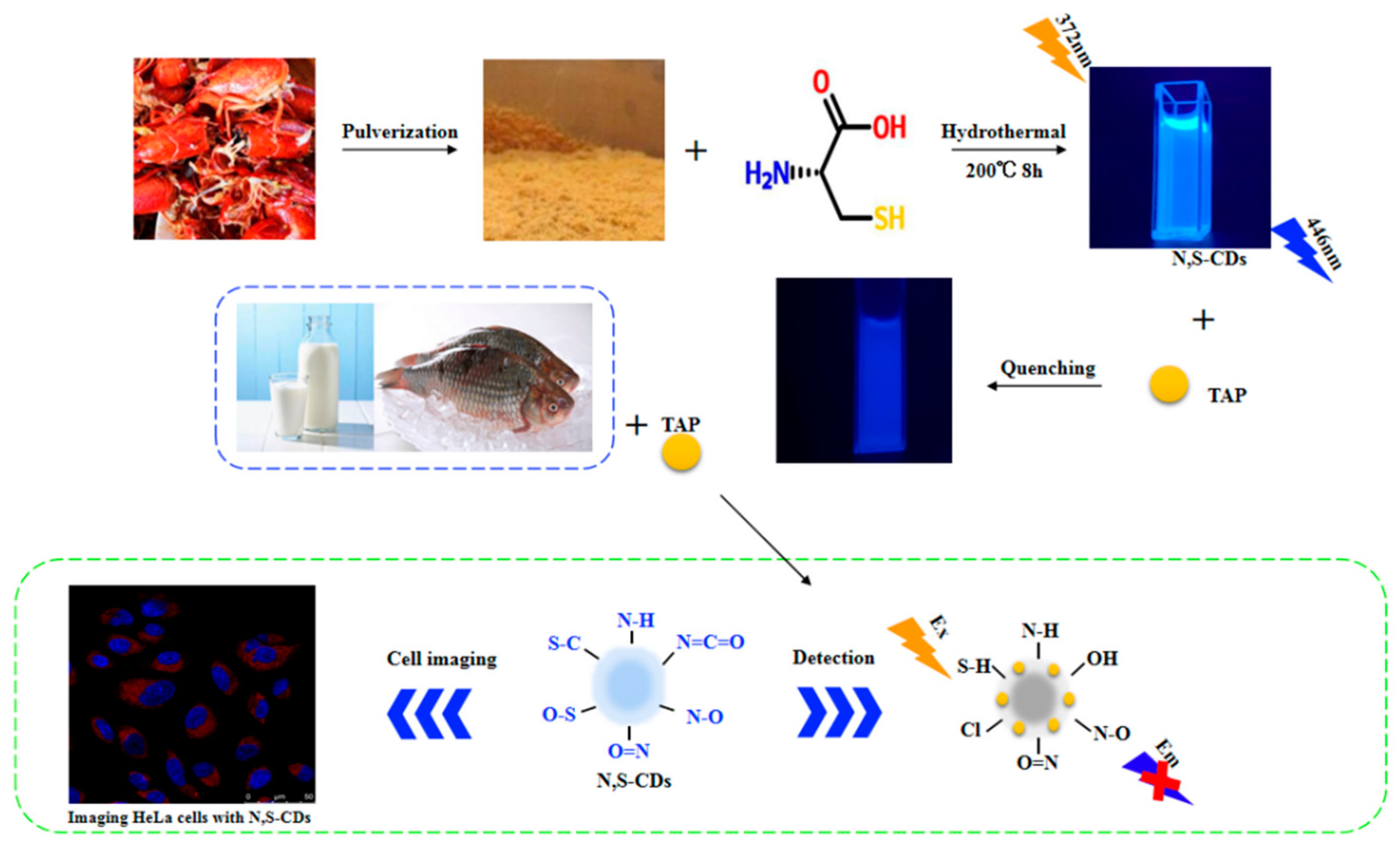
Facile Synthesis of N, S-Doped Carbon Quantum Dots from Food Waste as Fluorescent Probe for Sensitive Detection of Thiamphenicol and Its Analogues in Real Food Samples along with an Application in Bioimaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of N, S-CDs
2.3. Characterization of N, S-CDs
2.4. Sensing Approaches for TAP Determination
2.5. Analysis of Real Food Samples
2.6. Cellular Toxicity Test
2.7. Cellular Imaging and Intracellular Detection of TAP in Hela Cells
3. Results and Discussion
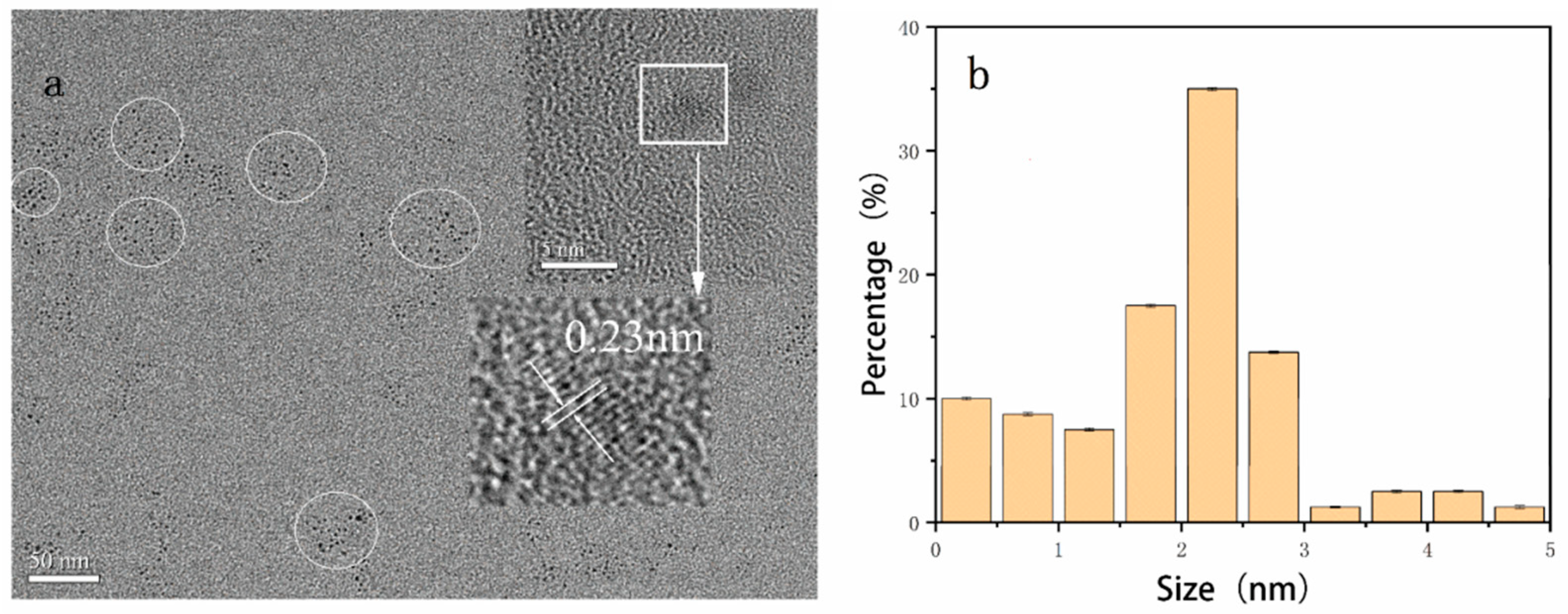
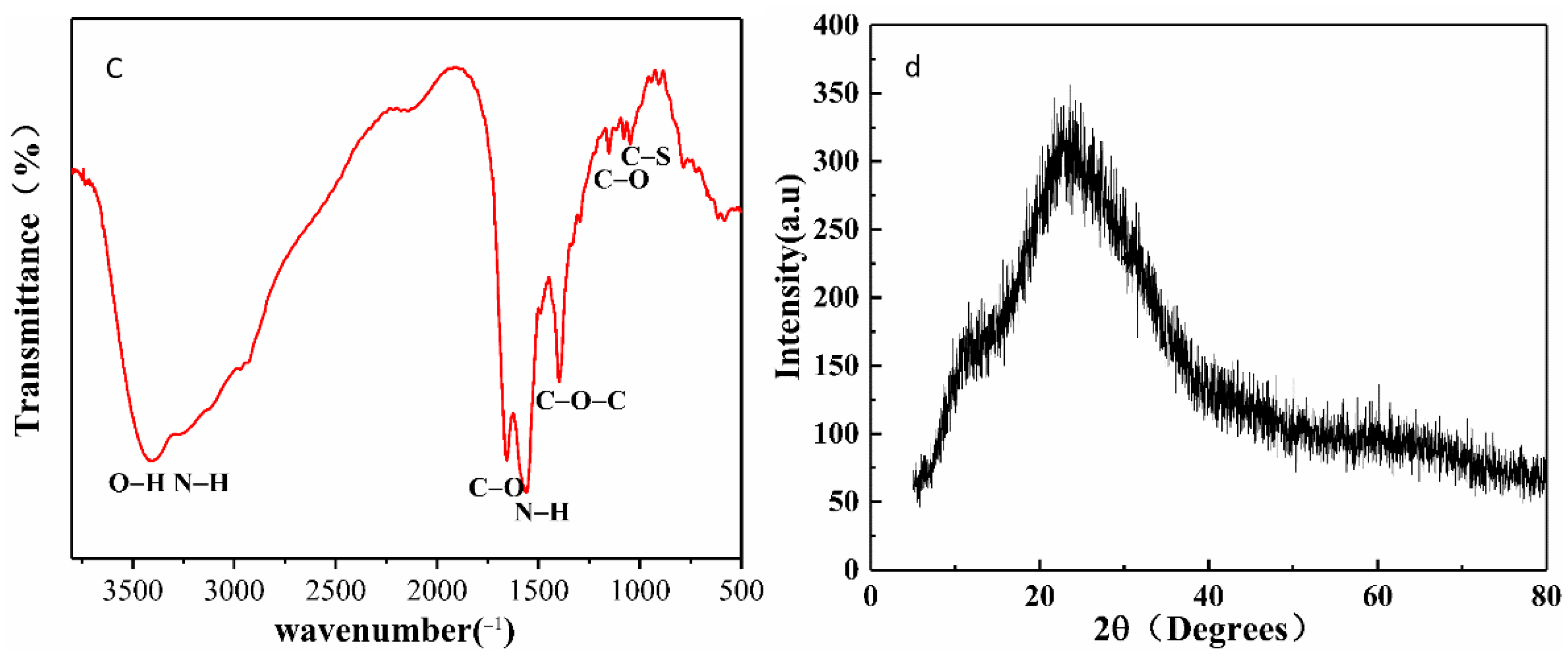
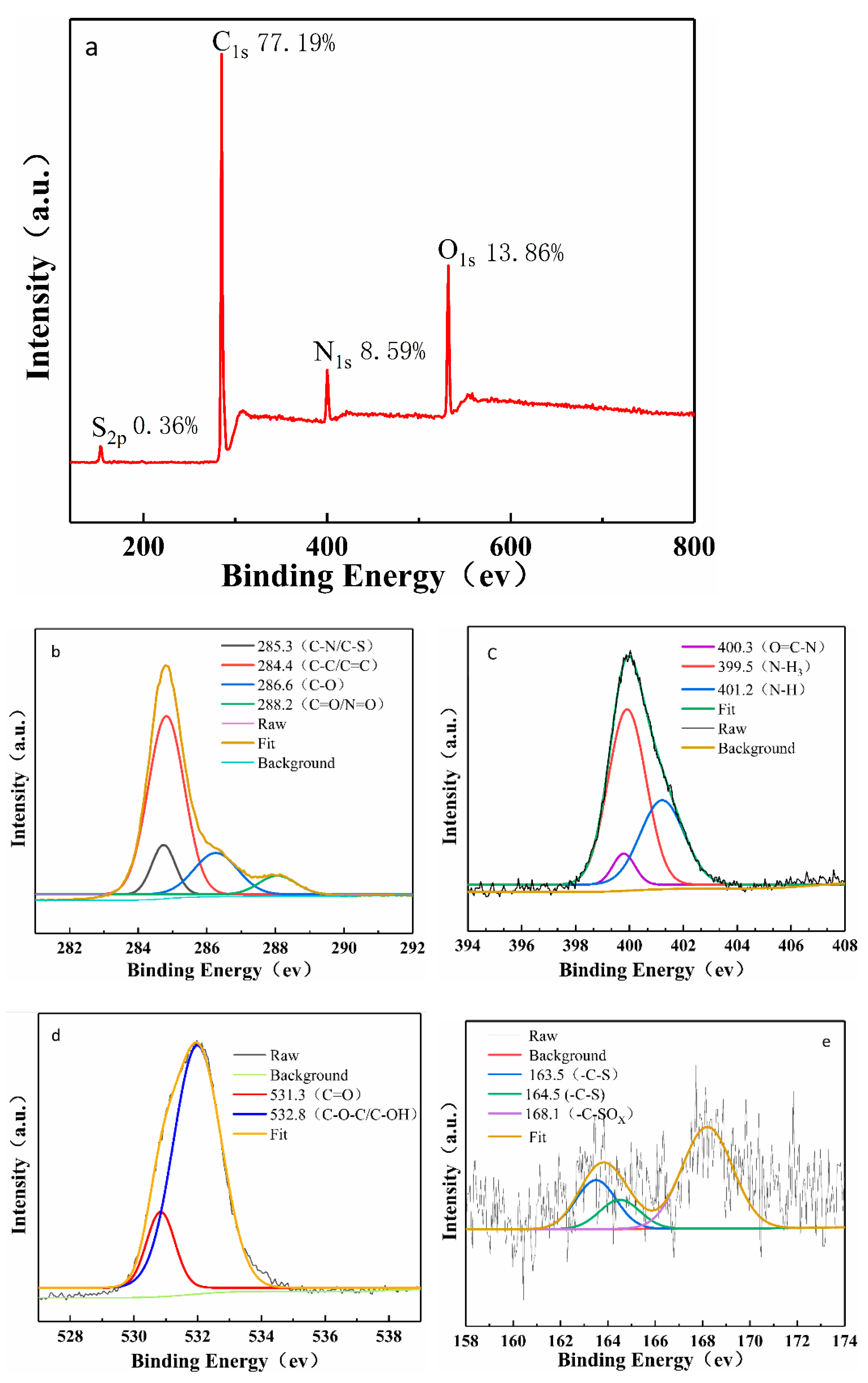
3.1. Characterization of N, S-CDs
3.2. Optical Properties of N, S-CDs
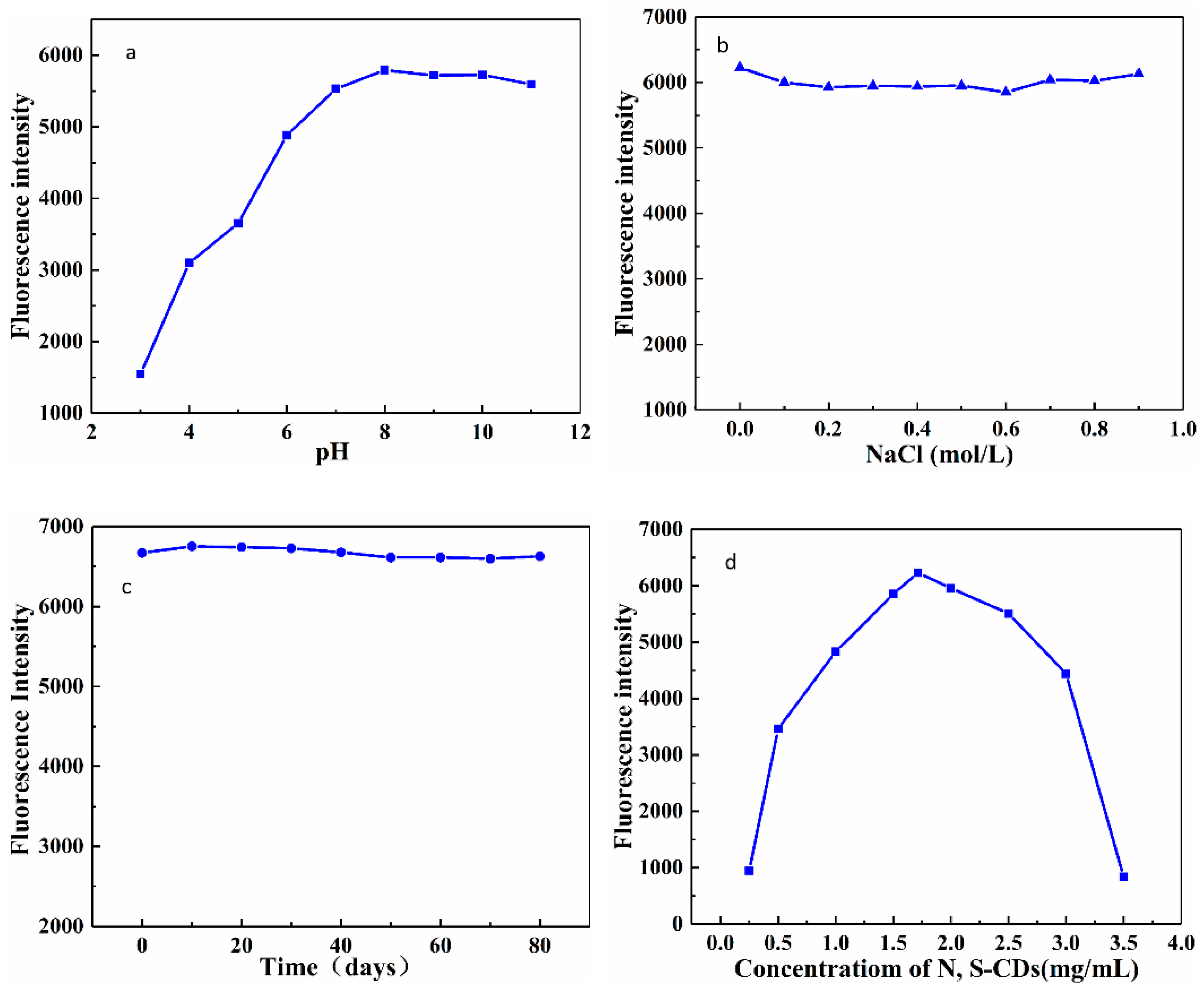
3.3. Stability of the N, S-CDs
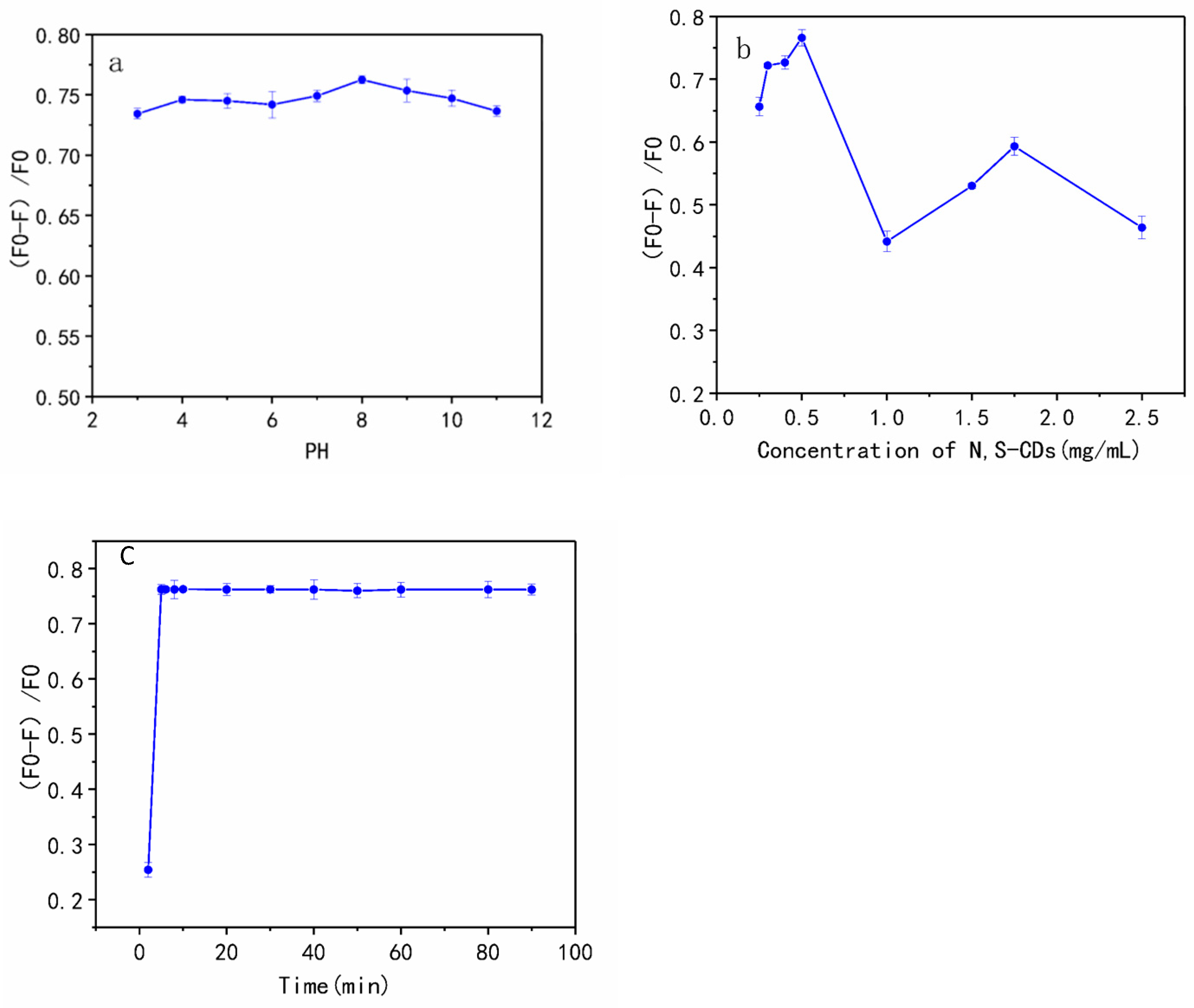
3.4. Optimization of the Sensing Conditions
3.5. Sensing of N, S-CDs to TAP
3.6. Selectivity of N, S-CDs to TAP
3.7. Sensing Mechanism of N, S-CDs to TAP
3.8. Detection of Actual Food Samples
| Technique | Pretreatments | Detection Time | Linear Range (μg·L−1) | LOD (μg·L−1) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|
| ELISA | Prepare and screen the polyclonal antibodies | >70 min | 0.41–11.2 | 0.15 | 77.2–116.0 | [7] |
| UV spectrophotometric | Purification | 55 min | 5000–25,000 | 590 | 99.26–100.56 | [8] |
| HPLC | Thin-layer chromatography (TLC) purification | >1 h | 50–10,000 | 20 | 82.0–114.9 | [9] |
| HPLC-DAD | dispersive solid phase extraction (SPE) | 2 h | 5–150 | 0.48 | 81.7–97.5 | [44] |
| UHPLC-MS | SPE | >1 h | 1–200 | 0.5 | 87.6–102.7 | [1] |
| HPLC-ESI/MS | Accelerated solvent extraction (ASE) | >1 h | 1–150 | 0.4 | 88.3–107 | [10] |
| Fe3O4 @ SiO2-MIPS | Purification and separation by organic solvents | 90 min | 100–300 | 10 | 92.6–95.6 | [5] |
| CNTs-AuNPs electrochemical sensor | Modification of screen printed electrode | >30 min | 35.5–10,650 | 1.07 | 94.0–97.0 | [45] |
| N, S-CDs | Purification | 45 min | 50–300 | 27.5 | 97.30–99.34 | This work |
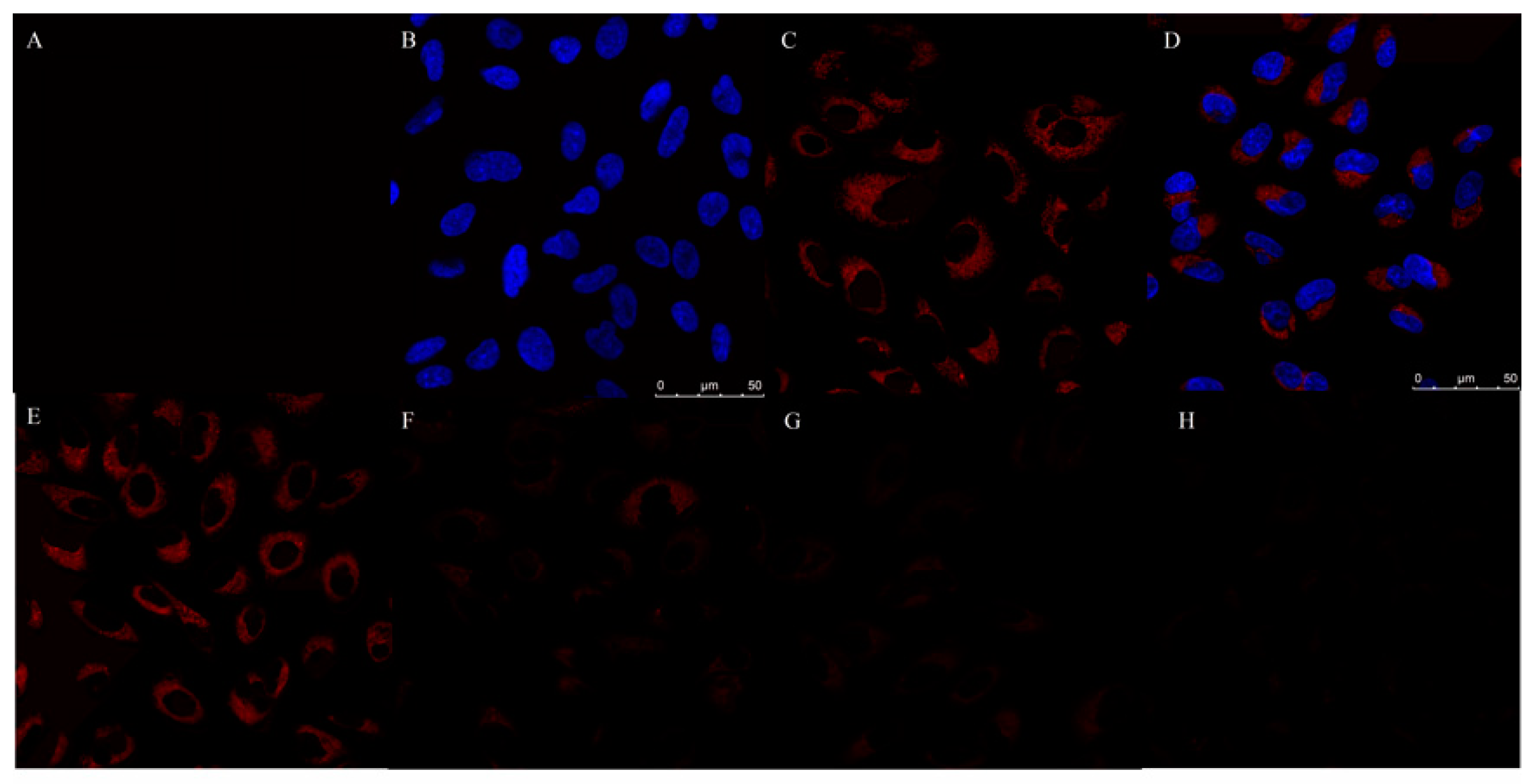
3.9. Cytotoxicity and Cellular Imaging
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Gong, J.; Yuan, H.; Xiao, G.; Wang, H.; Sun, L.; Qiu, H.; Chu, B.; Fang, R. Determination of Chloramphenicol, Thiamphenicol and Florfenicol in Chinese Gelatin Medicines using Dispersive Solid-Phase Extraction Coupled with Ultra High-Performance Liquid Chromatography-Mass Spectrometry. J. Chromatogr. Sci. 2020, 58, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Impens, S.; Reybroeck, W.; Vercammen, J.; Courtheyn, D.; Ooghe, S.; De Wasch, K.; Smedts, W.; De Brabander, H. Screening and confirmation of chloramphenicol in shrimp tissue using ELISA in combination with GC–MS2 and LC–MS2. Anal. Chim. Acta 2003, 483, 153–163. [Google Scholar] [CrossRef]
- Thiam, A.; Salazar, R.; Brillas, E.; Sires, I. In-situ dosage of Fe2+ catalyst using natural pyrite for thiamphenicol mineralization by photoelectro-Fenton process. J. Environ. Manag. 2020, 270, 110835. [Google Scholar] [CrossRef] [PubMed]
- Samsonova, J.V.; Cannavan, A.; Elliott, C.T. A Critical Review of Screening Methods for the Detection of Chloramphenicol, Thiamphenicol, and Florfenicol Residues in Foodstuffs. Crit. Rev. Anal. Chem. 2012, 42, 50–78. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Qi, Y.; Sun, Z.; Gong, B. Preparation of thiamphenicol magnetic surface molecularly imprinted polymers for its selective recognition of thiamphenicol in milk samples. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 868–879. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Off. J. Eur. Communities 2010, 15. Available online: http://data.europa.eu/eli/reg/2010/37(1)/oj (accessed on 26 May 2021).
- Li, R.; Lin, Z.-J.; Yang, J.-Y.; Xu, Z.-L.; Wang, H.; Lei, H.-T.; Sun, Y.-M.; Shen, Y.-D. An Indirect Competitive Enzyme-linked Immunosorbent Assay for Simultaneous Determination of Florfenicol and Thiamphenicol in Animal Meat and Urine. Chin. J. Anal. Chem. 2018, 46, 1321–1328. [Google Scholar] [CrossRef]
- Martins, Y.; Oliveira, D. Development and Validation of an UV Spectrophotometric Method for Determination of Thiamphenicol in Dosage Form. J. Appl. Spectrosc. 2019, 86, 629–635. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Qian, M.; Zhou, D.; Ke, X.; Sun, G.; Yang, B. A TLC-HPLC Method for Determination of Thiamphenicol in Pig, Chicken, and Fish Feedstuffs. Food Anal. Methods 2018, 11, 3103–3112. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.; Xie, X.; Xie, K.; Zhang, G.; Zhang, T. Development of an Accelerated Solvent Extraction Approach for Quantitative Analysis of Chloramphenicol, Thiamphenicol, Florfenicol, and Florfenicol Amine in Poultry Eggs. Food Anal. Methods 2019, 12, 1705–1714. [Google Scholar] [CrossRef]
- Tsai, H.; Hu, H.C.; Hsieh, C.C.; Lu, Y.H.; Chen, C.H.; Fuh, C.B. Fluorescence studies of the interaction between chloramphenicol and nitrogen-doped graphene quantum dots and determination of chloramphenicol in chicken feed. J. Chin. Chem. Soc. 2019, 67, 152–159. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Hou, J.; Huo, D.; Hou, C. A Simple and Rapid Phosphorescence Probe Based on Mn-Doped ZnS Quantum Dots for Chloramphenicol Detection. Nano 2019, 14, 1950144. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C.-H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a kind but different: Luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Weng, B.; Wang, B.; Li, C. Facile synthesis of nitrogen and sulfur co-doped carbon dots and application for Fe(III) ions detection and cell imaging. Sens. Actuators B Chem. 2016, 223, 689–696. [Google Scholar] [CrossRef]
- Wei, J.-M.; Liu, B.-T.; Zhang, X.; Song, C.-C. One-pot synthesis of N, S co-doped photoluminescent carbon quantum dots for Hg2+ ion detection. New Carbon Mater. 2018, 33, 333–340. [Google Scholar] [CrossRef]
- Wu, H.; Tong, C. Nitrogen- and Sulfur-Codoped Carbon Dots for Highly Selective and Sensitive Fluorescent Detection of Hg2+ Ions and Sulfide in Environmental Water Samples. J. Agric. Food Chem. 2019, 67, 2794–2800. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Han, M.; Yuan, S.; Guan, W.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. N,S co-doped carbon dots as a stable bio-imaging probe for detection of intracellular temperature and tetracycline. J. Mater. Chem. B 2017, 5, 3293–3299. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Chen, S.; Yuan, R.; Dong, L. A novel multifunctional fluorescent sensor based on N/S co-doped carbon dots for detecting Cr (VI) and toluene. Microchem. J. 2019, 151, 104246. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Li, J.; Xu, L.; Qiao, Y. One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J. Colloid Interface Sci. 2017, 485, 167–174. [Google Scholar] [CrossRef]
- Ding, C.; Deng, Z.; Chen, J.; Jin, Y. One-step microwave synthesis of N, S co-doped carbon dots from 1,6-hexanediamine dihydrochloride for cell imaging and ion detection. Colloids Surf. B Biointerfaces 2020, 189, 110838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, S.H.; Feng, L. Highly luminescent N, S- Co-doped carbon dots and their direct use as mercury(II) sensor. Anal. Chim. Acta 2015, 890, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Zhao, X.R. Research on Proteinous Adhesive Made of Protein Isolated from Crayfish Shell Waste. Adv. Mater. Res. 2013, 815, 299–304. [Google Scholar] [CrossRef]
- Liu, X.; Pang, J.; Xu, F.; Zhang, X. Simple Approach to Synthesize Amino-Functionalized Carbon Dots by Carbonization of Chitosan. Sci. Rep. 2016, 6, 31100. [Google Scholar] [CrossRef] [PubMed]
- GB 29689-2013; National Food Safety Standard-Determination of Thiamphenicol Residues in Milk-HPLC Method. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2013.
- The Ministry of Agriculture of the People’s Republic of China. Determination of Chloramphenicol, Thiamphenicol and Florfenicol Residues in Fishery Products Gas Chromatography Method (Announcement of the Ministry of Agriculture [13-2007] No. 958). Ministry of Agriculture: Beijing, China. 18 December 2006. Available online: http://jiuban.moa.gov.cn/nybgb/2008/deq/201806/t20180609_6151543.htm (accessed on 26 May 2021).
- Jalili, R.; Khataee, A.; Rashidi, M.R.; Razmjou, A. Detection of penicillin G residues in milk based on dual-emission carbon dots and molecularly imprinted polymers. Food Chem. 2020, 314, 126172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, Y.; Hou, S.; Zhao, L. Functionalized carbon quantum dots as fluorescent nanoprobe for determination of tetracyclines and cell imaging. Mikrochim. Acta 2020, 187, 351. [Google Scholar] [CrossRef]
- Kascakova, S.; Maigre, L.; Chevalier, J.; Refregiers, M.; Pages, J.M. Antibiotic transport in resistant bacteria: Synchrotron UV fluorescence microscopy to determine antibiotic accumulation with single cell resolution. PLoS ONE 2012, 7, e38624. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Yang, X.; Chang, J.; Liu, Z.; Jiang, K. Fish-scale-derived carbon dots as efficient fluorescent nanoprobes for detection of ferric ions. RSC Adv. 2019, 9, 940–949. [Google Scholar] [CrossRef]
- Liao, S.; Zhao, X.; Zhu, F.; Chen, M.; Wu, Z.; Song, X.; Yang, H.; Chen, X. Novel S, N-doped carbon quantum dot-based “off-on” fluorescent sensor for silver ion and cysteine. Talanta 2018, 180, 300–308. [Google Scholar] [CrossRef]
- Gu, D.; Hong, L.; Zhang, L.; Liu, H.; Shang, S. Nitrogen and sulfur co-doped highly luminescent carbon dots for sensitive detection of Cd (II) ions and living cell imaging applications. J. Photochem. Photobiol. B 2018, 186, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Konar, S.; Samanta, D.; Mandal, S.; Das, S.; Mahto, M.K.; Shaw, M.; Mandal, M.; Pathak, A. Selective and sensitive detection of cinnamaldehyde by nitrogen and sulphur co-doped carbon dots: A detailed systematic study. RSC Adv. 2018, 8, 42361–42373. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wu, Z.; Wang, L.; Liu, J.; Deng, Y.; Xiao, Z.; Fang, J.; Liang, Y. Green Preparation of Fluorescent Nitrogen-Doped Carbon Quantum Dots for Sensitive Detection of Oxytetracycline in Environmental Samples. Nanomaterials 2020, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ye, S.; Xu, Y.; Liu, F.; Zhou, Y.; Yang, Q.; Peng, H.; Xiong, H.; Zhang, Z. Microwave-assisted Synthesis of N,S-co-carbon Dots as Switch-on Fluorescent Sensor for Rapid and Sensitive Detection of Ascorbic Acid in Processed Fruit Juice. Anal. Sci. 2020, 36, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Dong, S.; Zhao, F.; Deng, M.; Fu, Y.; Lü, C. Tricolor emissive carbon dots for ultra-wide range pH test papers and bioimaging. Sens. Actuators B Chem. 2019, 298, 126869. [Google Scholar] [CrossRef]
- Porres, L.; Holland, A.; Palsson, L.O.; Monkman, A.P.; Kemp, C.; Beeby, A. Absolute measurements of photoluminescence quantum yields of solutions using an integrating sphere. J. Fluoresc. 2006, 16, 267–272. [Google Scholar] [CrossRef]
- Valenta, J. Photoluminescence of the integrating sphere walls, its influence on the absolute quantum yield measurements and correction methods. AIP Adv. 2018, 8, 105123. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, B.; Li, X.; Zhang, G.; Zhang, Y.; Dong, C.; Shuang, S. Green-fluorescent nitrogen-doped carbon nanodots for biological imaging and paper-based sensing. Anal. Methods 2017, 9, 2197–2204. [Google Scholar] [CrossRef]
- Sadeghi, S.; Olieaei, S. Capped cadmium sulfide quantum dots with a new ionic liquid as a fluorescent probe for sensitive detection of florfenicol in meat samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117349. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, L.; Rao, S.Q.; Yang, Z.Q.; Li, T.; Gong, X. Nitrogen and chlorine dual-doped carbon nanodots for determination of curcumin in food matrix via inner filter effect. Food Chem. 2019, 280, 195–202. [Google Scholar] [CrossRef]
- Röding, M.; Bradley, S.J.; Nydén, M.; Nann, T. Fluorescence Lifetime Analysis of Graphene Quantum Dots. J. Phys. Chem. C 2014, 118, 30282–30290. [Google Scholar] [CrossRef]
- Patyra, E.; Kwiatek, K. HPLC-DAD analysis of florfenicol and thiamphenicol in medicated feedingstuffs. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Hajian, R.; Yusof, N.A.; Shams, N.; Abdullah, J.; Woi, P.M.; Garmestani, H. A screen printed carbon electrode modified with carbon nanotubes and gold nanoparticles as a sensitive electrochemical sensor for determination of thiamphenicol residue in milk. RSC Adv. 2018, 8, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, N.-M.; Kalogianni, D.P.; Christopoulos, T.K. Posidonia oceanica (Mediterranean tapeweed) leaf litter as a source of fluorescent carbon dot preparations. Microchem. J. 2021, 161, 105787. [Google Scholar] [CrossRef]
- Hu, Q.; Paau, M.C.; Zhang, Y.; Gong, X.; Zhang, L.; Lu, D.; Liu, Y.; Liu, Q.; Yao, J.; Choi, M.M.F. Green synthesis of fluorescent nitrogen/sulfur-doped carbon dots and investigation of their properties by HPLC coupled with mass spectrometry. RSC Adv. 2014, 4, 18065–18073. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.; Tian, J.; Jia, L.; Yu, J.-S. Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon 2014, 77, 775–782. [Google Scholar] [CrossRef]


| Sample | Added (μg·L−1) | Found (μg·L−1) | Recovery | RSD (%) |
|---|---|---|---|---|
| Milk-1 | 0 | nd a | - | - |
| Milk-2 | 50 | 49.20 ± 0.77 | 98.39% | 1.54% |
| Milk-3 | 100 | 98.20 ± 1.04 | 98.20% | 1.04% |
| Milk-4 | 150 | 149.00 ± 1.33 | 99.34% | 0.89% |
| Fish-1 | 0 | nd a | - | - |
| Fish-2 | 50 | 48.91 ± 0.20 | 97.30% | 0.41% |
| Fish-3 | 100 | 96.16 ± 0.67 | 97.86% | 0.69% |
| Fish-4 | 150 | 148.64 ± 1.99 | 97.92% | 1.34% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Ouyang, W.; Zhu, Y.; He, L.; Zou, L.; Ao, X.; Liu, S.; Yang, Y.; Li, J. Facile Synthesis of N, S-Doped Carbon Quantum Dots from Food Waste as Fluorescent Probe for Sensitive Detection of Thiamphenicol and Its Analogues in Real Food Samples along with an Application in Bioimaging. Foods 2022, 11, 2414. https://doi.org/10.3390/foods11162414
Chen S, Ouyang W, Zhu Y, He L, Zou L, Ao X, Liu S, Yang Y, Li J. Facile Synthesis of N, S-Doped Carbon Quantum Dots from Food Waste as Fluorescent Probe for Sensitive Detection of Thiamphenicol and Its Analogues in Real Food Samples along with an Application in Bioimaging. Foods. 2022; 11(16):2414. https://doi.org/10.3390/foods11162414
Chicago/Turabian StyleChen, Shujuan, Wanlin Ouyang, Yiting Zhu, Li He, Likou Zou, Xiaolin Ao, Shuliang Liu, Yong Yang, and Jianlong Li. 2022. "Facile Synthesis of N, S-Doped Carbon Quantum Dots from Food Waste as Fluorescent Probe for Sensitive Detection of Thiamphenicol and Its Analogues in Real Food Samples along with an Application in Bioimaging" Foods 11, no. 16: 2414. https://doi.org/10.3390/foods11162414
APA StyleChen, S., Ouyang, W., Zhu, Y., He, L., Zou, L., Ao, X., Liu, S., Yang, Y., & Li, J. (2022). Facile Synthesis of N, S-Doped Carbon Quantum Dots from Food Waste as Fluorescent Probe for Sensitive Detection of Thiamphenicol and Its Analogues in Real Food Samples along with an Application in Bioimaging. Foods, 11(16), 2414. https://doi.org/10.3390/foods11162414






