Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Pullulan Films with Propolis Extracts
2.3. Physical and Mechanical Characteristics of Films
2.3.1. Thickness
2.3.2. Moisture Content
2.3.3. Water Solubility
2.3.4. Mechanical Characteristics
2.4. Optical Characteristics of Films
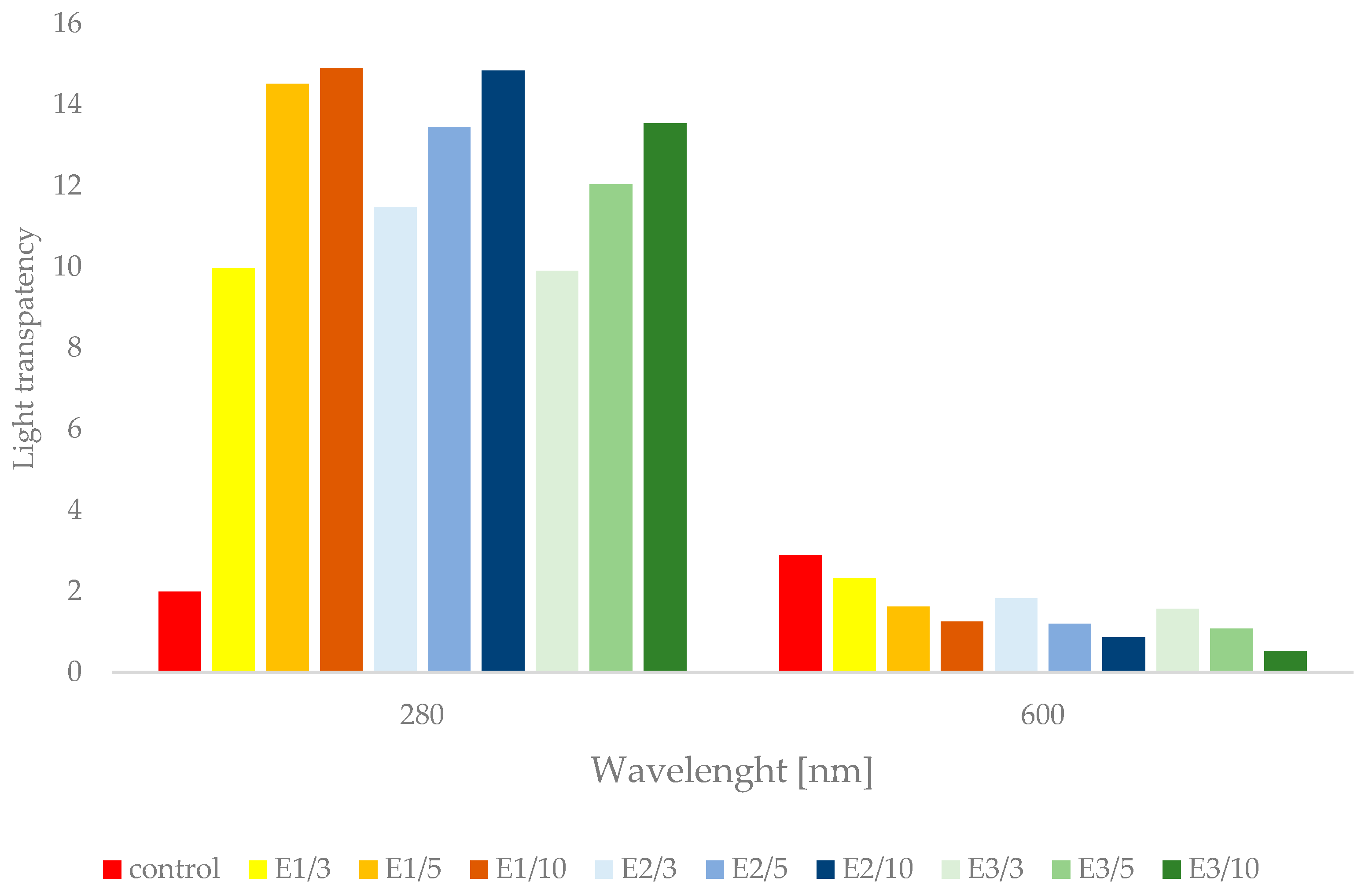
2.4.1. Transparency and UV-Barrier Properties
2.4.2. Color
2.5. Antifungal Activity Assay
2.5.1. Fungal Test Strains and Their Preparation
2.5.2. Disc Diffusion Method
2.6. Statistical Analysis
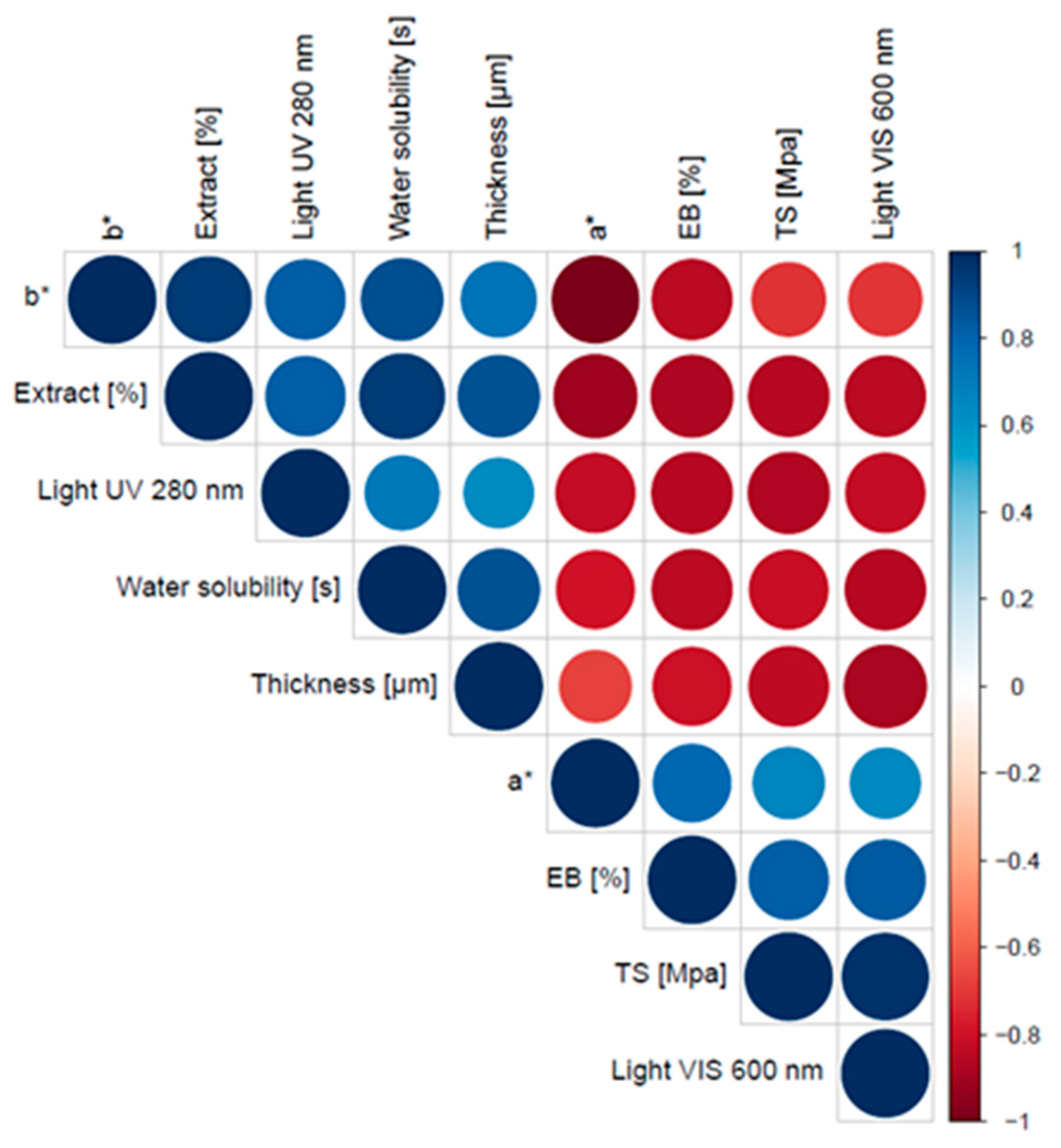
3. Results and Discussion
3.1. Appearance of Pullulan Films with Propolis Extract
3.2. Thickness, Moisture Content and Water Solubility of Films with Propolis Extract
3.3. Mechanical Properties of Films with Propolis Extract
3.4. Light Barrier Properties of Pullulan Films with Propolis Extract
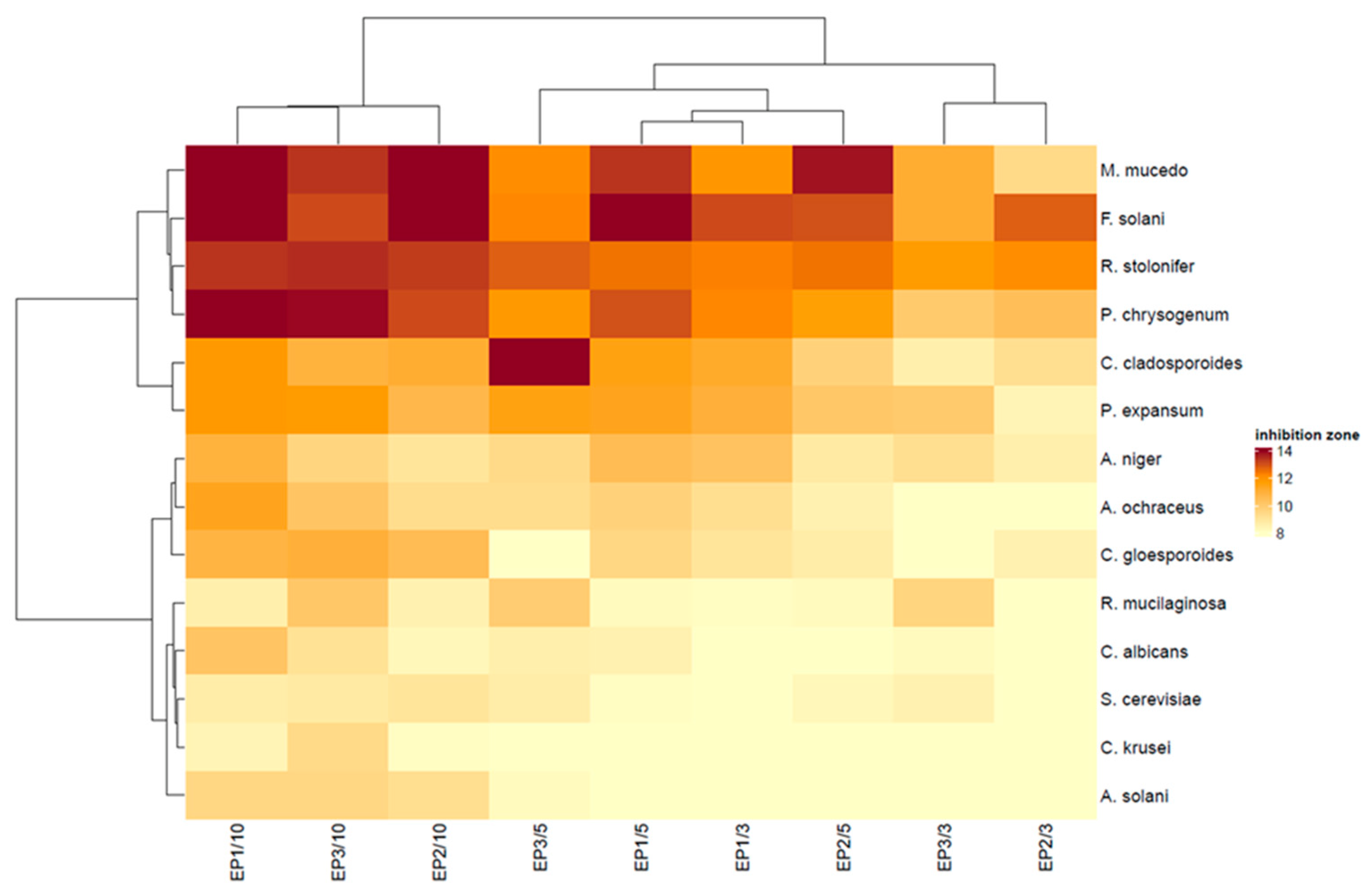
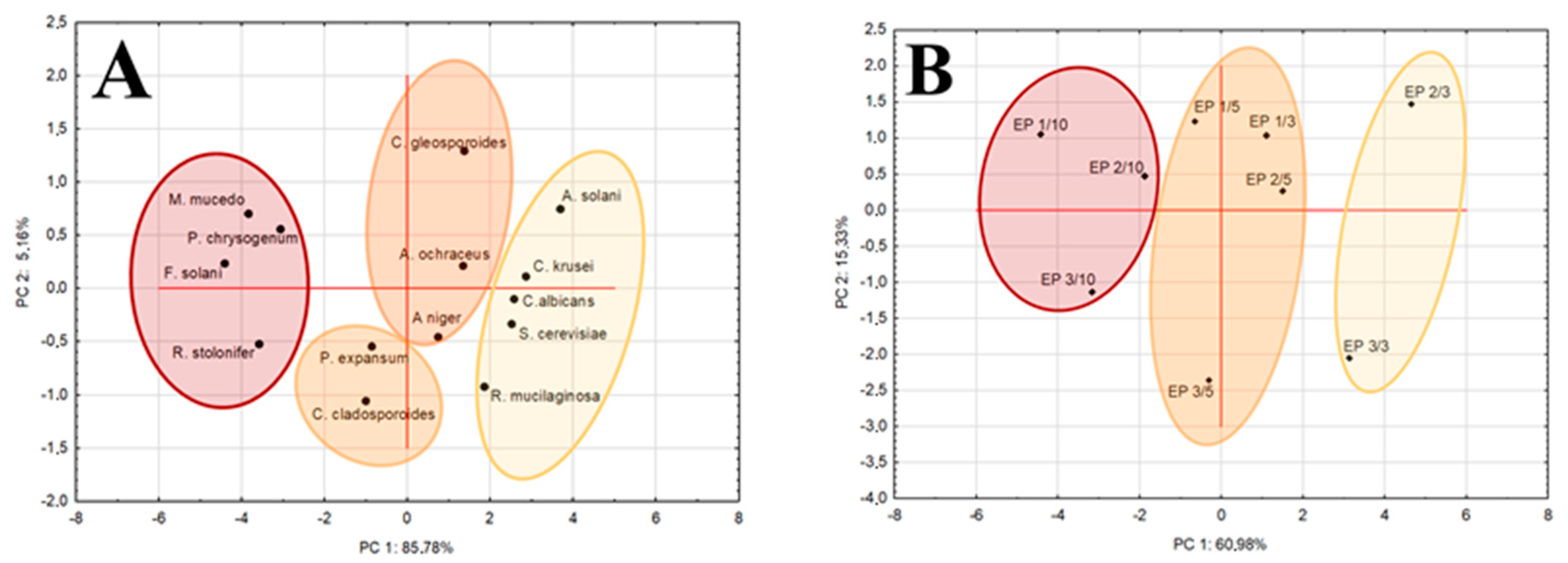
3.5. Antifungal Activity of Films with Propolis Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Domínguez, E.T.; Nguyen, P.H.; Hunt, H.K.; Mustapha, A. Antimicrobial coatings for food contact surfaces: Legal framework, mechanical properties, and potential applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1825–1858. [Google Scholar] [CrossRef] [PubMed]
- Aloui, H.; Khwaldia, K. Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Falah, F.; Vasiee, A.; Yazdi, F.T. Control of microbial growth and lipid oxidation in beef using a Lepidium perfoliatum seed mucilage edible coating incorporated with chicory essential oil. Food Sci. Nutr. 2021, 9, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdes, A.; Ramos, M.; Burgos, N.; del Carmen Garrigos, M.; Jimenez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133, 42631. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, D.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Akanele, A.E.; Scholastica, M.O.C.U.; Chukwu, M.A.B. Microbiological contamination of food: The mechanisms, impacts and prevention. Int. J. Sci. Technol. Res. 2016, 5, 65–78. [Google Scholar]
- Liu, Y.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. 2020, 19, 1521–1560. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Magan, N. Mycotoxin contamination of food in Europe: Early detection and prevention strategies. Mycopathologia 2006, 162, 245–253. [Google Scholar] [CrossRef]
- Garvey, M. Food pollution: A comprehensive review of chemical and biological sources of food contamination and impact on human health. Nutrire 2019, 44, 1. [Google Scholar] [CrossRef]
- Gniewosz, M.; Duszkiewicz-Reinhard, W. Comparative studies on pullulan synthesis, melanin synthesis and morphology of white mutant Aureobasidium pullulans B-1 and parent strain A.p.-3. Carbohydr. Polym. 2008, 72, 431–438. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan—Biopolymer with potential for use as food packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Mishra, B.; Vuppu, S.; Rath, K. The role of microbial pullulan, a biopolymer in pharmaceutical approaches: A review. J. Appl. Pharm. 2011, 1, 45–50. [Google Scholar]
- Taguchi, R.; Kikuchi, Y.; Sakano, Y.; Kobayashi, T. Structural uniformity of pullulan produced by several strains of Pullularia pullulans. Agric. Biol. Chem. 1973, 37, 1583–1588. [Google Scholar] [CrossRef][Green Version]
- Yuen, S. Pullulan and its applications. Process Biochem. 1974, 22, 7–9. [Google Scholar]
- Kimoto, T.; Shibuya, T.; Shiobara, S. Safety studies of a novel starch, pullulan: Chronic toxicity in rats and bacterial mutagenicity. Food Chem. Toxicol. 1997, 35, 323–329. [Google Scholar] [CrossRef]
- Roller, S.; Dea, I.C.M. Biotechnology in the production and modification of biopolymers for foods. Crit. Rev. Biotechnol. 1992, 12, 261–277. [Google Scholar] [CrossRef]
- Diab, T.; Biliaderis, C.G.; Gerasopoulos, D.; Sfakiotakis, E. Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. J. Sci. Food Agric. 2001, 81, 988–1000. [Google Scholar] [CrossRef]
- Chen, F.; Chi, C. Development of pullulan/carboxylated cellulose nanocrystal/tea polyphenol bionanocomposite films for active food packaging. Int. J. Biol. Macromol. 2021, 186, 405–413. [Google Scholar] [CrossRef]
- Duan, M.; Yu, S.; Sun, J.; Jiang, H.; Zhao, J.; Tong, C.; Hu, Y.; Pang, J.; Wu, C. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int. J. Biol. Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Gniewosz, M.; Kosakowska, O.; Cis, A. Preservation of brussels sprouts by pullulan coating containing oregano essential oil. J. Food Prot. 2016, 79, 493–500. [Google Scholar] [CrossRef]
- Synowiec, A.; Gniewosz, M.; Kraśniewska, K.; Chlebowska-Śmigiel, A.; Przybył, J.L.; Bączek, K.; Węglarz, Z. Effect of meadowsweet flower extract-pullulan coatings on rhizopus rot development and postharvest quality of cold-stored red peppers. Molecules 2014, 19, 12925–12939. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Bankova, V.; de Casto, S.; Marcucci, M. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Żubernik, J.; Witrowa-Rajchert, D.; Gniewosz, M. Growth biocontrol of foodborne pathogens and spoilage microorganisms of food by Polish propolis extracts. Molecules 2019, 24, 2965. [Google Scholar] [CrossRef]
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcaudkaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of beijing propolis extracts. Evid. Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 74, 117–124. [Google Scholar] [CrossRef] [PubMed]
- das Neves, M.V.M.; da Silva, T.M.S.; Lima, E.O.; Cunha, E.V.L.; Oliveira, E.J. Isoflavone formononetin from red propolis acts as a fungicide against Candida sp. Braz. J. Microbiol. 2016, 47, 159–166. [Google Scholar] [CrossRef]
- Freires, I.A.; de Alencar, S.M.; Rosalen, P.L. A pharmacological perspective on the use of Brazilian red propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 2016, 110, 267–279. [Google Scholar] [CrossRef]
- Bankova, V.; Galabov, A.S.; Antonova, D.; Vilhelmova, N.; Di Perri, B. Chemical composition of propolis extract ACF® and activity against herpes simplex virus. Phytomedicine 2014, 21, 1432–1438. [Google Scholar] [CrossRef]
- Nolkemper, S.; Reichling, J.; Sensch, K.H.; Schnitzler, P. Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine 2010, 17, 132–138. [Google Scholar] [CrossRef]
- Diaz, L.G.; Pereira, A.P.; Estevinho, L.M. Comparative study of different Portuguese samples of propolis: Pollinic, sensorial, physicochemical, microbiological characterization and antibacterial activity. Food Chem. Toxicol. 2012, 50, 4246–4253. [Google Scholar]
- Netíková, L.; Petr, B.; Petr, H. Czech ethanol-free propolis extract displays inhibitory activity against a broad spectrum of bacterial and fungal pathogens. J. Food Sci. 2013, 78, M1421–M1429. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef]
- Yang, S.Z.; Peng, L.T.; Su, X.J.; Chen, F.; Cheng, Y.J.; Fan, G.; Pan, S.Y. Bioassay-guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chem. 2011, 127, 210–215. [Google Scholar] [CrossRef]
- Vasilaki, A.; Hatzikamari, M.; Stagkos-Georgiadis, A.; Goula, A.M.; Mourtzinos, I. A natural approach in food preservation: Propolis extract as sorbate alternative in non-carbonated beverage. Food Chem. 2019, 298, 125080. [Google Scholar] [CrossRef]
- Dudoit, A.; Mertz, C.; Chillet, M.; Cardinault, N.; Brat, P. Antifungal activity of Brazilian red propolis extract and isolation of bioactive fractions by thin-layer chromatography-bioautography. Food Chem. 2020, 327, 127060. [Google Scholar] [CrossRef]
- Aldayed, M.F.S.; Hashem, A.; Al-Hazzani, A.A.; Abd-Allah, E.F. Biological control of yeast contamination of industrial foods by propolis. Saudi J. Biol. Sci. 2020, 27, 935–946. [Google Scholar] [CrossRef]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the shelf life of cherry tomatoes by pullulan coating with ethanol extract of propolis during refrigerated storage. Food Bioproc. Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The use of pullulan coatings with propolis extract to extend the shelf life of blueberry (Vaccinium corymbosum) fruit. Int. J. Food Sci. Technol. 2021, 56, 1013–1020. [Google Scholar] [CrossRef]
- Bodoni, R.B.; Sobral, P.J.A.; Favaro-Trindade, C.S.; Carvalho, R.A. Properties of gelatin-based films with added ethanolepropolis extract. LWT-Food Sci. Technol. 2013, 51, 104–110. [Google Scholar] [CrossRef]
- Omar-Aziz, M.; Khodaiyan, F.; Yarmand, M.S.; Mousavi, M.; Gharaghani, M.; Kennedy, J.F.; Hosseini, S.S. Combined effects of octenylsuccination and beeswax on pullulan films: Water-resistant and mechanical properties. Carbohydr. Polym. 2021, 255, 117471. [Google Scholar] [CrossRef]
- Method ASTM D882–02; Standard Test Methods for Tensile Properties of Plastics. American Society for Testing and Materials: Philadelphia, PA, USA, 2002.
- Djenane, D.; Yangüela, J.; Montañés, L.; Mouloud Djerbal, M.; Roncalés, P. Antimicrobial activity of Pistacia lentiscus and Satureja montana essential oils against Listeria monocytogenes CECT 935 using laboratory media: Efficacy and synergistic potential in minced beef. Food Control 2011, 22, 1046–1053. [Google Scholar] [CrossRef]
- Frazão, G.G.S.; Blank, A.F.; de Aquino Santana, L.C.L. Optimisation of edible chitosan coatings formulations incorporating Myrcia ovata Cambessedes essential oil with antimicrobial potential against foodborne bacteria and natural microflora of mangaba fruits. LWT 2017, 79, 1–10. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Gniewosz, M.; Synowiec, A.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Węglarz, Z. The antimicrobial activity of pullulan film incorporated with meadowsweet flower extracts (Filipendulae ulmariae flos) on postharvest quality of apples. Food Control 2014, 37, 351–361. [Google Scholar] [CrossRef]
- Reyes, L.M.; Landgraf, M.; Sobral, P.J.A. Gelatin-based films activated with red propolis ethanolic extract and essential oils. Food Packag. Shelf Life 2021, 27, 100607. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and antifungal properties of hydroxypropylmethylcellulose based films containing propolis as affected by moisture content. Carbohydr. Polym. 2010, 82, 1174–1183. [Google Scholar] [CrossRef]
- Khanzadi, M.; Jafari, S.M.; Mirzaei, H.; Chegini, F.K.; Maghsoudloua, Y.; Dehnad, D. Physical and mechanical properties in biodegradable films of whey protein concentrate–pullulan by application of beeswax. Carbohydr. Polym. 2015, 118, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Perez, O.B.; Ventura-Sobrevilla, J.M.; Torres-León, C.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Aguilar-González, M.A.; Aguilar, C.N. Development and characterization of whey protein films incorporated with tarbush polyphenols and candelilla wax. Food Biosci. 2022, 45, 101505. [Google Scholar] [CrossRef]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; Hadi Nezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef]
- Pérez-Vergara, L.D.; Cifuentes, M.T.; Franco, A.P.; Pérez-Cervera, C.E.; Andrade-Pizarro, R.D. Development and characterization of edible films based on native cassava starch, beeswax, and propolis. NFS J. 2020, 21, 39–49. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; de Lorena Ramos-García, M.; del Carmen Martínez-González, M.; Hernández-Romano, J. Physicochemical characterization and antimicrobial activity of edible propolis-chitosan nanoparticle films. Prog. Org. Coat. 2019, 137, 105326. [Google Scholar] [CrossRef]
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J.R. Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6-331.2K: Fundamental data to design polyphenol extraction processes. Fluid Phase Equilib. 2014, 382, 279–285. [Google Scholar] [CrossRef]
- Chen, C.R.; Lee, Y.N.; Chang, C.M.J.; Lee, M.R.; Wei, I.C. Hot-pressurized fluid extraction of flavonoids and phenolic acids from Brazilian propolis and their cytotoxic assay in vitro. Chin. J. Chem. Eng. 2007, 38, 191–196. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Physical, barrier and antioxidant properties of a novel plasticized edible film from quince seed mucilage. Int. J. Biol. Macromol. 2013, 62, 500–507. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Ihl, M.; Bifani, V.; Silva, A.; Montero, P. Edible films made from tuna-fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugni molinae Turcz). Food Hydrocoll. 2007, 21, 1133–1143. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Rosa, M.D.; Siracusa, V. Characterization of composite edible films based on pectin/alginate/whey protein concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Trajkovska-Petkoska, A.; Khojah, E.; Sami, R.; Al-Mushhin, A.A.M. Chitosan edible films enhanced with pomegranate peel extract: Study on physical, biological, thermal, and barrier properties. Materials 2021, 14, 3305. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- de Rigo, D.; Enescu, C.M.; Houston Durrant, T.; Caudullo, G. Populus nigra in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; SanMiguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 136–137. [Google Scholar]
- Caudullo, G.; de Rigo, D. Populus alba in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; pp. 134–135. [Google Scholar]
- Gucwa, K.; Kusznierewicz, B.; Milewski, S.; Van Dijck, P.; Szweda, P. Antifungal activity and synergism with azoles of Polish propolis. Pathogens 2018, 7, 56. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M.; Alam, R.; Łochyńska, M. Antifungal properties of chemically defined propolis from various geographical regions. Microorganisms 2022, 10, 364. [Google Scholar] [CrossRef]
- Corrêa, J.L.; Veiga, F.F.; Jarros, I.C.; Costa, M.I.; Castilho, P.F.; de Oliveira, K.M.P.; Rosseto, H.C.; Bruschi, M.L.; Svidzinski, T.I.E.; Negri, M. Propolis extract has bioactivity on the wall and cell membrane of Candida albicans. J. Ethnopharmacol. 2020, 256, 112791. [Google Scholar] [CrossRef]
- Stähli, A.; Schröter, H.; Bullitta, S.; Serralutzu, F.; Dore, A.; Nietzsche, S.; Milia, E.; Sculean, A.; Eick, S. In vitro activity of propolis on oral microorganisms and biofilms. Antibiotics 2021, 10, 1045. [Google Scholar] [CrossRef]

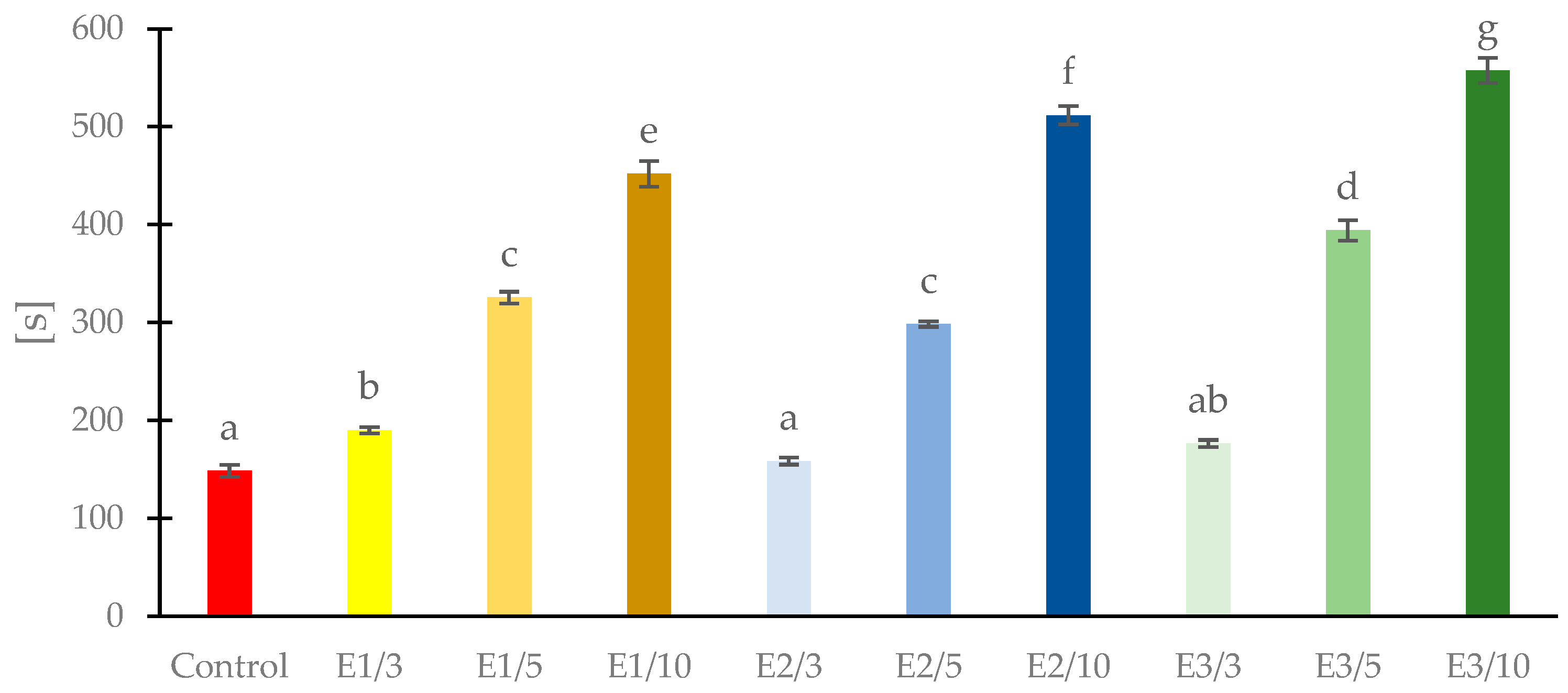
| Film Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Control 1 | 90.98 ± 0.41d | −0.47 ± 0.06 a | 0.87 ± 0.04 a | 1.28 ± 0.39 a |
| E1/3 | 90.52 ± 0.30 cd | −0.94 ± 0.05 bc | 3.17 ± 0.41 bc | 3.11 ± 0.45 bc |
| E1/5 | 89.45 ± 0.55 ab | −1.52 ± 0.11 d | 5.72 ± 0.60 d | 8.12 ± 1.39 e |
| E1/10 | 89.67 ± 0.32 ab | −2.05 ± 0.28 e | 8.75 ± 1.09 e | 9.16 ± 0.46 e |
| E2/3 | 90.95 ± 0.25 d | −0.79 ± 0.04 b | 2.45 ± 0.16 b | 2.26 ± 0.24 ab |
| E2/5 | 90.54 ± 0.26 cd | −1.08 ± 0.06 c | 3.65 ± 0.26 c | 3.53 ± 0.25 c |
| E2/10 | 89.32 ± 0.30 a | −1.73 ± 0.24 de | 7.78 ± 0.76 e | 7.84 ± 0.85 e |
| E3/3 | 91.01 ± 0.15 d | −0.82 ± 0.04 b | 2.41 ± 0.19 b | 2.20 ± 0.22 ab |
| E3/5 | 90.46 ± 0.29 cd | −0.93 ± 0.17 bc | 3.86 ± 0.74 c | 3.73 ± 0.77 c |
| E3/10 | 90.04 ± 0.42 bc | −1.45 ± 0.15 d | 6.43 ± 0.87 d | 6.29 ± 0.95 d |
| Film Sample | Thickness | Moisture Content | Mechanical Parameters | |
|---|---|---|---|---|
| (µm) | (%) | TS (MPa) | EB (%) | |
| Control 1 | 86.0 ± 8.5 a | 10.60 ± 0.37 a | 24.62 ± 2.12 f | 21.00± 0.92 c |
| E1/3 | 173.7 ± 4.6 bcde | 9.98 ± 0.38 a | 14.42 ± 1.99 cde | 15.92 ± 1.51 b |
| E1/5 | 183.3 ± 25.4 bcde | 10.68 ± 0.46 a | 13.13 ± 2.44 bcd | 12.00 ± 2.78 ab |
| E1/10 | 248.8 ± 19.8 e | 9.63 ± 0.58 a | 8.52 ± 1.36 ab | 12.15 ±1.45 ab |
| E2/3 | 115.1 ± 23.9 abc | 10.62 ± 0.32 a | 19.4 ± 1.94 e | 14.56 ± 1.15 ab |
| E2/5 | 134.7 ± 12.8 abc | 9.44 ± 0.12 a | 15.46 ± 1.60 cde | 14.86 ± 1.10 ab |
| E2/10 | 214.08 ± 11.9 de | 9.86 ± 0.07 a | 12.98 ± 0.61 bcd | 11.57 ± 1.08 a |
| E3/3 | 102.0 ± 18.2 ab | 10.60 ± 0.33 a | 16.81 ± 1.71 de | 16.80 ± 3.24 bc |
| E3/5 | 158.0 ± 14.7 abcd | 9.55 ± 0.37 a | 10.91± 1.54 abc | 15.57 ± 2.53 ab |
| E3/10 | 189.3 ± 16.0 cde | 9.91 ± 0.54 a | 7.66 ± 1.54 a | 10.72 ± 1.62 a |
| EP1/3 | EP1/5 | EP1/10 | EP2/3 | EP2/5 | EP2/10 | EP3/3 | EP3/5 | EP3/10 | |
|---|---|---|---|---|---|---|---|---|---|
| (mm ± SD) | |||||||||
| Yeast | |||||||||
| R. mucilaginosa | 8.1 ± 0.6 bcAB | 8.2 ± 0.4 bcB | 8.6 ± 0.5 aB | 7.3 ± 0.8 cA | 8.2 ± 0.5 bcB | 8.5 ± 0.6 aB | 9.6 ± 0.7 efC | 9.9 ± 0.9 dC | 10.1 ± 0.8 C |
| C. albicans | 7.8 ± 0.6 abBC | 8.5 ± 0.7 bcCDE | 10.2 ± 0.7 bF | 6.2 ± 0.5 aA | 7.6 ± 0.7 abB | 8.3 ± 0.7 aBCDE | 8.2 ± 0.5 bcBCD | 8.6 ± 0.5 bcDE | 9.1 ± 0.3 abE |
| C. krusei | 7.7 ± 0.6 abA | 7.9 ± 0.7 bAB | 8.4 ± 0.7 aB | 7.6 ± 0.5 abA | 7.9 ± 0.7 bcAB | 8.1 ± 0.7 aB | 7.6 ± 0.4 abcA | 7.6 ± 0.5 abA | 9.4 ± 1.0 abC |
| S. cerevisiae | 7.4 ± 0.6 abAB | 8.1 ± 0.6 bBC | 8.7 ± 0.7 aC | 7.2 ± 0.6 bcA | 8.3 ± 0.5 bcC | 9.0 ± 0.5 aC | 8.5 ± 0.7 cdC | 8.7 ± 0.7 bcC | 8.8 ± 0.5 aC |
| Mold | |||||||||
| C. gloeosporioides | 9.0 ± 0.5 cBC | 9.5 ± 0.4 cdC | 10.8 ± 0.6 bcD | 8.5 ± 0.9 deB | 8.7 ± 0.5 cBC | 10.5 ± 1.1 bcD | 6.7 ± 0.2 abA | 7.6 ± 0.6 aA | 11.0 ± 0.7 deD |
| A. solani | 6.1 ± 0.1 aA | 6.3 ± 0.1 aA | 9.5 ± 0.1 abC | 6.1 ± 0.0 abcA | 6.4 ± 0.1 aA | 9.2 ± 0.1 abcBC | 6.2 ± 0.1 aA | 8.2 ± 0.8 abcB | 9.5 ± 0.6 abcC |
| F. solani | 13.1 ± 1.0 gB | 14.7 ± 1.0 hC | 15.5 ± 1.2 eC | 12.8 ± 0.7 gB | 13.0 ± 0.6 ghB | 14.7 ± 1.0 eC | 11.1 ± 0.6 gA | 12.2 ± 1.2 fgB | 13.1 ± 1.2 fgB |
| R. stolonifer | 12.3 ± 1.1 fgABC | 12.5 ± 0.5 fgABC | 13.4 ± 0.9 dBC | 12.1 ± 1.2 gBC | 12.5 ± 0.7 fgABC | 13.3 ± 1.2 dBC | 11.7 ± 0.8 gA | 12.8 ± 0.5 gABC | 13.5 ± 1.4 gC |
| C. cladosporioides | 11.2 ± 0.7 defDE | 11.5 ± 0.4 efE | 11.8 ± 0.3 bcdE | 9.2 ± 0.5 deAB | 9.7 ± 0.7 deBC | 11.1 ± 1.0 cDE | 8.6 ± 0.8c dA | 10.4 ± 0.3 deCD | 10.9 ± 0.6 cdeDE |
| A. niger | 10.3 ± 1.1 dCD | 10.5 ± 0.8 deD | 10.9 ± 0.6 bcD | 8.6 ± 0.3 deA | 8.8 ± 0.4 cdAB | 9.0 ± 0.4 aAB | 9.2 ± 0.5 deAB | 9.4 ± 0.5 bcAB | 9.6 ± 0.3 abcdBC |
| A. ochraceus | 9.2 ± 0.6 cC | 9.7 ± 0.7 dCD | 11.4 ± 1.1 bcE | 7.0 ± 0.5 abcA | 8.5 ± 0.3 bcBC | 9.3 ± 0.6 abBCD | 8.0 ± 0.8 bcB | 9.3 ± 0.9 bcCD | 10.2 ± 1.1 bcdeD |
| M. mucedo | 11.9 ± 1.1 efBC | 13.4 ± 1.4 gC | 15.3 ± 1.6 eDE | 9.4 ± 0.9 eA | 13.8 ± 1.4 hCD | 16.5 ± 1.5 fE | 11.1 ± 0.9 gB | 12.1 ± 1.2 fgBC | 13.4 ± 1.3 gC |
| P. expansum | 11.0 ± 0.8 deCD | 11.4 ± 0.5 efCDE | 11.8 ± 0.5 cE | 8.4 ± 0.8 dA | 10.1 ± 0.5 eB | 10.7 ± 0.4 bcBC | 10.0 ± 0.6 fB | 11.5 ± 0.6 efCDE | 11.7 ± 0.2 efDE |
| P. chrysogenum | 12.2 ± 1.1 efgCD | 13.0 ± 1.1 gCDE | 15.3 ± 1.2 eF | 10.4 ± 0.9 fAB | 11.6 ± 0.9 fBC | 13.1 ± 1.4 dDE | 10.0 ± 1.0 efA | 11.8 ± 1.0 fgC | 13.9 ± 1.0 gE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gniewosz, M.; Pobiega, K.; Kraśniewska, K.; Synowiec, A.; Chaberek, M.; Galus, S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods 2022, 11, 2319. https://doi.org/10.3390/foods11152319
Gniewosz M, Pobiega K, Kraśniewska K, Synowiec A, Chaberek M, Galus S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods. 2022; 11(15):2319. https://doi.org/10.3390/foods11152319
Chicago/Turabian StyleGniewosz, Małgorzata, Katarzyna Pobiega, Karolina Kraśniewska, Alicja Synowiec, Marta Chaberek, and Sabina Galus. 2022. "Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging" Foods 11, no. 15: 2319. https://doi.org/10.3390/foods11152319
APA StyleGniewosz, M., Pobiega, K., Kraśniewska, K., Synowiec, A., Chaberek, M., & Galus, S. (2022). Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods, 11(15), 2319. https://doi.org/10.3390/foods11152319









