Ethanol at Subinhibitory Concentrations Enhances Biofilm Formation in Salmonella Enteritidis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Target Bacteria
2.2. Antibacterial Activity Test
2.3. Biofilm Formation Assay
2.4. Bacterial Attachment Assay
2.5. Cell Motility Test
2.6. Cell Surface Hydrophobicity Study
2.7. Cell Auto-Aggregation Assay
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results and Discussion
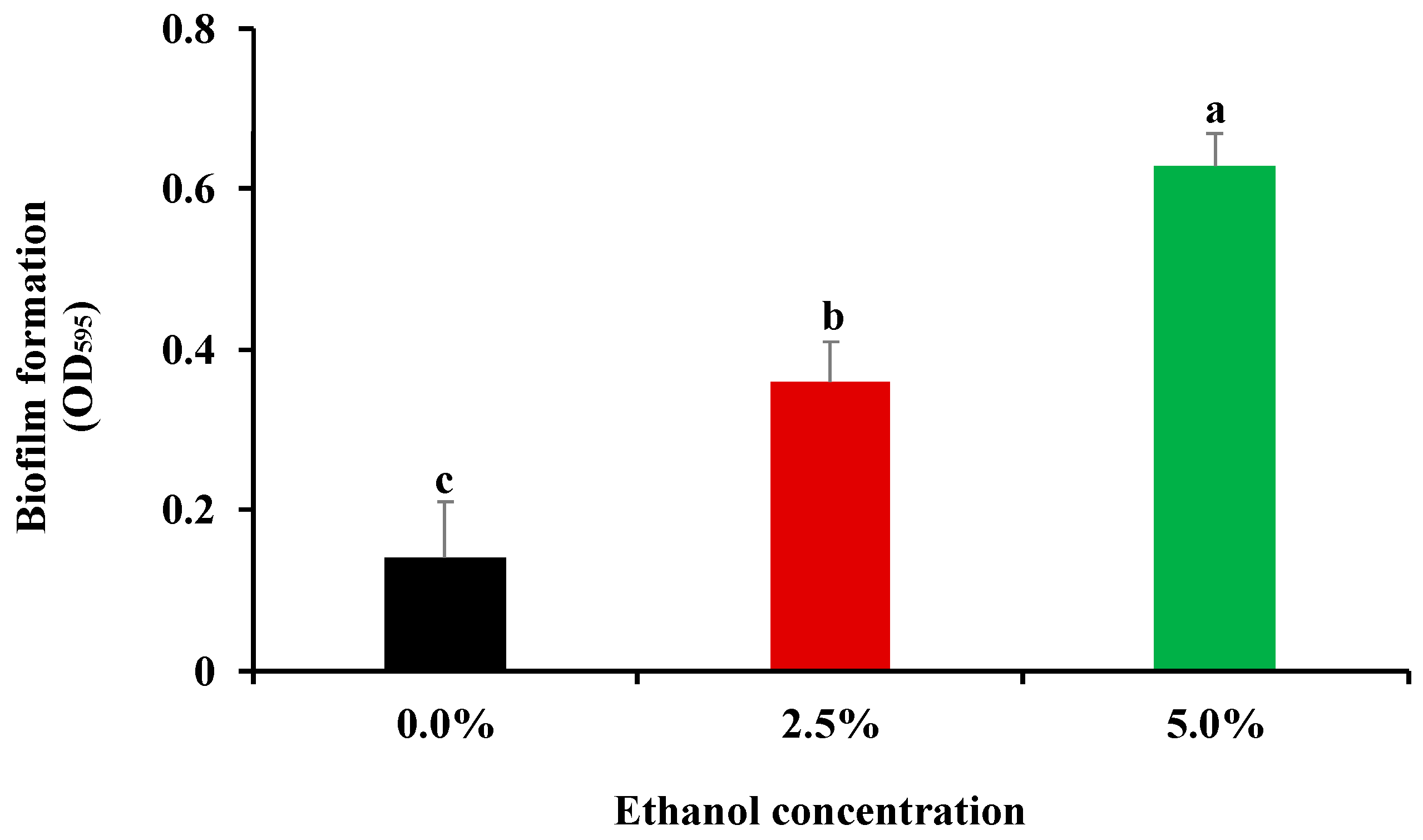
3.1. Influence of Ethanol at Subinhibitory Concentrations on Bacterial Biofilm Formation
3.2. Influence of Ethanol at Subinhibitory Concentrations on Cell Attachment Ability
3.3. Influence of Ethanol at Subinhibitory Concentrations on Bacterial Motility Capacity
3.4. Influence of Ethanol at Subinhibitory Concentrations on Bacterial Surface Characteristics
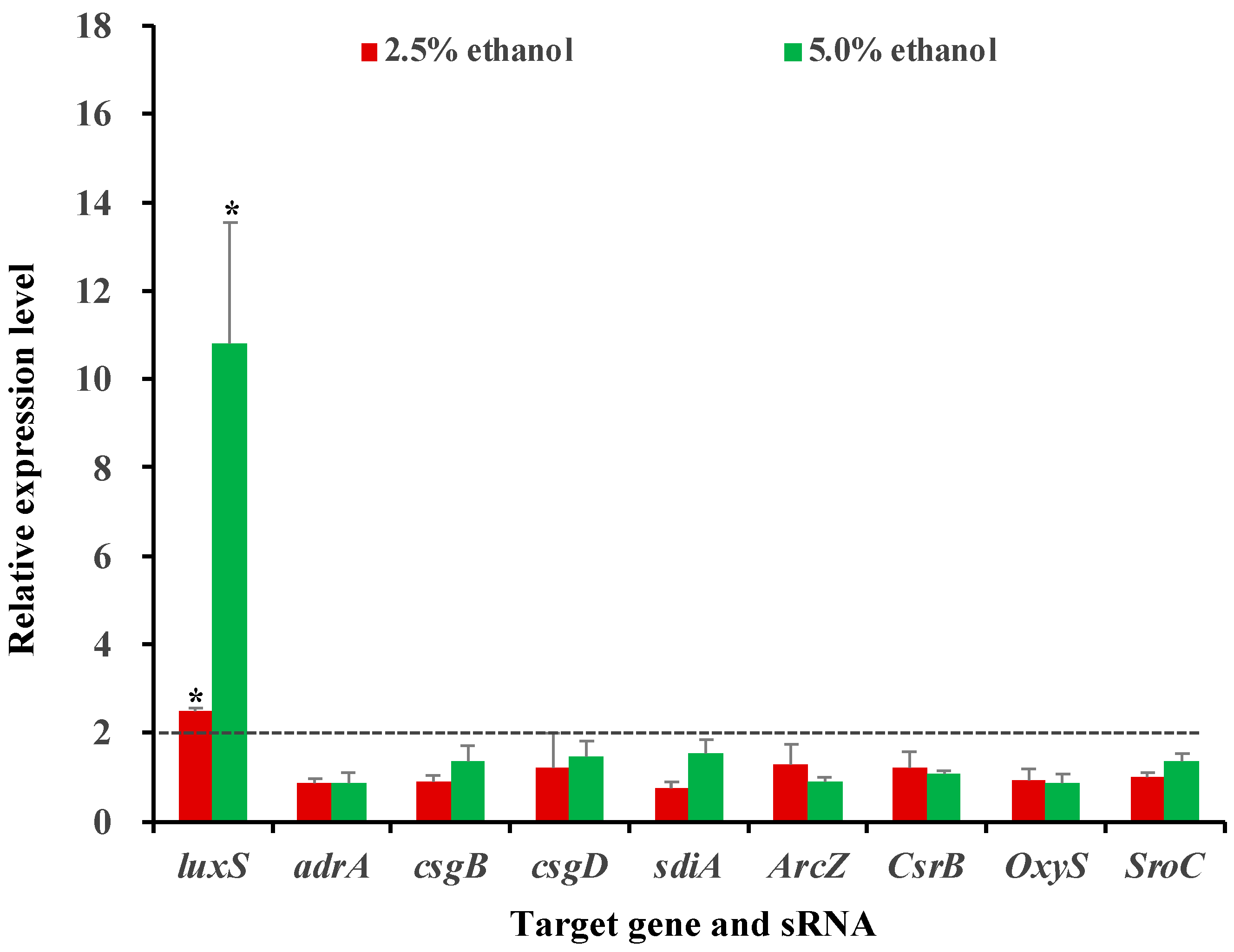
3.5. Influence of Ethanol at Subinhibitory Concentrations on the Expression of Selected Genes and sRNAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzeer, J.; Abou Hadeed, K. Ethanol and its Halal status in food industries. Trends Food Sci. Tech. 2016, 58, 14–20. [Google Scholar] [CrossRef]
- Chiou, R.Y.; Phillips, R.D.; Zhao, P.; Doyle, M.P.; Beuchat, L.R. Ethanol-mediated variations in cellular fatty acid composition and protein profiles of two genotypically different strains of Escherichia coli O157: H7. Appl. Environ. Microbiol. 2004, 70, 2204–2210. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.K.; Distefano, S. Ethanol content of various foods and soft drinks and their potential for interference with a breath-alcohol test. J. Anal. Toxicol. 1998, 22, 181–183. [Google Scholar] [CrossRef] [Green Version]
- Dev Kumar, G.; Mishra, A.; Dunn, L.; Townsend, A.; Oguadinma, I.C.; Bright, K.R.; Gerba, C.P. Biocides and novel antimicrobial agents for the mitigation of coronaviruses. Front. Microbiol. 2020, 11, 1351. [Google Scholar] [CrossRef]
- Fagerlund, A.; Møretrø, T.; Heir, E.; Briandet, R.; Langsrud, S. Cleaning and disinfection of biofilms composed of Listeria monocytogenes and background microbiota from meat processing surfaces. Appl. Environ. Microbiol. 2017, 83, e01046-17. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Fong, K.; Wang, S.; Shi, X. Ethanol adaptation in foodborne bacterial pathogens. Crit. Rev. Food Sci. Nutr. 2021, 61, 777–787. [Google Scholar] [CrossRef]
- Dao, T.; Dantigny, P. Control of food spoilage fungi by ethanol. Food Control 2011, 22, 360–368. [Google Scholar] [CrossRef]
- Tango, C.N.; Akkermans, S.; Hussain, M.S.; Khan, I.; Van Impe, J.; Jin, Y.G.; Oh, D.H. Modeling the effect of pH, water activity, and ethanol concentration on biofilm formation of Staphylococcus aureus. Food Microbiol. 2018, 76, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Inagaki, A.; Ono, K.; Inaba, T.; Yawata, Y.; Uchiyama, H.; Nomura, N. Low concentrations of ethanol stimulate biofilm and pellicle formation in Pseudomonas aeruginosa. Biosci. Biotech. Bioch. 2014, 78, 178–181. [Google Scholar] [CrossRef]
- Dhivya, R.; Rajakrishnapriya, V.C.; Sruthi, K.; Chidanand, D.V.; Sunil, C.K.; Rawson, A. Biofilm combating in the food industry: Overview, non-thermal approaches, and mechanisms. J. Food Process. Pres. 2022, 00, e16282. [Google Scholar] [CrossRef]
- Díez-García, M.; Capita, R.; Alonso-Calleja, C. Influence of serotype on the growth kinetics and the ability to form biofilms of Salmonella isolates from poultry. Food Microbiol. 2012, 31, 173–180. [Google Scholar] [CrossRef]
- Wang, H.H.; Ye, K.P.; Zhang, Q.Q.; Dong, Y.; Xu, X.L.; Zhou, G.H. Biofilm formation of meat-borne Salmonella enterica and inhibition by the cell-free supernatant from Pseudomonas aeruginosa. Food Control 2013, 32, 650–658. [Google Scholar] [CrossRef]
- Wang, H.; Ding, S.; Dong, Y.; Ye, K.; Xu, X.; Zhou, G. Biofilm formation of Salmonella serotypes in simulated meat processing environments and its relationship to cell characteristics. J. Food Protect. 2013, 76, 1784–1789. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Y.; Wang, G.; Xu, X.; Zhou, G. Effect of growth media on gene expression levels in Salmonella Typhimurium biofilm formed on stainless steel surface. Food Control 2016, 59, 546–552. [Google Scholar] [CrossRef]
- Lamas, A.; Paz-Mendez, A.M.; Regal, P.; Vazquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Food preservatives influence biofilm formation, gene expression and small RNAs in Salmonella enterica. LWT-Food Sci. Technol. 2018, 97, 1–8. [Google Scholar] [CrossRef]
- Fan, Q.; He, Q.; Zhang, T.; Song, W.; Sheng, Q.; Yuan, Y.; Yue, T. Antibiofilm potential of lactobionic acid against Salmonella Typhimurium. LWT-Food Sci. Technol. 2022, 162, 113461. [Google Scholar] [CrossRef]
- Kim, Y.K.; Roy, P.K.; Ashrafudoulla, M.; Nahar, S.; Toushik, S.H.; Hossain, M.I.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression. Food Control 2022, 137, 108964. [Google Scholar] [CrossRef]
- Song, X.; Sun, Y.; Zhang, Q.; Yang, X.; Zheng, F.; He, S.; Wang, Y. Failure of Staphylococcus aureus to acquire direct and cross tolerance after habituation to cinnamon essential oil. Microorganisms 2019, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Von Hertwig, A.M.; Prestes, F.S.; Nascimento, M.S. Biofilm formation and resistance to sanitizers by Salmonella spp. isolated from the peanut supply chain. Food Res. Int. 2022, 152, 110882. [Google Scholar] [CrossRef]
- Dhakal, J.; Sharma, C.S.; Nannapaneni, R.; McDANIEL, C.D.; Kim, T.; Kiess, A. Effect of chlorine-induced sublethal oxidative stress on the biofilm-forming ability of Salmonella at different temperatures, nutrient conditions, and substrates. J. Food Protect. 2019, 82, 78–92. [Google Scholar] [CrossRef]
- Obe, T.; Nannapaneni, R.; Sharma, C.S.; Kiess, A. Homologous stress adaptation, antibiotic resistance, and biofilm forming ability of Salmonella enterica serovar Heidelberg ATCC8326 on different food-contact surfaces following exposure to sublethal chlorine concentrations. Poultry Sci. 2018, 97, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.E.; Ross, T.; Bowman, J.P. Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int. J. Food Microbiol. 2011, 150, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Do Valle Gomes, M.Z.; Nitschke, M. Evaluation of rhamnolipid and surfactin to reduce the adhesion and remove biofilms of individual and mixed cultures of food pathogenic bacteria. Food Control 2012, 25, 441–447. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. Impact of quercetin against Salmonella Typhimurium biofilm formation on food-contact surfaces and molecular mechanism pattern. Foods 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, Y.; Phale, P.S. Biodegradation of phenanthrene by Pseudomonas sp. strain PP2: Novel metabolic pathway, role of biosurfactant and cell surface hydrophobicity in hydrocarbon assimilation. Appl. Microbiol. Biot. 2003, 61, 342–351. [Google Scholar] [CrossRef]
- Xu, H.; Zou, Y.; Lee, H.Y.; Ahn, J. Effect of NaCl on the biofilm formation by foodborne pathogens. J. Food Sci. 2010, 75, M580–M585. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, Y.J.; Yu, H.H.; Jung, S.C.; Park, J.H.; Lee, D.H.; Lee, N.K.; Paik, H.D. Antimicrobial and antibiofilm effect of ε-polylysine against Salmonella Enteritidis, Listeria monocytogenes, and Escherichia coli in tryptic soy broth and chicken juice. Foods 2021, 10, 2211. [Google Scholar] [CrossRef]
- He, S.; Cui, Y.; Qin, X.; Zhang, F.; Shi, C.; Paoli, G.C.; Shi, X. Influence of ethanol adaptation on Salmonella enterica serovar Enteritidis survival in acidic environments and expression of acid tolerance-related genes. Food Microbiol. 2018, 72, 193–198. [Google Scholar] [CrossRef]
- He, S.; Qin, X.; Wong, C.W.; Shi, C.; Wang, S.; Shi, X. Ethanol adaptation strategies in Salmonella enterica serovar Enteritidis revealed by global proteomic and mutagenic analyses. Appl. Environ. Microbiol. 2019, 85, e01107-19. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Roy, P.K.; Ha, A.J.W.; Mizan, M.F.R.; Hossain, M.I.; Ashrafudoulla, M.; Toushik, S.H.; Nahar, S.; Kim, Y.K.; Ha, S.D. Effects of environmental conditions (temperature, pH, and glucose) on biofilm formation of Salmonella enterica serotype Kentucky and virulence gene expression. Poultry Sci. 2021, 100, 101209. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Influence of milk, chicken residues and oxygen levels on biofilm formation on stainless steel, gene expression and small RNAs in Salmonella enterica. Food Control 2018, 90, 1–9. [Google Scholar] [CrossRef]
- Ranieri, M.R.; Whitchurch, C.B.; Burrows, L.L. Mechanisms of biofilm stimulation by subinhibitory concentrations of antimicrobials. Curr. Opin. Microbiol. 2018, 45, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Bae, Y.M.; Lee, S.Y. Effect of environmental conditions on biofilm formation and related characteristics of Staphylococcus aureus. J. Food Saf. 2016, 36, 412–422. [Google Scholar] [CrossRef]
- Chang, Y.; Gu, W.; McLandsborough, L. Low concentration of ethylenediaminetetraacetic acid (EDTA) affects biofilm formation of Listeria monocytogenes by inhibiting its initial adherence. Food Microbiol. 2012, 29, 10–17. [Google Scholar] [CrossRef]
- Chathoth, K.; Fostier, L.; Martin, B.; Baysse, C.; Mahé, F. A multi-skilled mathematical model of bacterial attachment in initiation of biofilms. Microorganisms 2022, 10, 686. [Google Scholar] [CrossRef]
- Li, Y.; Bai, F.; Xia, H.; Zhuang, L.; Xu, H.; Jin, Y.; Zhang, X.; Bai, Y.; Qiao, M. A novel regulator PA5022 (aefA) is involved in swimming motility, biofilm formation and elastase activity of Pseudomonas aeruginosa. Microbiol. Res. 2015, 176, 14–20. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, B.; Shi, C.; Shi, X. Mutation of a Salmonella serogroup-C1-specific gene abrogates O7-antigen biosynthesis and triggers NaCl-dependent motility deficiency. PLoS ONE 2014, 9, e106708. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Ding, X.; Zhao, B.; An, Q.; Guo, J. The essential role of hydrophobic interaction within extracellular polymeric substances in auto-aggregation of P. stutzeri strain XL-2. Int. Biodeter. Biodegr. 2022, 171, 105404. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Quorum sensing inhibitory effect of hexanal on Autoinducer-2 (AI-2) and corresponding impacts on biofilm formation and enzyme activity in Erwinia carotovora and Pseudomonas fluorescens isolated from vegetables. J. Food Process. Pres. 2022, 46, e16293. [Google Scholar] [CrossRef]
- Ju, X.; Li, J.; Zhu, M.; Lu, Z.; Lv, F.; Zhu, X.; Bie, X. Effect of the luxS gene on biofilm formation and antibiotic resistance by Salmonella serovar Dublin. Food Res. Int. 2018, 107, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Ma, M.; Sun, Y.; Xu, X.; Qiu, S.; Yin, J.; Chen, L. The effect of sublethal concentrations of benzalkonium chloride on the LuxS/AI-2 quorum sensing system, biofilm formation and motility of Escherichia coli. Int. J. Food Microbiol. 2021, 353, 109313. [Google Scholar] [CrossRef]
- Jesudhasan, P.R.; Cepeda, M.L.; Widmer, K.; Dowd, S.E.; Soni, K.A.; Hume, M.E.; Zhu, J.; Pillai, S.D. Transcriptome analysis of genes controlled by luxS/autoinducer-2 in Salmonella enterica serovar Typhimurium. Foodborne Pathog. Dis. 2010, 7, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial biofilms in the food industry-a comprehensive review. Int. J. Env. Res. Pub. He. 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]


| Gene/sRNA | NCBI Accession No. or Gene ID | Sequence (5′ to 3′) | Reference |
|---|---|---|---|
| luxS | CAR34242.1 | F: ATGCCATTATTAGATAGCTT | [14] |
| R: GAGATGGTCGCGCATAAAGCCAGC | |||
| adrA | CAR31954.1 | F: GAAGCTCGTCGCTGGAAGTC | [15] |
| R: TTCCGCTTAATTTAATGGCCG | |||
| csgB | CAR33485.1 | F: TCCTGGTCTTCAGTAGCGTAA | [14] |
| R: TATGATGGAAGCGGATAAGAA | |||
| csgD | CAR33486.1 | F: TCCTGGTCTTCAGTAGCGTAA | [15] |
| R: TATGATGGAAGCGGATAAGAA | |||
| sdiA | CAR32642.1 | F: AATATCGCTTCGTACCAC | [15] |
| R: GTAGGTAAACGAGGAGCAG | |||
| ArcZ | 2847690 | F: ACTGCGCCTTTGACATCATC | [15] |
| R: CGAATACTGCGCCAACACCA | |||
| CsrB | 1254489 | F: CAAAGTGGAAAGCGCAGGAT | [15] |
| R: TGACCTTACGGCCTGTTCAT | |||
| OxyS | 6797054 | F: TAACCCTTGAAGACACCGCC | [15] |
| R: ACCAGAGGTCCGCAAAAGTT | |||
| SroC | 6793706 | F: GGGACTCCTGTCCTCTCGAT | [15] |
| R: CAGCGCTACCCTCGAAGATT | |||
| 16S rRNA | X80676.1 | F: AGGCCTTCGGGTTGTAAAGT | [15] |
| R: GTTAGCCGGTGCTTCTTCTG |
| Properties | 0.0% Ethanol | 2.5% Ethanol | 5.0% Ethanol |
|---|---|---|---|
| Hydrophobicity (%) | 3.92 ± 1.33 a | 4.98 ± 0.65 a | 3.60 ± 1.16 a |
| Auto-aggregation (%) | 40.53 ± 1.35 c | 58.92 ± 1.06 b | 72.32 ± 0.45 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Zhan, Z.; Shi, C.; Wang, S.; Shi, X. Ethanol at Subinhibitory Concentrations Enhances Biofilm Formation in Salmonella Enteritidis. Foods 2022, 11, 2237. https://doi.org/10.3390/foods11152237
He S, Zhan Z, Shi C, Wang S, Shi X. Ethanol at Subinhibitory Concentrations Enhances Biofilm Formation in Salmonella Enteritidis. Foods. 2022; 11(15):2237. https://doi.org/10.3390/foods11152237
Chicago/Turabian StyleHe, Shoukui, Zeqiang Zhan, Chunlei Shi, Siyun Wang, and Xianming Shi. 2022. "Ethanol at Subinhibitory Concentrations Enhances Biofilm Formation in Salmonella Enteritidis" Foods 11, no. 15: 2237. https://doi.org/10.3390/foods11152237
APA StyleHe, S., Zhan, Z., Shi, C., Wang, S., & Shi, X. (2022). Ethanol at Subinhibitory Concentrations Enhances Biofilm Formation in Salmonella Enteritidis. Foods, 11(15), 2237. https://doi.org/10.3390/foods11152237







