Trends in the Exposure, Distribution, and Health Risk Assessment of Perchlorate among Crayfish in the Middle and Lower Reaches of the Yangtze River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
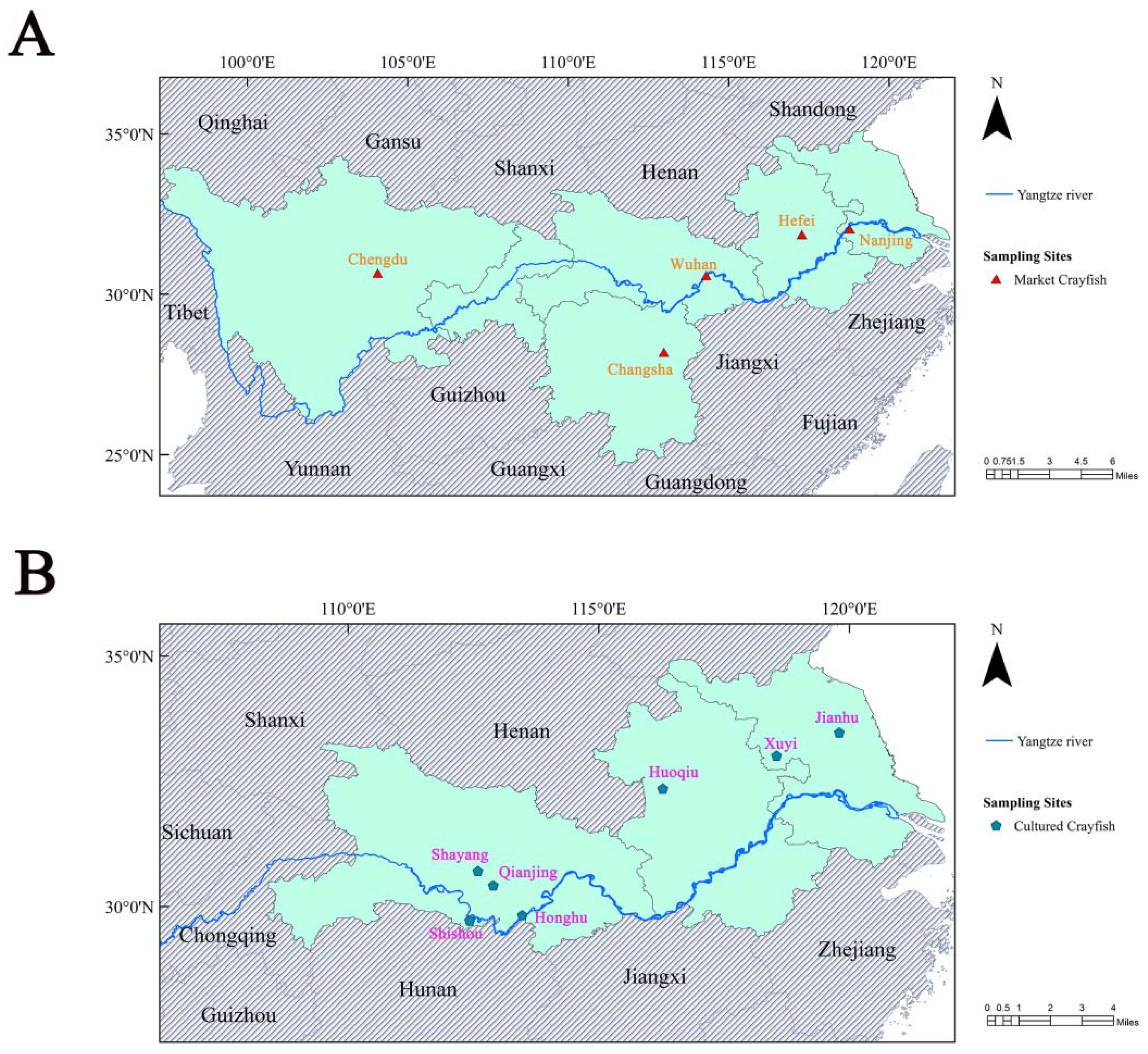
2.2. Sample Collection
2.3. Sample Extraction and Purification
2.4. UPLC–MS Analysis
2.5. Probabilistic Exposure and Risk Assessment
2.6. Quality Assurance and Quality Control
2.7. Statistical Analysis
3. Results and Discussion
3.1. Detection Frequencies and Concentrations of Perchlorate among Crayfish
3.2. Investigation of Perchlorate Concentration in Crayfish Tissues
3.3. Risk Characterization of Daily Exposure to Perchlorate via Crayfish
3.4. Probabilistic Assessment of Dietary Perchlorate Exposure from Crayfish
3.5. Sensitivity Analysis of Perchlorate Exposure from Crayfish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calderon, R.; Rajendiran, K.; U.J., K.; Palma, P.; Arancibia-Miranda, N.; Silva-Moreno, E.; Corradini, F. Sources and fates of perchlorate in soils in Chile: A case study of perchlorate dynamics in soil-crop systems using lettuce (Lactuca sativa) fields. Environ. Pollut. 2020, 264, 114682. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, H.; Zhou, L.; Luo, F.; Zhang, X.; Chen, Z. Quantitative determination and contamination pattern of perchlorate in tea by ultra performance liquid chromatography and tandem mass spectrometry. Food Chem. 2018, 274, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, J.; Chen, M.; Tian, Y.; Liu, X.; Liu, L.; Wu, Y.; Gong, Z. Dietary exposure and risk assessment of perchlorate in diverse food from Wuhan, China. Food Chem. 2021, 358, 129881. [Google Scholar] [CrossRef] [PubMed]
- Kumarathilaka, P.; Oze, C.; Indraratne, S.P.; Vithanage, M. Perchlorate as an emerging contaminant in soil, water and food. Chemosphere 2016, 150, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, H.; Liu, S.; Fang, M.; Wu, Y.; Gong, Z. Study on the bioaccessibility and bioavailability of perchlorate in different food matrices in vitro. Food Chem. 2020, 333, 127470. [Google Scholar] [CrossRef]
- Chang, W.H.; Chen, H.L.; Lee, C.C. Dietary exposure assessment to perchlorate in the Taiwanese population: A risk assessment based on the probabilistic approach. Environ. Pollut. 2020, 267, 115486. [Google Scholar] [CrossRef]
- Wolff, J. Perchlorate and the thyroid gland. Pharmacol. Rev. 1998, 50, 89–105. [Google Scholar]
- Wang, Q.; Song, W.; Tian, Y.; Hu, P.; Liu, X.; Xu, L.; Gong, Z. Targeted Lipidomics Reveal the Effect of Perchlorate on Lipid Profiles in Liver of High-Fat Diet Mice. Front. Nutr. 2022, 9, 837601. [Google Scholar] [CrossRef]
- Panseri, S.; Nobile, M.; Arioli, F.; Biolatti, C.; Pavlovic, R.; Chiesa, L.M. Occurrence of perchlorate, chlorate and polar herbicides in different baby food commodities. Food Chem. 2020, 330, 127205. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ren, J.; Zhang, Y.; Li, L.; Zhao, Y.; Chen, D.; Wu, Y. A highly-efficient and cost-effective pretreatment method for selective extraction and detection of perchlorate in tea and dairy products. Food Chem. 2020, 328, 127113. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Pi, L.; Li, Y.; Hu, W.; Su, S.; Qin, X.; Ding, S.; Sun, H. Occurrence and exposure evaluation of perchlorate in indoor dust and diverse food from Chengdu, China. Sci. Total Environ. 2015, 536, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Ji-Woo, L.; Sung-Hee, O.; Jeong-Eun, O. Monitoring of perchlorate in diverse foods and its estimated dietary exposure for Korea populations. J. Hazard. Mater. 2012, 243, 52–58. [Google Scholar]
- Wang, Z.; Forsyth, D.; Lau, B.P.; Pelletier, L.; Bronson, R.; Gaertner, D. Estimated dietary exposure of Canadians to perchlorate through the consumption of fruits and vegetables available in Ottawa markets. J. Agric. Food Chem. 2009, 57, 9250–9255. [Google Scholar] [CrossRef]
- Steinmaus, C.M. Perchlorate in Water Supplies: Sources, Exposures, and Health Effects. Curr. Environ. Health Rep. 2016, 3, 136–143. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Z.; Zhao, Y.; Wu, X.; Xiao, Q.; Deng, Y.; Li, M.; Li, C.; Qiu, H.; Lu, S. Perchlorate in shellfish from South China Sea and implications for human exposure. Mar. Pollut. Bull. 2021, 170, 112672. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Xu, T.; Li, R.; Johnson, D.; Ren, D.; Liu, H.; Xi, Y.; Huang, Y. Heavy metal accumulation and health risk assessment of crayfish collected from cultivated and uncultivated ponds in the Middle Reach of Yangtze River. Sci. Total Environ. 2020, 739, 139963. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Peng, B.; Wu, Y.; Xiong, L.; Sun, J.; Peng, G.; Bai, X. Human health risk assessment of toxic heavy metal and metalloid intake via consumption of red swamp crayfish (Procambarus clarkii) from rice-crayfish co-culture fields in China. Food Control 2021, 128, 108181. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Chen, C.; Feng, Y.; Ren, N.; Sun, K. Effects of cadmium on intestinal histology and microbiota in freshwater crayfish (Procambarus clarkii). Chemosphere 2020, 242, 125105. [Google Scholar] [CrossRef]
- Yao, Q.; Yan, S.; Liu, W.; Huang, M.; Lin, Q. Perchlorate contamination of tea leaves and a corresponding probabilistic dietary risk assessment using Monte Carlo simulation. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2021, 39, 341–350. [Google Scholar] [CrossRef] [PubMed]
- van Klaveren, J.D.; Boon, P.E. Probabilistic risk assessment of dietary exposure to single and multiple pesticide residues or contaminants: Summary of the work performed within the SAFE FOODS project. Food Chem. Toxicol. 2009, 47, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.A.; Paoli, G.M. Recent Advances in Probabilistic Dose-Response Assessment to Inform Risk-Based Decision Making. Risk Anal. Off. Publ. Soc. Risk Anal. 2020, 41, 596–609. [Google Scholar] [CrossRef]
- Tong, R.; Yang, X.; Su, H.; Pan, Y.; Zhang, Q.; Wang, J.; Long, M. Levels, sources and probabilistic health risks of polycyclic aromatic hydrocarbons in the agricultural soils from sites neighboring suburban industries in Shanghai. Sci. Total Environ. 2018, 616–617, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Du, H.; Xiao, G.; Guo, L. Heavy metal accumulation and health risk assessment of crayfish in the middle and lower reaches of Yangtze River during 2015–2017. Environ. Monit. Assess. 2021, 194, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, Y.; Sun, J.; Li, X.; Geng, X.; Zhao, M.; Sun, T.; Fan, Z. Health risk assessment of heavy metal(loid)s in park soils of the largest megacity in China by using Monte Carlo simulation coupled with Positive matrix factorization model. J. Hazard. Mater. 2021, 415, 125629. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Yang, D.; Li, C.; Zhao, Y.; Ma, H.; Luo, X.; Lu, S. Trace elements in shellfish from Shenzhen, China: Implication of coastal water pollution and human exposure. Environ. Pollut. 2020, 263 Pt B, 114582. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, T.; Gan, Z.; Sun, H. Spatial distribution of perchlorate, iodide and thiocyanate in the aquatic environment of Tianjin, China: Environmental source analysis. Chemosphere 2014, 111, 201–208. [Google Scholar] [CrossRef]
- Kuklina, I.; Kouba, A.; Buřič, M.; Horká, I.; Duriš, Z.; Kozák, P. Accumulation of heavy metals in crayfish and fish from selected Czech reservoirs. BioMed Res. Int. 2014, 2014, 306103. [Google Scholar] [CrossRef] [Green Version]
- Zarei, A.R.; Poormohammadi, A.; Rezaeivahidian, H.; Nejad, A.S.; Seid-Mohammadi, A. Effect of Coagulation Process in Presence of Chitosan and Psyllium Plantago in Removal of Perchlorate at High Concentrations. Asian J. Chem. 2016, 28, 2299–2302. [Google Scholar] [CrossRef]
- Alomirah, H.F.; Al-Zenki, S.F.; Alaswad, M.C.; Alruwaih, N.A.; Wu, Q.; Kannan, K. Widespread occurrence of perchlorate in water, foodstuffs and human urine collected from Kuwait and its contribution to human exposure. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1016–1025. [Google Scholar] [CrossRef]
- Vejdovszky, K.; Grossgut, R.; Unterluggauer, H.; Inreiter, N.; Steinwider, J. Risk assessment of dietary exposure to perchlorate for the Austrian population. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 623–631. [Google Scholar] [CrossRef]
- Maffini, M.V.; Trasande, L.; Neltner, T.G. Perchlorate and Diet: Human Exposures, Risks, and Mitigation Strategies. Curr. Environ. Health Rep. 2016, 3, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Tian, Y.; Fang, M.; Xu, L.; Wang, Q.; Li, J.; Shen, H.; Wu, Y.; Gong, Z. Bioavailability Evaluation of Perchlorate in Different Foods In Vivo: Comparison with In Vitro Assays and Implications for Human Health Risk Assessment. J. Agric. Food Chem. 2021, 69, 5189–5197. [Google Scholar] [CrossRef] [PubMed]



| Source | Region | Mean ± SD | Median | Detection Rate (100%) | Range |
|---|---|---|---|---|---|
| Cultivated ponds (n = 35) | Xuyi | 14.46 ± 4.70 | 11.96 | 100 | 7.74–43.71 |
| Jianhu | 21.76 ± 6.45 | 25.61 | 100 | ||
| Huoqiu | 29.25 ± 10.19 | 26.49 | 100 | ||
| Honghu | 10.27 ± 3.78 | 8.58 | 100 | ||
| Qianjiang | 19.65 ± 8.70 | 16.47 | 100 | ||
| Shayang | 18.00 ± 8.75 | 17.52 | 100 | ||
| Shishou | 10.00 ± 3.14 | 9.42 | 100 | ||
| Markets (n = 14) | Nanjing | 6.90 ± 1.05 | 6.90 | 100 | 4.90–16.73 |
| Hefei | 10.21 ± 5.86 | 8.51 | 100 | ||
| Wuhan | 5.17 ± 0.35 | 5.04 | 100 | ||
| Changsha | 7.79 ± 1.49 | 8.18 | 100 | ||
| Chengdu | 6.07 ± 0.26 | 5.93 | 100 |
| Edible Parts | Perchlorate Levels (μg/kg) | Adults | Children | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | P95 | HQ | Mean (SD) | P95 | HQ | ||
| Hepatopancreas | 10.21 ± 3.30 | 0.0014 (0.0009) | 0.0029 | 0.002 | 0.0049 (0.0031) | 0.0102 | 0.007 |
| Abdominal muscle | 20.98 ± 10.49 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wang, M.; Zhou, B.; Zhou, M.; Wang, Q.; Liu, X.; Liu, Y.; Wu, Y.; Zhao, X.; Gong, Z. Trends in the Exposure, Distribution, and Health Risk Assessment of Perchlorate among Crayfish in the Middle and Lower Reaches of the Yangtze River. Foods 2022, 11, 2238. https://doi.org/10.3390/foods11152238
Chen M, Wang M, Zhou B, Zhou M, Wang Q, Liu X, Liu Y, Wu Y, Zhao X, Gong Z. Trends in the Exposure, Distribution, and Health Risk Assessment of Perchlorate among Crayfish in the Middle and Lower Reaches of the Yangtze River. Foods. 2022; 11(15):2238. https://doi.org/10.3390/foods11152238
Chicago/Turabian StyleChen, Mengyuan, Manman Wang, Bingjie Zhou, Mengxin Zhou, Qiao Wang, Xin Liu, Yan Liu, Yongning Wu, Xiaole Zhao, and Zhiyong Gong. 2022. "Trends in the Exposure, Distribution, and Health Risk Assessment of Perchlorate among Crayfish in the Middle and Lower Reaches of the Yangtze River" Foods 11, no. 15: 2238. https://doi.org/10.3390/foods11152238
APA StyleChen, M., Wang, M., Zhou, B., Zhou, M., Wang, Q., Liu, X., Liu, Y., Wu, Y., Zhao, X., & Gong, Z. (2022). Trends in the Exposure, Distribution, and Health Risk Assessment of Perchlorate among Crayfish in the Middle and Lower Reaches of the Yangtze River. Foods, 11(15), 2238. https://doi.org/10.3390/foods11152238






