci. Technol. 2013, 47, 8434–8442. [Google Scholar] [CrossRef]
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, I.B.; Öberg, L.; Haglund, P.; Tysklind, M. Sources, fate, and toxic hazards of oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) at PAH-contaminated sites. Ambio 2007, 36, 475–485. [Google Scholar] [CrossRef]
- Tomruk, A.; Guven, K.C. Biotransformation of 1-methylnaphthalene and anthracene in mussels (Mytilus galloprovincialis Lamarck, 1819). Fresenius Environ. Bull. 2008, 17, 256–259. [Google Scholar]
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The occurrence of 16 EPA PAHs in food—A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef]
- Schlemitz, S.; Pfannhauser, W. Analysis of nitro-PAHs in food matrices by on-line reduction and high performance liquid chromatography. Food Addit. Contam. 1996, 13, 969–977. [Google Scholar] [CrossRef]
- Schlemitz, S.; Pfannhauser, W. Monitoring of nitropolycyclic aromatic hydrocarbons in food using gas chromatography. Z. Fur Lebensm.-Unters. Und-Forsch. 1996, 203, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Schlemitz, S.; Pfannhauser, W. Supercritical fluid extraction of mononitrated polycyclic aromatic hydrocarbons from tea-Correlation with the PAH concentration. Eur. Food Res. Technol. 1997, 205, 305–310. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Diesel and Gasoline Engine Exhausts and Some Nitroarenes. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1989; Volume 46. [Google Scholar]
- International Agency for Research on Cancer. Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-water. In IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 101. [Google Scholar]
- International Agency for Research on Cancer. Polynuclear Aromatic Compounds, Part 1, Chemical, Environmental and Experimental Data, IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1983; Volume 32.
- International Agency for Research on Cancer. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. In IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 1987; Suppl. 7. [Google Scholar]
- International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 92.
- Nicol, S.; Dugay, J.; Hennion, M.-C. Simultaneous determination of polycyclic aromatic hydrocarbons and their nitrated derivatives in airborne particulate matter using gas chromatography-tandem mass spectrometry. J. Sep. Sci. 2001, 24, 451–458. Available online: https://doi.org/10.1002/1615-9314(20010601)24:6<451::AID-JSSC451>3.0.CO;2-D (accessed on 1 June 2021). [CrossRef]
- Ringuet, J.; Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E. Diurnal/nocturnal concentrations and sources of particulate-bound PAHs, OPAHs and NPAHs at traffic and suburban sites in the region of Paris (France). Sci. Total Environ. 2012, 437, 297–305. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment—A review. Sci. Total Environ. 2017, 581–582, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, P.; Riccardi, C.; Pomata, D.; Buiarelli, F. Concentrations of PAHs, and nitro-and methyl-derivatives associated with a size-segregated urban aerosol. Atmos. Environ. 2010, 44, 2742–2749. [Google Scholar] [CrossRef]
- Zastrow, L.; Schwind, K.H.; Schwägele, F.; Speer, K. Influence of Smoking and Barbecuing on the Contents of Anthraquinone (ATQ) and Polycyclic Aromatic Hydrocarbons (PAHs) in Frankfurter-Type Sausages. J. Agric. Food Chem. 2019, 67, 13998–14004. [Google Scholar] [CrossRef]
- Kinouchi, T.; Tsutsui, H.; Ohnishi, Y. Detection of 1-nitropyrene in yakitori (grilled chicken). Mutat. Res./Genet. Toxicol. 1986, 171, 105–113. [Google Scholar] [CrossRef]
- Larsson, B.K.; Pyysalo, H.; Sauri, M. Class separation of mutagenic polycyclic organic material in grilled and smoked foods. Z. Für Lebensm.-Unters. Und-Forsch. 1988, 187, 546–551. [Google Scholar] [CrossRef]
- Resende dos Santos, R.; Leal Vidotti, L.D.; de Lourdes Cardeal, Z.; Menezes Costa, H. Determination of polycyclic aromatic hydrocarbons and their nitrated and oxygenated derivatives in coffee brews using an efficient cold fiber-solid phase microextraction and gas chromatography mass spectrometry method. J. Chromatogr. A 2019, 1584, 64–71. [Google Scholar] [CrossRef]
- Ko, J.H.; Das, G.; Kim, J.E.; Shin, H.S. Study on formation of nitrated polycyclic aromatic hydrocarbons from different roasting condition in coffee. J. Food Sci. Technol. 2018, 55, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- Anggraini, T.; Neswati Nanda, R.F.; Syukri, D. Identification of 9,10-anthraquinone contamination during black and green tea processing in Indonesia. Food Chem. 2020, 327, 127092. [Google Scholar] [CrossRef] [PubMed]
- Resende dos Santos, R.; Orlando, R.M.; de Lourdes Cardeal, Z.; Menezes, H.C. Assessment of polycyclic aromatic hydrocarbons and derivatives in beer using a new cold fiber-solid phase microextraction system. Food Control 2021, 126, 108104. [Google Scholar] [CrossRef]
- Deng, K.; Chan, W. Development of a QuEChERS-Based Method for Determination of Carcinogenic 2-Nitrofluorene and 1-Nitropyrene in Rice Grains and Vegetables: A Comparative Study with Benzo[a]pyrene. J. Agric. Food Chem. 2017, 65, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Dafflon, O.; Scheurer, L.K.H. Quantification of nitrated polycyclic aromatic hydrocarbons in fish, meat products and cheese using high performance liquid chromatography. Mitt. Aus Lebensm. Und Hyg. 2000, 91, 158–171. [Google Scholar]
- Siegmund, B.; Weiss, R.; Pfannhauser, W. Sensitive method for the determination of nitrated polycyclic aromatic hydrocarbons in the human diet. Anal. Bioanal. Chem. 2003, 375, 175–181. [Google Scholar] [CrossRef]
- Yan, K.; Wu, S.; Gong, G.; Xin, L.; Ge, Y. Simultaneous Determination of Typical Chlorinated, Oxygenated, and European Union Priority Polycyclic Aromatic Hydrocarbons in Milk Samples and Milk Powders. J. Agric. Food Chem. 2021, 69, 3923–3931. [Google Scholar] [CrossRef]
- Gong, G.; Wu, S.; Wu, X. Effects of storage time and temperature on toxic aldehydes and polycyclic aromatic hydrocarbons in flavouring oil gravy during storage. Lwt 2019, 116, 108510. [Google Scholar] [CrossRef]
- Gong, G.; Wu, S.; Wu, X. Influences of Light Intensity and β-Carotene on Polycyclic Aromatic Hydrocarbons and Aldehydes in Vegetable Oil: A Case Study Using Palm Oil. J. Agric. Food Chem. 2018, 66, 11124–11132. [Google Scholar] [CrossRef]
- Hua, H.; Zhao, X.; Wu, S.; Li, G. Impact of refining on the levels of 4-hydroxy-trans-alkenals, parent and oxygenated polycyclic aromatic hydrocarbons in soybean and rapeseed oils. Food Control 2016, 67, 82–89. [Google Scholar] [CrossRef]
- Teng, C.; Wu, S.; Sun, Y.; Gong, G. Determination of parent and oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) in waste cooking oil and oil deodorizer distillate by GC-QQQ-MS. J. AOAC Int. 2019, 102, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gong, G.; Wu, S. Effect of storage time and temperature on parent and oxygenated polycyclic aromatic hydrocarbons in crude and refined vegetable oils. Food Chem. 2018, 239, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, S.; Gong, G.; Li, G.; Zhuang, L. TBHQ and peanut skin inhibit accumulation of PAHs and oxygenated PAHs in peanuts during frying. Food Control 2017, 75, 99–107. [Google Scholar] [CrossRef]
- Li, G.; Wu, S.; Zeng, J.; Wang, L.; Yu, W. Effect of frying and aluminium on the levels and migration of parent and oxygenated PAHs in a popular Chinese fried bread youtiao. Food Chem. 2016, 209, 123–130. [Google Scholar] [CrossRef]
- Gong, G.; Zhao, X.; Shimin, W. Effect of natural antioxidants on inhibition of parent and oxygenated polycyclic aromatic hydrocarbons in Chinese fried bread youtiao. Food Control 2018, 87, 117–125. [Google Scholar] [CrossRef]
- Zastrow, L.; Speer, K.; Schwind, K.; Jira, W. A sensitive GC–HRMS method for the simultaneous determination of parent and oxygenated polycyclic aromatic hydrocarbons in barbecued meat and meat substitutes. Food Chem. 2021, 365, 130625. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yu, H.; Yin, S.; Li, Y.; Sun, C. Solid-Phase Extraction Combined with Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry for the Determination of 5 Trace Nitro-Polycyclic Aromatic Hydrocarbons in Barbecued Foods. J. AOAC Int. 2020, 103, 1512–1520. [Google Scholar] [CrossRef]
- Williams, T.T.J.; Perreault, H. Selective detection of nitrated polycyclic aromatic hydrocarbons by electrospray ionization mass spectrometry and constant neutral loss scanning. Rapid Commun. Mass Spectrom. 2000, 14, 1474–1481. [Google Scholar] [CrossRef]
- Deng, K.; Wong, T.Y.; Wang, Y.; Leung, E.M.K.; Chan, W. Combination of precolumn nitro-reduction and ultraperformance liquid chromatography with fluorescence detection for the sensitive quantification of 1-nitronaphthalene, 2-nitrofluorene, and 1-nitropyrene in meat products. J. Agric. Food Chem. 2015, 63, 3161–3167. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, Y.; Zhao, M.; Wang, Z.; Chen, X.; Wang, M. Core–shell indium (III) sulfide@metal-organic framework nanocomposite as an adsorbent for the dispersive solid-phase extraction of nitro-polycyclic aromatic hydrocarbons. J. Chromatogr. A 2018, 1551, 21–28. [Google Scholar] [CrossRef]
- Sun, C.; Qu, L.; Wu, L.; Wu, X.; Sun, R.; Li, Y. Advances in analysis of nitrated polycyclic aromatic hydrocarbons in various matrices. TrAC-Trends Anal. Chem. 2020, 127, 115878. [Google Scholar] [CrossRef]
- [EC] European Commission. Regulation No. 1881/2006/EC of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs with amendments. Off. J. Eur. Union. 2006, L364, 5. [Google Scholar]
- [EC] European Commision Regulation (EU) No 1146/2014 of 23 October 2014 Amending Annexes 561 II, III, IV and V to Regulation (EC) No 396/2005 of the European Parliament and of 562 the Council. Off. J. Eur. Union 2014, L308, 3.
- Lund, M.; Duedahl-Olesen, L.; Christensen, J.H. Extraction of polycyclic aromatic hydrocarbons from smoked fish using pressurized liquid extraction with integral fat removal. Talanta. 2009, 79, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Duedahl-Olesen, L.; Christensen, J.H.; Højgard, A.; Granby, K.; Timm-Heinrich, M. Influence of smoking parameters on the concentration of polycyclic aromatic hydrocarbons (PAHs) in Danish smoked fish. Food Addit. Contam-Part A Chem. Anal. Control Expo. Risk Assess 2010, 27, 1294–1305. [Google Scholar] [CrossRef]
- Fromberg, A.; Højgård, A.; Duedahl-Olesen, L. Analysis of polycyclic aromatic hydrocarbons in vegetable oils combining gel permeation chromatography with solid-phase extraction clean-up. Food Addit. Contam. 2007, 24, 758–767. [Google Scholar] [CrossRef] [PubMed]
- [ISO] International Organization for Standardization 5725-2:2019. Accuracy (Truness and precision) of measurement methods and results–Part2: Basic method for the determination of repeatability and reproducibility of a standard measurement method. 2019. Available online: https://www.iso.org/standard/69419.html (accessed on 1 June 2021).
- [EC] European Commission Regulation No. 333/2007 of 28 March 2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo(a)pyrene in foodstuffs with amendments in EC regulation 836/2011. Off. J. Eur. Union 2007, L88, 29.
- Duedahl-Olesen, L.; Iversen, N.M.; Kelmo, C.; Jensen, L.K. Validation of QuEChERS for screening of 4 marker polycyclic aromatic hydrocarbons in fish and malt. Food Control 2020, 108, 106434. [Google Scholar] [CrossRef]
- Barreto, R.P.; Albuquerque, F.C.; Netto, A.D.P. Optimization of an improved analytical method for the determination of 1-nitropyrene in milligram diesel soot samples by high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 2007, 1163, 219–227. [Google Scholar] [CrossRef]
- De Witte, B.; Walgraeve, C.; Demeestere, K.; Van Langenhove, H. Oxygenated polycyclic aromatic hydrocarbons in mussels: Analytical method development and occurrence in the Belgian coastal zone. Environ. Sci. Pollut. Res. 2019, 26, 9065–9078. [Google Scholar] [CrossRef]
- Dušek, B.; Hajšlová, J.; Kocourek, V. Determination of nitrated polycyclic aromatic hydrocarbons and their precursors in biotic matrices. J. Chromatogr. A 2002, 982, 127–143. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Results of the monitoring of non-dioxin-like PCBs in food and feed. EFSA J. 2010, 8, 1701. Available online: www.efsa.europa.eu (accessed on 1 June 2021). [CrossRef]
- Jafarabadi, A.R.; Riyahi Bakhtiari, A.; Yaghoobi, Z.; Kong Yap, C.; Maisano, M.; Cappello, T. Distributions and compositional patterns of polycyclic aromatic hydrocarbons (PAHs) and their derivatives in three edible fishes from Kharg coral Island, Persian Gulf, Iran. Chemosphere 2019, 215, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Durant, J.L.; Busby, W.F.; Lafleur, A.L.; Penman, B.W.; Crespi, C.L. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat. Res.-Genet. Toxicol. 1996, 371, 123–157. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Sánchez-Uría, J.E.; Castillo-Busto, E.D. Analytical performance of microwave-assisted solvent extraction (MASE) for the routine determination of PAHs in polluted soils by gas chromatographic-mass spectrometry (GC-MS). Rev. Int. De Contam. Ambient. 2018, 34, 355–366. [Google Scholar] [CrossRef]
- Duedahl-Olesen LAaslyng, M.; Meinert, L.; Christensen, T.; Jensen, A.H.; Binderup, M.-L. Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control 2015, 57, 169–176. [Google Scholar] [CrossRef]
- van der Burgt, P.J.; Dunne, M.; Gradziel, M.L. Comparison of the fragmentations of phenanthrene and anthracene by low-energy electron impact. In Journal of Physics: Conference Series; IOP Publishing: Dublin, Ireland, 2019; Volume 1289, p. 012008. [Google Scholar]
- Seitz, F.; Holm, A.I.; Zettergren, H.; Johansson, H.A.; Rosén, S.; Schmidt, H.T.; Cederquist, H. Polycyclic aromatic hydrocarbon-isomer fragmentation pathways: Case study for pyrene and fluoranthene molecules and clusters. J. Chem. Phys. 2011, 135, 064302. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Bigalke, M.; Boamah, L.; Nyarko, E.; Saalia, F.K.; Wilcke, W. Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): Bioaccumulation and health risk assessment. Environ. Int. 2014, 65, 135–146. [Google Scholar] [CrossRef]
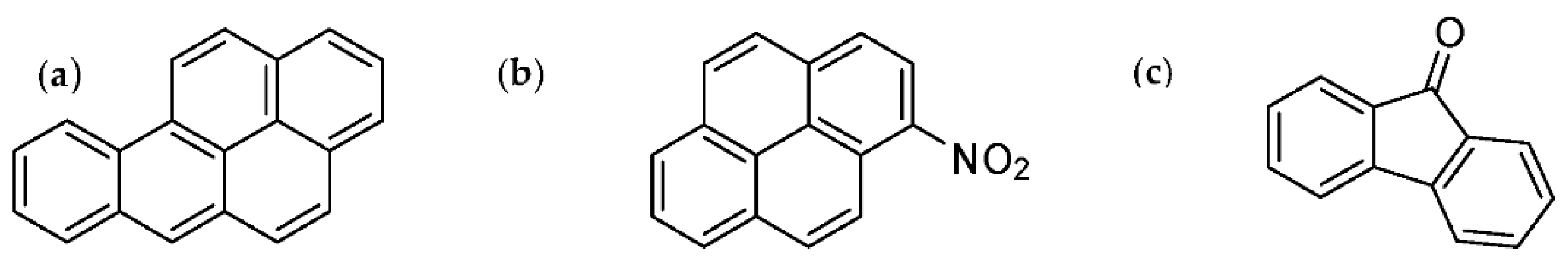

| Compound | Molecular Formula | Abbreviation | Compound Class | IARC Group a | CAS Num |
|---|---|---|---|---|---|
| 1-Indanone | C9H8O | 1-Ind-one | OPAH | * | 83-33-0 |
| 1-Nitro-naphthalene | C10H7NO2 | 1NNap | NPAH | 3 | 86-57-7 |
| 2-Nitro-naphthalene | C10H7NO2 | 2NNap | NPAH | 3 | 581-89-5 |
| Acenaphthylene | C12H8 | Acy | PAH | * | 208-96-8 |
| 4-Acenaphthylenol | C12H8O | 4-Acy-ol | OPAH | * | 111013-09-3 |
| Acenaphthene | C12H10 | Acn | PAH | 3 | 83-32-9 |
| 5-Nitro-acenaphthene | C12H9NO2 | 5NAce | NPAH | 2B | 602-87-9 |
| Fluorene | C13H10 | Flu | PAH | 3 | 86-73-7. |
| 2-Nitro-fluorene | C13H9NO2 | 2NFlu | NPAH | 2B | 607-57-8 |
| 9-Fluorenone | C13H8O | 9FLO | OPAH | * | 486-25-9 |
| 2-Hydroxyfluorene | C13H10O | 2-OH-Flu | OPAH | * | 2443-58-5 |
| Xantene | C13H10O | Xan | OPAH | * | 92-83-1 |
| 4-Methylfluorene | C14H12 | 2-Me-Flu | Methylated-PAH | * | 1556-99-6 |
| Antracene | C14H10 | Ant | PAH | 3 | 1719-06-8. |
| 9- Nitro-anthracene | C14H9NO2 | 9NAnt | NPAH | 3 | 602-60-8 |
| 9,10-Antraquinone | C14H8O2 | ATQ | OPAH | 2B | 84-65-1 |
| Phenanthrene | C14H10 | Phe | PAH | 3 | 85-01-8 |
| 3- Nitro-phenanthrene | C14H9NO2 | 3NPhe | NPAH | * | 17024-19-0 |
| 9-Nitro-phenanthrene | C14H9NO2 | 9NPhe | NPAH | * | 954-46-1 |
| 4-Methylphenanthrene | C15H10 | 4-Me-Phe | Methylated-PAH | * | 832-64-4 |
| Fluoranthene | C16H10 | Fla | PAH | 3 | 206-44-0 |
| 3-Nitro-fluoranthene | C16H9NO2 | 3NFla | NPAH | 3 | 892-21-7 |
| Pyrene | C16H10 | Pyr | PAH | 3 | 129-00-0 |
| 1-Nitro-pyrene | C16H9NO2 | 1NPyr | NPAH | 2A | 5522-43-0 |
| 4-Nitro-pyrene | C16H9NO2 | 4NPyr | NPAH | 2B | 57835-92-4 |
| 1,3-Dinitro-pyrene | C16H8N2O4 | 1,3DNPyr | NPAH | 2B | 75321-20-9 |
| 1,6-Dinitro-pyrene | C16H8N2O4 | 1,6DNPyr | NPAH | 2B | 42397-64-8 |
| 1,8-Dinitro-pyrene | C16H8N2O4 | 1,8DNPyr | NPAH | 2B | 42397-65-9 |
| Benz[a]anthracene | C18H12 | BaA | PAH | 2B | 56-55-3 |
| 7-Nitro-benz[a]anthracene | C18H11NO2 | 7NBaA | NPAH | 3 | 20268-51-3 |
| Chrysene | C18H12 | Chr | PAH | 2B | 218-01-9 |
| 6- Nitro-chrysene | C18H11NO2 | 6NChr | NPAH | 2A | 7496-02-8 |
| Benzo[b]fluoranthene | C20H12 | BbF | PAH | 2B | 205-99-2 |
| Benzo[a]pyrene | C20H12 | BaP | PAH | 1 | 50-32-8 |
| 6-Nitro-benzo[a]pyrene | C20H11NO2 | 6NBaP | NPAH | 3 | 63041-90-7 |
| Compounds | QTOF (m/z) | Retention Time (Min) | RSDR | RSDr | Total Recovery (%) | LOD (µg/kg) | LOQ (µg/kg) | Matrix Effect (%) |
|---|---|---|---|---|---|---|---|---|
| 1NNap-d7 | 134.097 | 8.61 | - | - | - | - | - | - |
| 1NNap | 127.053 | 8.65 | 9 | 6 | 101 | 0.70 | 1.40 | 93 |
| 2NNap | 127.053 | 9.08 | 8 | 8 | 93 | 0.38 | 0.75 | 92 |
| 9FLO | 180.057 | 10.15 | 14 | 11 | 96 | 0.80 | 1.60 | 113 |
| ATQ | 208.052 | 14.13 | 13 | 9 | 64 | 0.45 | 0.89 | 90 |
| 5NAce | 152.061 | 14.80 | 8 | 8 | 94 | 0.43 | 0.86 | - |
| 2NFlu-d9 | 174.126 | 16.42 | - | - | - | - | - | - |
| 2NFlu | 165.069 | 16.56 | 8 | 6 | 101 | 0.54 | 1.08 | 113 |
| 9NAnt-d9 | 232.118 | 16.78 | - | - | - | - | - | - |
| 9NAnt | 176.062 | 16.87 | 6 | 5 | 96 | 0.29 | 0.58 | 100 |
| 9NPhe | 176.062 | 18.08 | 6 | 5 | 97 | 0.19 | 0.39 | 114 |
| 3NPhe | 223.062 | 18.73 | 8 | 6 | 98 | 0.49 | 0.98 | 114 |
| 3NFla-d9 | 226.143 | 22.00 | - | - | - | - | - | - |
| 3NFla | 247.062 | 22.06 | 6 | 5 | 93 | 0.69 | 1.38 | 90 |
| 4NPyr | 247.062 | 22.26 | 10 | 6 | 103 | 0.55 | 1.11 | 97 |
| 1NPyr-d9 | 226.142 | 22.73 | - | - | - | - | - | - |
| 1NPyr | 247.062 | 22.79 | 9 | 4 | 99 | 0.59 | 1.18 | 103 |
| 6NChr-d11 | 284.145 | 24.11 | - | - | - | - | - | - |
| 7NBaA | 215.086 | 24.59 | 10 | 9 | 107 | 0.44 | 0.88 | 98 |
| 6NChr | 273.079 | 25.61 | 6 | 4 | 97 | 0.47 | 0.94 | 98 |
| 1,3DNPyr * | 200.061 | 26.83 | 18 | 8 | 70 | 1.55 | 3.10 | 82 |
| 1,6DNpyr * | 292.046 | 28.19 | 32 | 8 | 91 | 3.46 | 6.93 | 106 |
| 1,8DNpyr * | 292.046 | 28.56 | 70 | 25 | - | 3.21 | 6.42 | 90 |
| 6NBaP | 297.079 | 30.40 | 13 | 13 | 88 | 0.35 | 0.71 | 90 |
| Fish/Meat | Cod (C1) | Cod (C2) | Cod (C3) | Cod (C4) | Halibut (HA1) | Halibut (HA2) | Herring (HE1) | Herring (HE2) | Mackerel (MA1) | Mackerel (MA2) | Mackerel (MA3) | Salmon (SA1) | Salmon (SA2) | Salmon (SA3) | Salmon (SA4) | Bacon (B1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoking Process | Boiled | Pan Fried | Hot (200 °C) | Hot (360 °C) | Traditional | Hot | Hot | Hot | Hot | Hot | Hot | Hot | Cold | Cold | Hot | Hot |
| 1NNap | nd | nd | nd | nd | nd | nd | 5.64 | nd | nd | nd | nd | nd | nd | 0.46 * | nd | nd |
| 2NNap | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.70 * | nd | nd | nd | nd | nd |
| 5NAce | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.43 * | nd | nd | nd | nd | 0.86 * |
| 2NFlu | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 9NAnt | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.02 |
| 9NPhe | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 3NPhe | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 3NFla | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 4NPyr | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.16 * | nd | nd | nd | nd |
| 1NPyr | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 7NBaA | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 6NChr | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 1,3DNPyr | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 1,6DNPyr | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 6NBaP | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| ΣNPAH | nd | nd | nd | nd | nd | nd | 5.64 | nd | nd | nd | 1.13 | 0.16 | nd | 0.46 | nd | 1.88 |
| 9FLO | 3.40 | 4.92 | 14.83 | 23.41 | 13.36 | 10.17 | 15.56 | 21.25 | nd | nd | 8.20 | 7.24 | 1.14 * | 2.02 | 15.82 | 162.12 ** |
| ATQ | 2.31 | 2.90 | 6.63 | 10.54 | 1.50 | 2.54 | 8.63 | 4.32 | nd | nd | 1.35 | 1.50 | 0.97 | 0.82 * | 5.92 | 25.63 |
| ΣOPAH | 5.71 | 7.82 | 21.47 | 33.95 | 14.86 | 12.71 | 24.19 | 25.58 | nd | nd | 9.56 | 8.73 | 2.11 | 2.84 | 21.75 | 187.74 |
| BaA | nd | nd | 0.10 * | 0.04 * | nd | nd | nd | 0.38 | nd | nd | nd | nd | nd | nd | nd | 13.86 |
| CHR | nd | nd | 0.24 | 0.13 * | nd | nd | nd | 0.21 * | 0.67 | nd | nd | nd | nd | nd | nd | 14.39 |
| BbF | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 4.78 |
| BaP | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.30 |
| ΣPAH4 | nd | nd | 0.34 | 0.17 | nd | nd | nd | 0.59 | 0.67 | nd | nd | nd | nd | nd | nd | 38.33 |
| S No. | Compound | Mol. Formula | m/z | Rt (Min) | Sample Name |
|---|---|---|---|---|---|
| 1 | 1-Ind-one | C9H8O | 132.057 | 6.39 | SA1, SA2, SA3, SA4, HE1, HE2, HA1, MA2, B1 |
| 2 | Acy | C12H8 | 152.063 | 7.23 | HE2, MA2 |
| 3 | Acn | C12H10 | 153.070 | 7.40 | HE2 |
| 4 | 4-Acy-ol | C12H8O | 168.059 | 7.58 | B1 |
| 5 | Flu | C13H10 | 166.077 | 8.16 | HA1, HE2, B1, SA1, MA1 |
| 6 | 2-OH-Flu | C13H10O | 181.066 | 8.46 | B1 |
| 7 | Xan | C13H10O | 182.074 | 8.47 | HE2, MA1 |
| 8 | 2-Me-Flu | C14H12 | 165.071 | 9.19 | HE2 |
| 9 | Phe | C14H10 | 178.079 | 10.60 | C2, HE2, MA1, HA1 |
| 10 | Ant | C14H10 | 178.079 | 10.73 | B1 |
| 11 | 4-Me-Phe | C15H12 | 192.095 | 12.07 | B1 |
| 12 | Fla | C16H10 | 202.08 | 15.28 | HE2, B1, MA1 |
| 13 | Pyr | C16H10 | 202.079 | 16.45 | HE2, B1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonego, E.; Bhattarai, B.; Duedahl-Olesen, L. Detection of Nitrated, Oxygenated and Hydrogenated Polycyclic Aromatic Compounds in Smoked Fish and Meat Products. Foods 2022, 11, 2446. https://doi.org/10.3390/foods11162446
Sonego E, Bhattarai B, Duedahl-Olesen L. Detection of Nitrated, Oxygenated and Hydrogenated Polycyclic Aromatic Compounds in Smoked Fish and Meat Products. Foods. 2022; 11(16):2446. https://doi.org/10.3390/foods11162446
Chicago/Turabian StyleSonego, Elisa, Bina Bhattarai, and Lene Duedahl-Olesen. 2022. "Detection of Nitrated, Oxygenated and Hydrogenated Polycyclic Aromatic Compounds in Smoked Fish and Meat Products" Foods 11, no. 16: 2446. https://doi.org/10.3390/foods11162446
APA StyleSonego, E., Bhattarai, B., & Duedahl-Olesen, L. (2022). Detection of Nitrated, Oxygenated and Hydrogenated Polycyclic Aromatic Compounds in Smoked Fish and Meat Products. Foods, 11(16), 2446. https://doi.org/10.3390/foods11162446