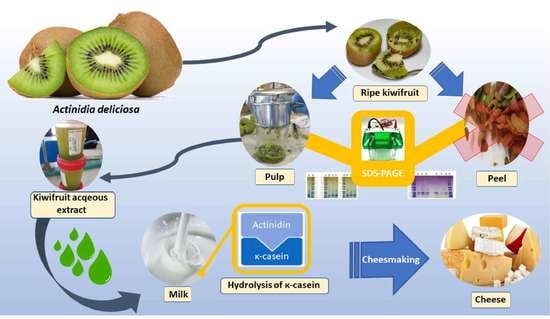
An Easy and Cheap Kiwi-Based Preparation as Vegetable Milk Coagulant: Preliminary Study at the Laboratory Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Kiwifruit Aqueous Extracts Preparation
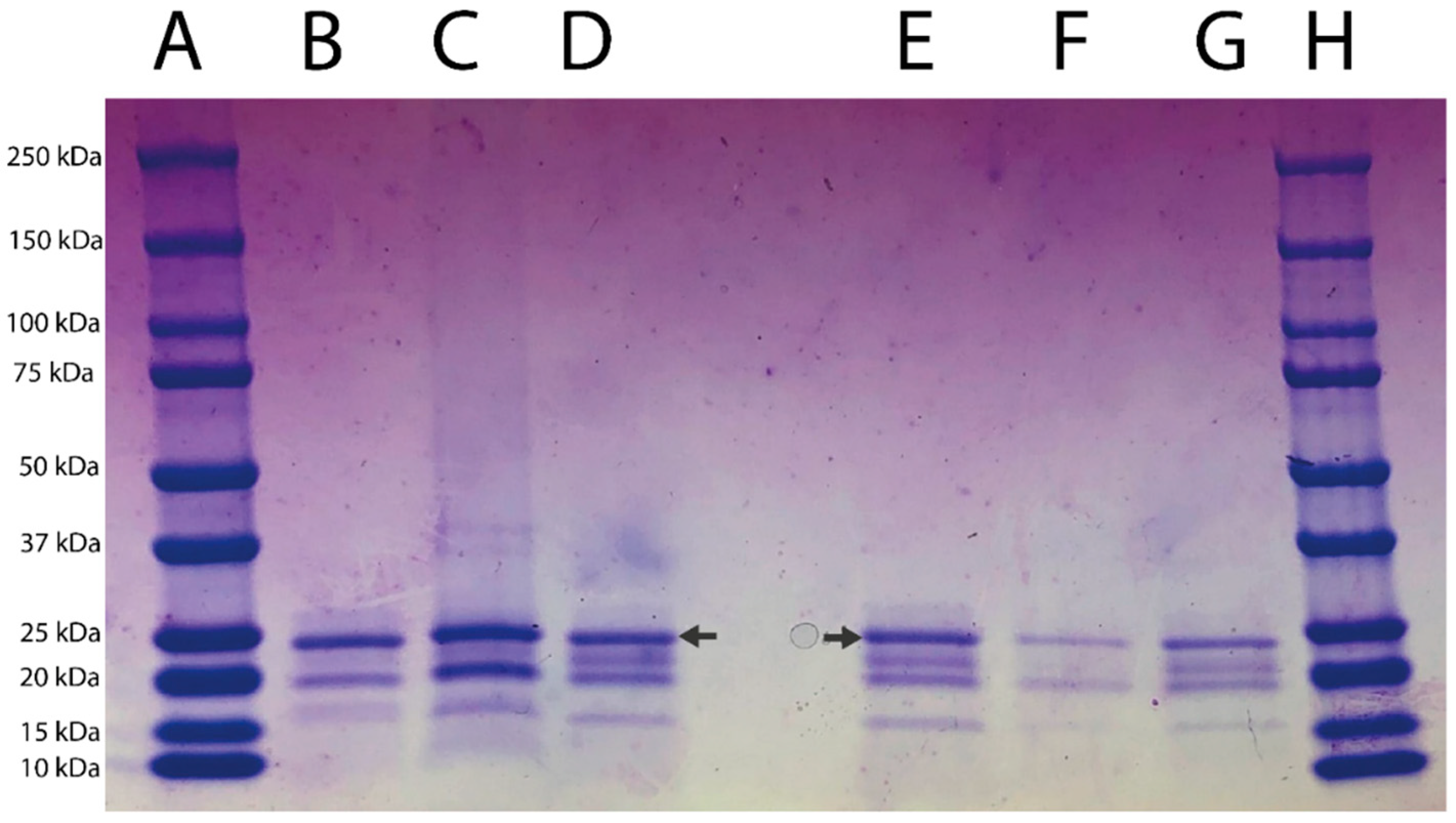
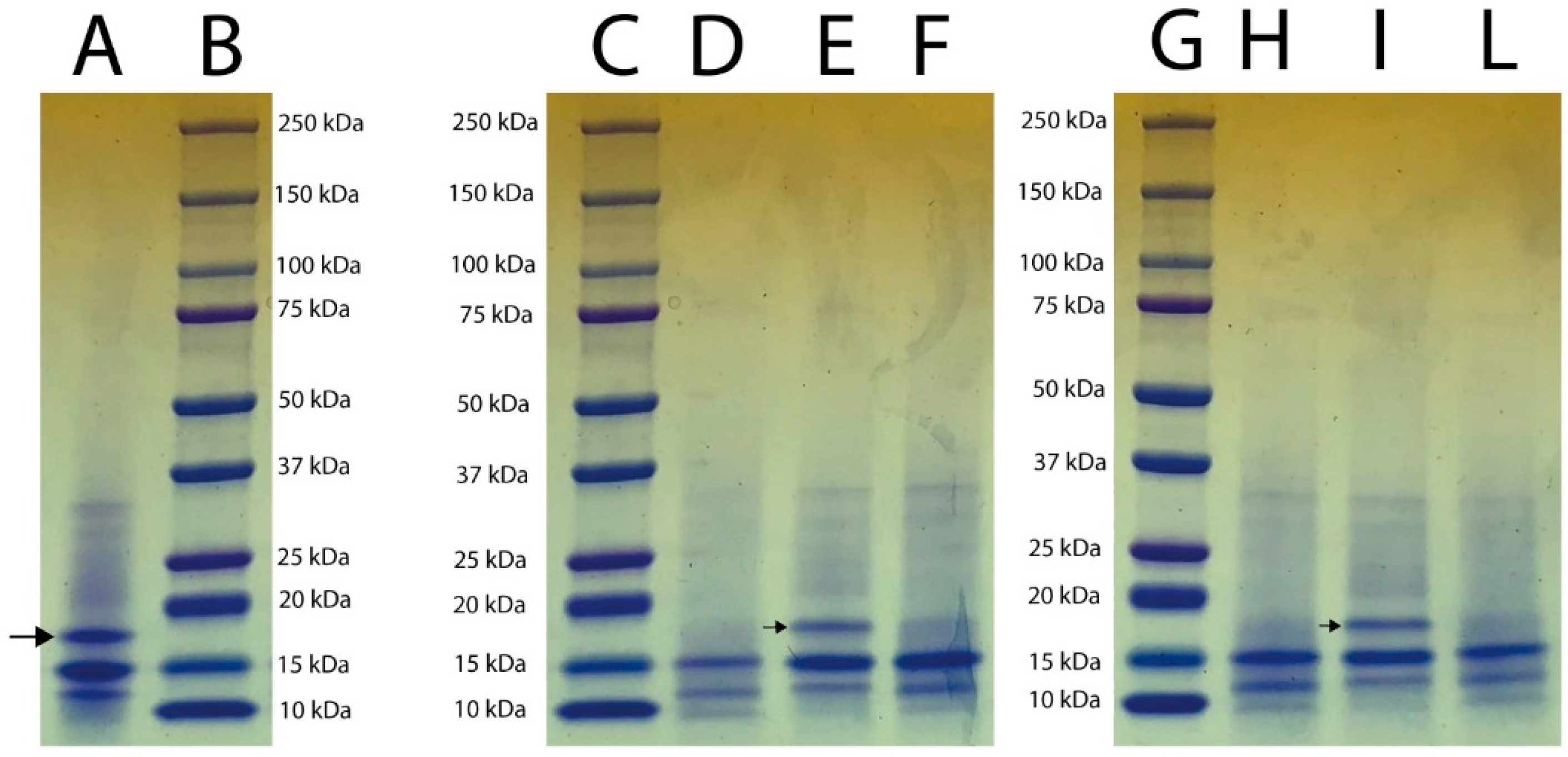
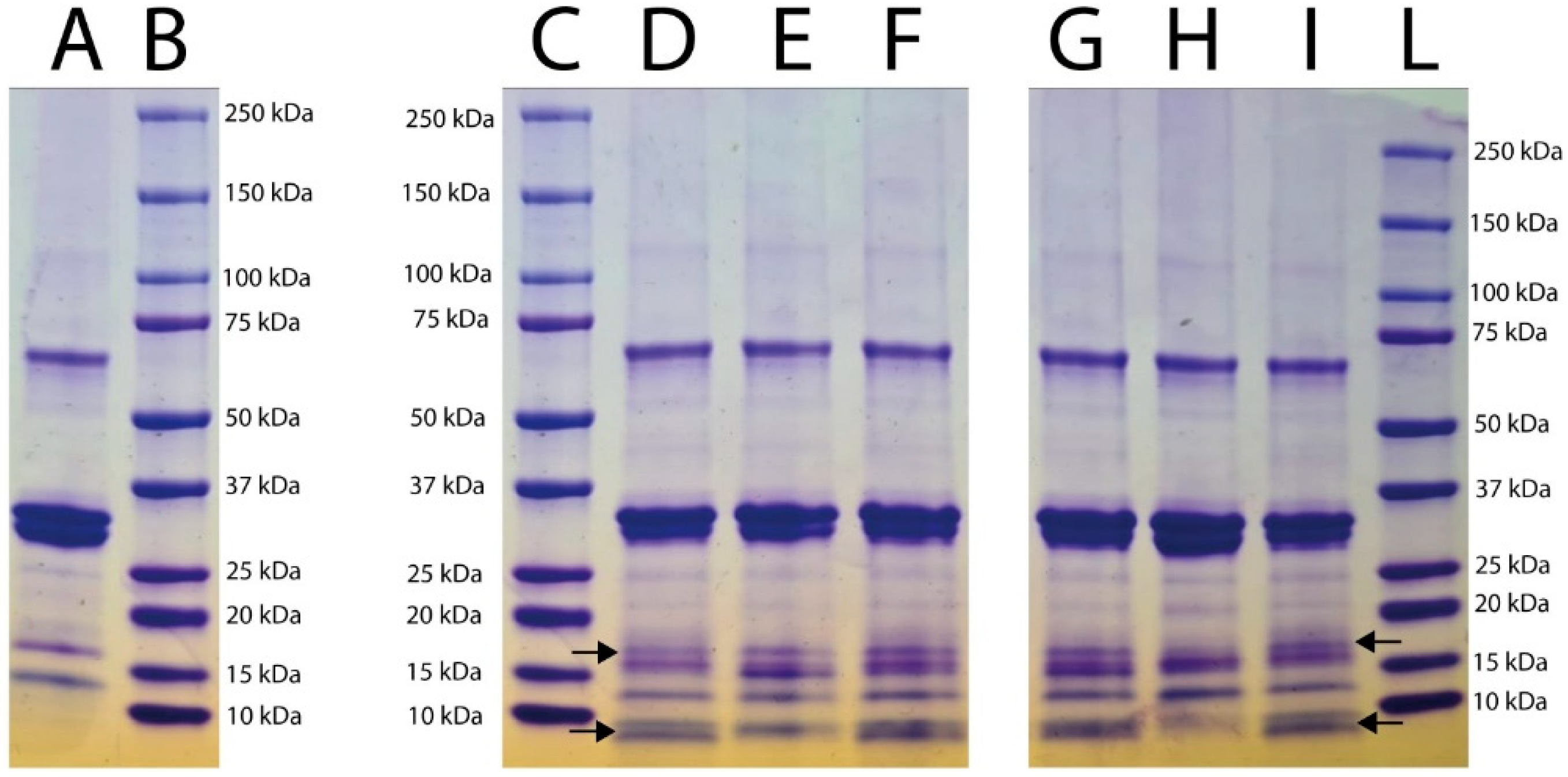
2.3. Electrophoretic Analyses
2.4. Milk-Clotting Activity Determination
2.5. Proteolytic Activity Determination
2.6. Cheesemaking at the Laboratory Scale
2.7. Statistical Analysis
3. Results
3.1. Kiwifruit Aqueous Extracts Preparation
3.2. SDS-PAGE Electrophoretic Profile
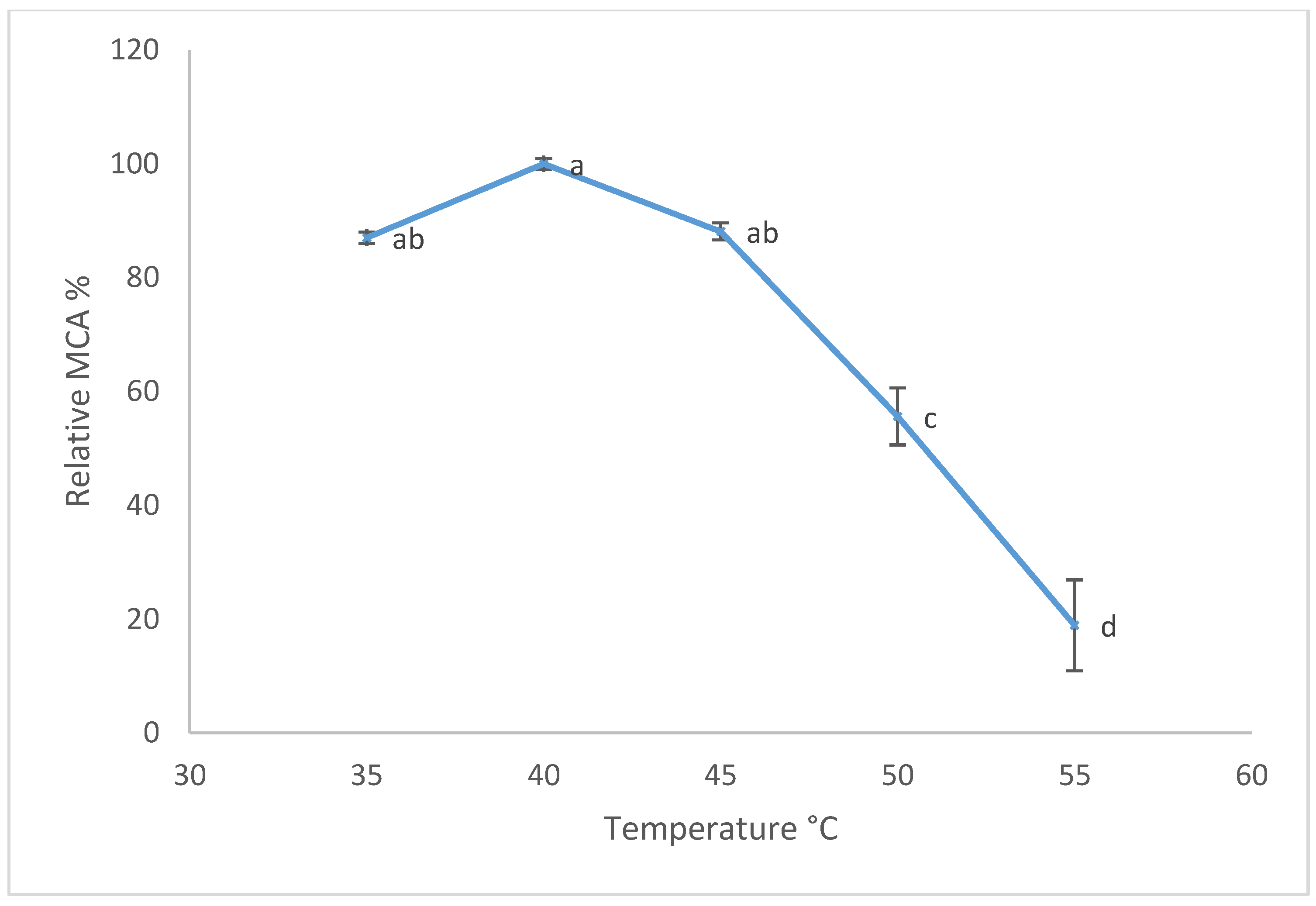
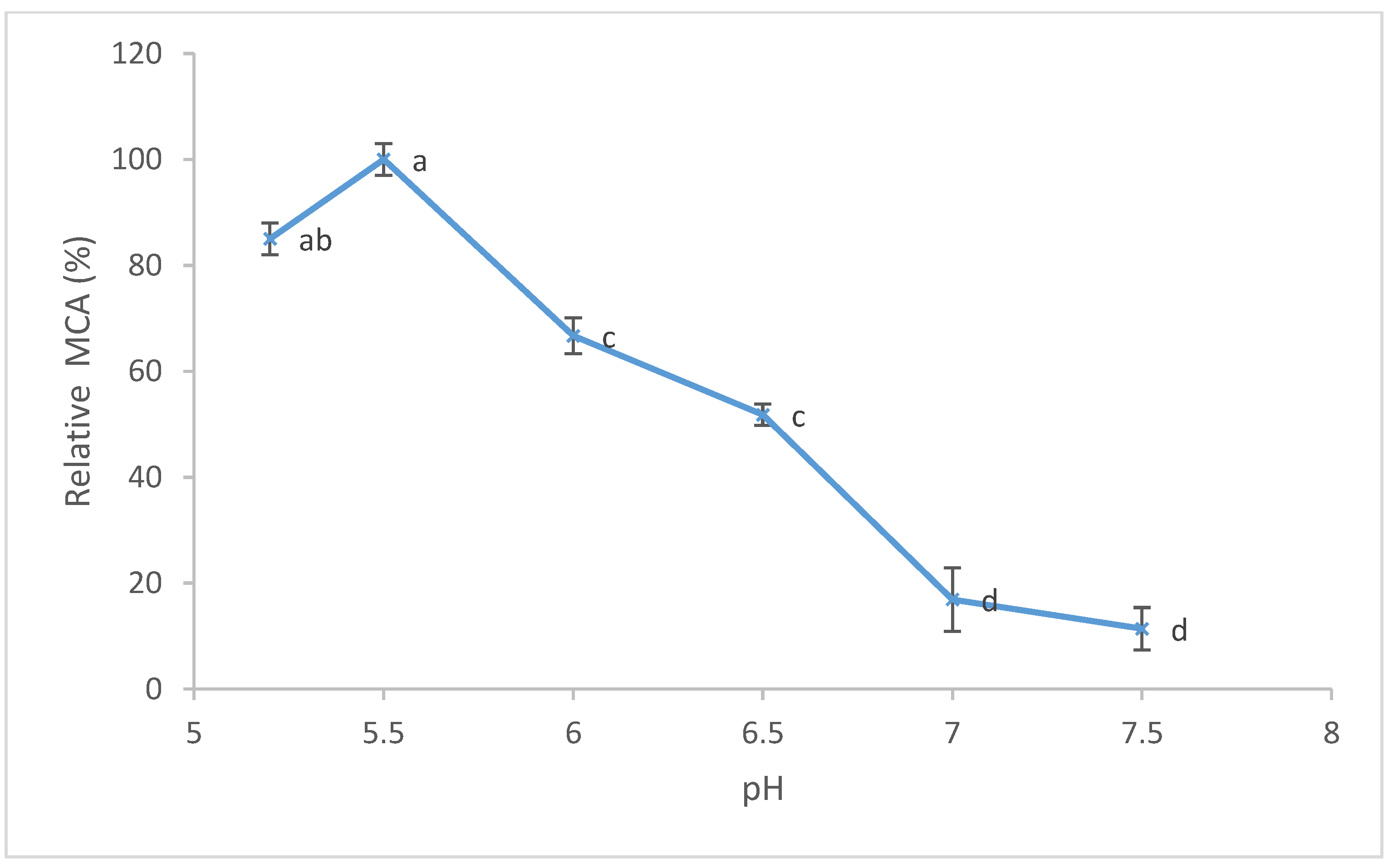
3.3. Temperature and pH Effects on Milk-Clotting Activity (MCA)
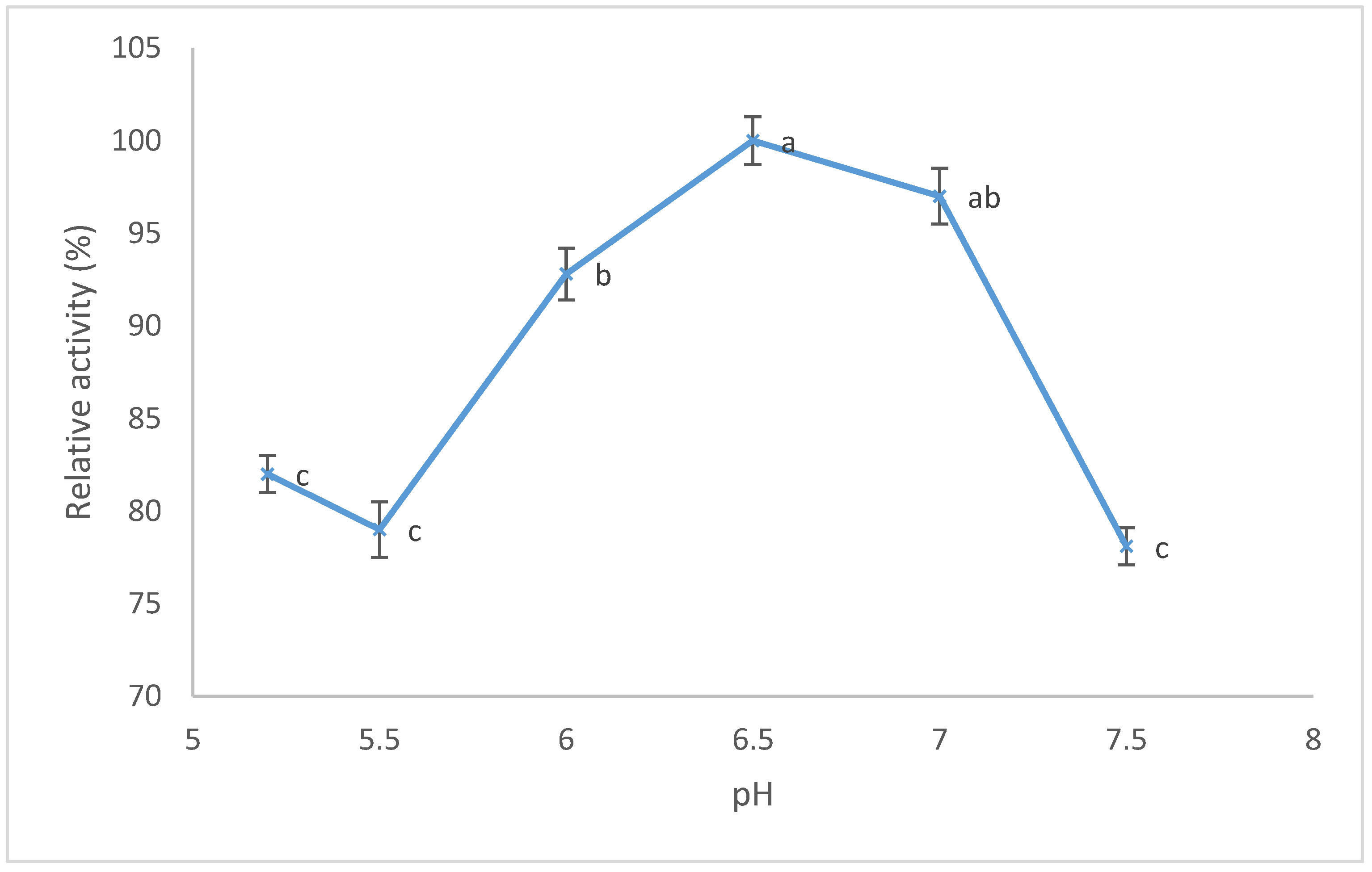
3.4. Effects of Different pHs on Proteolytic Activity (PA) and MCA/PA Ratio
3.5. Cheesemaking Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Grover, S.; Sharma, J.; Batish, V.K. Chymosin and Other Milk Coagulants: Sources and Biotechnological Interventions. Crit. Rev. Biotechnol. 2010, 30, 243–258. [Google Scholar] [CrossRef]
- García-Gómez, B.; Vázquez-Odériz, L.; Muñoz-Ferreiro, N.; Romero-Rodríguez, Á.; Vázquez, M. Rennet Type and Microbial Transglutaminase in Cheese: Effect on Sensory Properties. Eur. Food Res. Technol. 2020, 246, 513–526. [Google Scholar] [CrossRef]
- Colombo, M.L.; Cimino, C.V.; Bruno, M.A.; Hugo, A.; Liggieri, C.; Fernández, A.; Vairo-Cavalli, S. Artichoke cv. Francés flower extract as a rennet substitute: Effect on textural, microstructural, microbiological, antioxidant properties, and sensory acceptance of miniature cheeses. J. Sci. Food Agric. 2021, 101, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Mamo, J.; Assefa, F. The Role of Microbial Aspartic Protease Enzyme in Food and Beverage Industries. J. Food Qual. 2018, 2018, 7957269. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, Y.; Guan, R.; Jia, G.; Ma, Y.; Zhang, Y. Advances in Research on Calf Rennet Substitutes and Their Effects on Cheese Quality. Food Res. Int. 2021, 149, 110704. [Google Scholar] [CrossRef] [PubMed]
- Dekker, P. Dairy Enzymes. In Industrial Enzyme Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 143–166. [Google Scholar]
- Nicosia, F.D.; Puglisi, I.; Pino, A.; Caggia, C.; Randazzo, C.L. Plant Milk-Clotting Enzymes for Cheesemaking. Foods 2022, 11, 871. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Barbosa, M.; Ames, J.M.; Wilbey, R.A. Cheesemaking with Vegetable Coagulants-the Use of Cynara L. for the Production of Ovine Milk Cheeses. Int. J. Dairy Technol. 2003, 56, 76–85. [Google Scholar] [CrossRef]
- Zikiou, A.; Esteves, A.C.; Esteves, E.; Rosa, N.; Gomes, S.; Louro Martins, A.P.; Zidoune, M.N.; Barros, M. Algerian Cardoon Flowers Express a Large Spectrum of Coagulant Enzymes with Potential Applications in Cheesemaking. Int. Dairy J. 2020, 105, 104689. [Google Scholar] [CrossRef]
- Bueno-Gavilá, E.; Abellán, A.; Bermejo, M.S.; Salazar, E.; Cayuela, J.M.; Prieto-Merino, D.; Tejada, L. Characterization of Proteolytic Activity of Artichoke (Cynara scolymus L.) Flower Extracts on Bovine Casein to Obtain Bioactive Peptides. Animals 2020, 10, 914. [Google Scholar] [CrossRef]
- Hashim, M.M.; Mingsheng, D.; Iqbal, M.F.; Xiaohong, C. Ginger Rhizome as a Potential Source of Milk Coagulating Cysteine Protease. Phytochemistry 2011, 72, 458–464. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, C.; Shi, Y.; Wei, G.; Yang, K.; Wang, X.; Huang, A. Proteomics Analysis of the Bio-Functions of Dregea Sinensis Stems Provides Insights Regarding Milk-Clotting Enzyme. Food Res. Int. 2021, 144, 110340. [Google Scholar] [CrossRef] [PubMed]
- Mazorra-Manzano, M.A.; Moreno-Hernández, J.M.; Ramírez-Suarez, J.C.; de Jesús Torres-Llanez, M.; González-Córdova, A.F.; Vallejo-Córdoba, B. Sour Orange Citrus aurantium L. Flowers: A New Vegetable Source of Milk-Clotting Proteases. LWT—Food Sci. Technol. 2013, 54, 325–330. [Google Scholar] [CrossRef]
- Puglisi, I.; Petrone, G.; Lo Piero, A.R. A Kiwi Juice Aqueous Solution as Coagulant of Bovine Milk and Its Potential in Mozzarella Cheese Manufacture. Food Bioprod. Process. 2014, 92, 67–72. [Google Scholar] [CrossRef]
- Carne, A.; Moore, C.H. The Amino Acid Sequence of the Tryptic Peptides from Actinidin, a Proteolytic Enzyme from the Fruit of Actinidia chinensis. Biochem. J. 1978, 173, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Lo Piero, A.R.; Puglisi, I.; Petrone, G. Characterization of the Purified Actinidin as a Plant Coagulant of Bovine Milk. Eur. Food Res. Technol. 2011, 233, 517–524. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Perea-Gutiérrez, T.C.; Lugo-Sánchez, M.E.; Ramirez-Suarez, J.C.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Cordoba, B. Comparison of the Milk-Clotting Properties of Three Plant Extracts. Food Chem. 2013, 141, 1902–1907. [Google Scholar] [CrossRef]
- Serra, A.; Conte, G.; Corrales-Retana, L.; Casarosa, L.; Ciucci, F.; Mele, M. Nutraceutical and Technological Properties of Buffalo and Sheep Cheese Produced by the Addition of Kiwi Juice as a Coagulant. Foods 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Arima, K.; Yu, J.; Iwasaki, S. Milk-clotting enzyme from Mucor pusillus var. Lindt. In Methods in Enzymology; Perlmann, G., Lorand, L., Eds.; Academic Press: New York, NY, USA, 1970; Volume 19, pp. 446–459. [Google Scholar] [CrossRef]
- Kunitz, M. Crystalline Soybean Trypsin Inhibitor. J. Gen. Physiol. 1947, 30, 291–310. [Google Scholar] [CrossRef]
- Cologna, N.; Dal Zotto, R.; Penasa, M.; Gallo, L.; Bittante, G. A Laboratory Micro-Manufacturing Method for Assessing Individual Cheese Yield. Ital. J. Animal Sci. 2009, 8, 393–395. [Google Scholar] [CrossRef]
- Grozdanovic, M.M.; Burazer, L.; Gavrovic-Jankulovic, M. Kiwifruit (Actinidia deliciosa) Extract Shows Potential as a Low-Cost and Efficient Milk-Clotting Agent. Int. Dairy J. 2013, 32, 46–52. [Google Scholar] [CrossRef]
- Raquib, M.; Borpuzar, T.; Hazarika, M.; Kumar Laskar, S.; Saikia, G.K.; Ahmed Hazarika, R. Effect of Coagulating Enzymes and Types of Milk on the Physico-Chemical, Proximate Composition and Sensory Attributes of Iron Fortified Mozzarella Cheese. Emir. J. Food Agric. 2022, 34, 180–187. [Google Scholar] [CrossRef]
- Nishiyama, I.; Oota, T. Varietal Difference in Actinidin Concentration and Protease Activity in the Kiwifruit Juice. J. Jpn. Soc. Food Sci. Technol. 2002, 49, 401–408. [Google Scholar] [CrossRef]
- Salzano, A.M.; Renzone, G.; Sobolev, A.P.; Carbone, V.; Petriccione, M.; Capitani, D.; Vitale, M.; Novi, G.; Zambrano, N.; Pasquariello, M.S.; et al. Unveiling Kiwifruit Metabolite and Protein Changes in the Course of Postharvest Cold Storage. Front. Plant Sci. 2019, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Farias, V.A.; da Rocha Lima, A.D.; Santos Costa, A.; de Freitas, C.D.T.; da Silva Araújo, I.M.; dos Santos Garruti, D.; de Figueiredo, E.A.T.; de Oliveira, H.D. Noni (Morinda citrifolia L.) Fruit as a New Source of Milk-Clotting Cysteine Proteases. Food Res. Int. 2020, 127, 108689. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Méndez, N.; Balderrama-Carmona, A.; García-Sandoval, S.; Ramírez-Vigil, P.; Leal-Ramos, M.; García-Triana, A. Proteolysis and Rheological Properties of Cream Cheese Made with a Plant-Derived Coagulant from Solanum elaeagnifolium. Foods 2019, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, A.; Ojha, P. Quality Evaluation of Kiwi Juice Coagulated Mozzarella Cheese. J. Food Sci. Technol. Nepal 2018, 10, 7–10. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.J.; Beuning, L.L.; Sutherland, P.W.; Sharma, N.N.; Cooney, J.M.; Bieleski, L.R.F.; Schröder, R.; MacRae, E.A.; Atkinson, R.G. Identification and Characterisation of Acidic and Novel Basic Forms of Actinidin, the Highly Abundant Cysteine Protease from Kiwifruit. Funct. Plant Biol. 2007, 34, 946. [Google Scholar] [CrossRef]
- Lewis, D.A.; Luh, B.S. Application OF Actinidin From Kiwifruit to Meat Tenderization and Characterization of Beef Muscle Protein Hydrolysis. J. Food Biochem. 1988, 12, 147–158. [Google Scholar] [CrossRef]
- Chalabi, M.; Khademi, F.; Yarani, R.; Mostafaie, A. Proteolytic Activities of Kiwifruit Actinidin (Actinidia deliciosa cv. Hayward) on Different Fibrous and Globular Proteins: A Comparative Study of Actinidin with Papain. Appl. Biochem. Biotechnol. 2014, 172, 4025–4037. [Google Scholar] [CrossRef]
- Mahdian Dehkordi, A.; Rezazadeh Bari, M.; Babaie, G.; Amiri, S. Application of Actinidin as Coagulants to Produce Iranian White Brined Cheese: Investigating the Technological, Textural, and Sensorial Properties. J. Food Meas. Charact. 2022, 16, 957–963. [Google Scholar] [CrossRef]
- Katsaros, G.I.; Tavantzis, G.; Taoukis, P.S. Production of Novel Dairy Products Using Actinidin and High Pressure as Enzyme Activity Regulator. Innov. Food Sci. Emerg. Technol. 2010, 11, 47–51. [Google Scholar] [CrossRef]
- Amira, A.; Besbes, S.; Attia, H.; Blecker, C. Milk-Clotting Properties of Plant Rennets and Their Enzymatic, Rheological, and Sensory Role in Cheese Making: A Review. Int. J. Food Prop. 2017, 20, S76–S93. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.; Jaros, D.; Rohm, H. Recent Advances in Milk Clotting Enzymes. Int. J. Dairy Technol. 2011, 64, 14–33. [Google Scholar] [CrossRef]
- Ojha, P.; Dhakal, S.; Subedi, U. Comparative Quality Evaluation of Goat Cheese Made from Kiwi Juice and Rennet. Natl. Workshop Livest. Fish. Res. Nepal 2021, 3, 183. [Google Scholar]
- Mahajan, R.T.; Chaudhari, G.M. Plant latex as vegetable source for milk clotting enzymes and their use in cheese preparation. Int. J. Adv. Res. 2014, 2, 1173–1181. [Google Scholar]
- Nasr, A.I.A.M.; Mohamed Ahmed, I.A.; Hamid, O.I.A. Characterization of Partially Purified Milk-clotting Enzyme from Sunflower (Helianthus annuus) Seeds. Food Sci. Nutr. 2016, 4, 733–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez-Garay, D.R.; Gutiérrez-Méndez, N.; Valenzuela-Soto, M.E.; García-Triana, A. Partial Characterization of a Plant Coagulant Obtained from the Berries of Solanum elaeagnifolium. CyTA J. Food. 2016, 14, 200–205. [Google Scholar] [CrossRef]
| Tissue | Ripe | Unripe | ||
|---|---|---|---|---|
| Extract (mL/kg) | DM (%) | Extract (mL/kg) | DM (%) | |
| Pulp | 457 ± 2.5 | 17.5 ± 0.3 | 364 ± 3.1 | 14.5 ± 0.6 |
| Peel | 15 ± 2.1 | 12.4 ± 1.1 | 11 ± 1.8 | 10.8 ± 0.8 |
| Whole fruits | 469 ± 1.5 | 16.8 ± 0.4 | 371 ± 1.1 | 15.7 ± 0.9 |
| pH | ||||||
|---|---|---|---|---|---|---|
| 5.2 | 5.5 | 6.0 | 6.5 | 7.0 | 7.5 | |
| MCA/PA | 7.43 ± 0.4 b | 9.1 ± 0.5 a | 5.1 ± 0.5 c | 3.7 ± 0.9 c | 1.3 ± 0.8 d | 1.1 ± 0.5 d |
| Ripe Aqueous Extract | Chymosin | Microbial | ||||
|---|---|---|---|---|---|---|
| Inoculum (v/v) | 4% | 3% | 2% | 1.6% | 0.04% | 0.1% |
| Yield (%) | 20.03 ± 1.84 a | 20.27 ± 1.16 a | 11.43 ± 1.96 b | 10.90 ± 0.79 b | 20.93 ± 0.90 a | 20.06 ± 0.70 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicosia, F.D.; Puglisi, I.; Pino, A.; Baglieri, A.; La Cava, R.; Caggia, C.; Fernandes de Carvalho, A.; Randazzo, C.L. An Easy and Cheap Kiwi-Based Preparation as Vegetable Milk Coagulant: Preliminary Study at the Laboratory Scale. Foods 2022, 11, 2255. https://doi.org/10.3390/foods11152255
Nicosia FD, Puglisi I, Pino A, Baglieri A, La Cava R, Caggia C, Fernandes de Carvalho A, Randazzo CL. An Easy and Cheap Kiwi-Based Preparation as Vegetable Milk Coagulant: Preliminary Study at the Laboratory Scale. Foods. 2022; 11(15):2255. https://doi.org/10.3390/foods11152255
Chicago/Turabian StyleNicosia, Fabrizio Domenico, Ivana Puglisi, Alessandra Pino, Andrea Baglieri, Rosita La Cava, Cinzia Caggia, Antonio Fernandes de Carvalho, and Cinzia Lucia Randazzo. 2022. "An Easy and Cheap Kiwi-Based Preparation as Vegetable Milk Coagulant: Preliminary Study at the Laboratory Scale" Foods 11, no. 15: 2255. https://doi.org/10.3390/foods11152255
APA StyleNicosia, F. D., Puglisi, I., Pino, A., Baglieri, A., La Cava, R., Caggia, C., Fernandes de Carvalho, A., & Randazzo, C. L. (2022). An Easy and Cheap Kiwi-Based Preparation as Vegetable Milk Coagulant: Preliminary Study at the Laboratory Scale. Foods, 11(15), 2255. https://doi.org/10.3390/foods11152255