Hibiscus sabdariffa in Diabetes Prevention and Treatment—Does It Work? An Evidence-Based Review
Abstract
1. Hibiscus sabdariffa—Characteristic
1.1. Morphology
1.2. Roselle as a Functional Food and Nutraceutical
1.3. Hibiscus sabdariffa Active Compounds
1.4. Medicinal Properties and Usage of Roselle
2. Diabetes Mellitus
3. Hibiscus sabdariffa and Its Therapeutic Effects Studied In Vitro
4. Hibiscus sabdariffa in Diabetic and Pre-Diabetic Animal Studies
4.1. Hibiscus sabdariffa and Its Hypoglycemic Effects in Animal Studies
4.2. Hibiscus sabdariffa and Pancreatic Support
4.3. Hibiscus sabdariffa and Anti-Lipidemic Activity
4.4. Hibiscus sabdariffa and Hepatoprotective Effect
4.5. Hibiscus sabdariffa and Kidney Protective Effect
4.6. Hibiscus sabdariffa and Cardiovascular Protective Effect
4.7. Hibiscus sabdariffa and Protective Effect on Fertility
4.8. Hibiscus sabdariffa and Its Effects on Other Organs
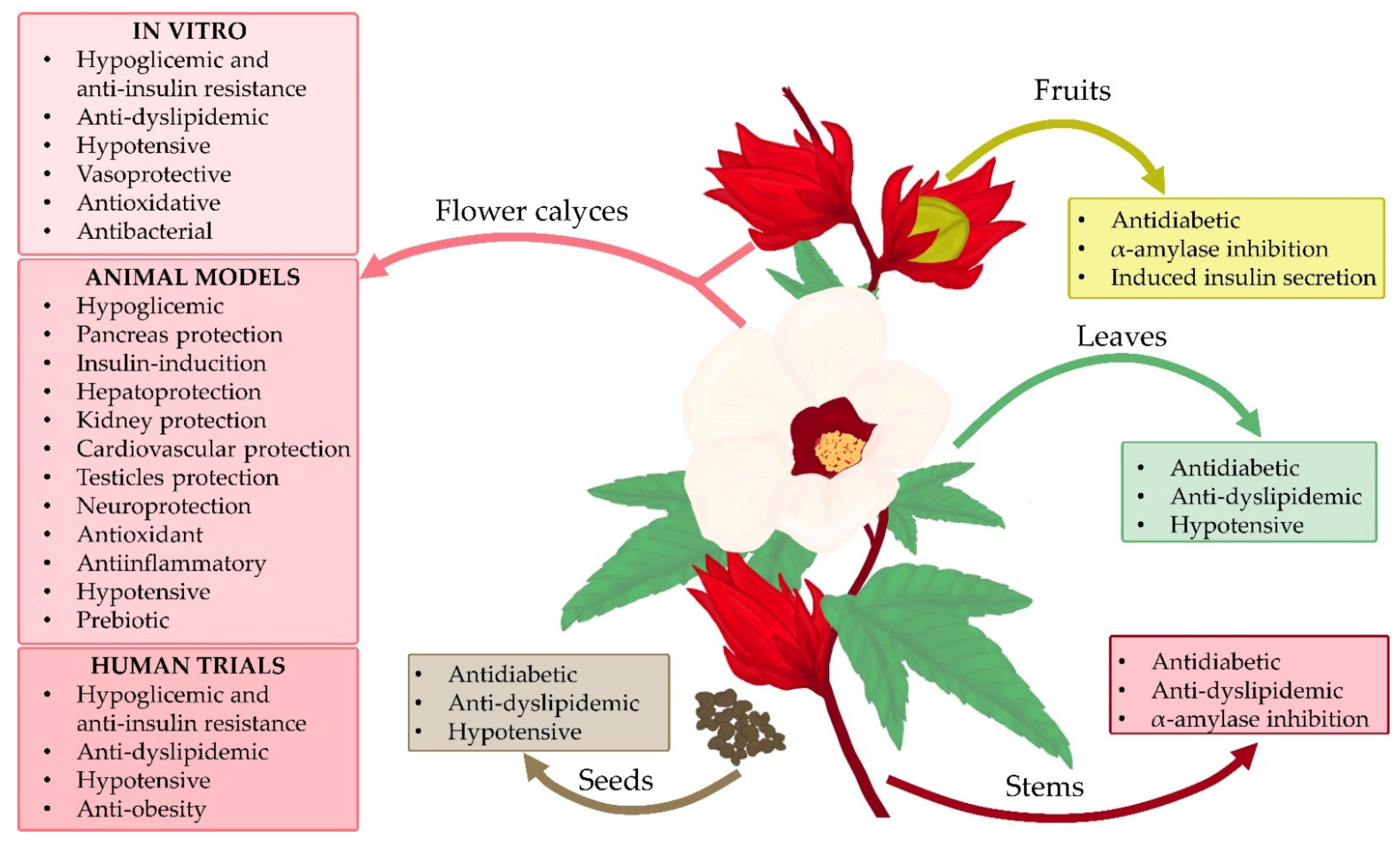
4.9. Hibiscus sabdariffa Is Not Only a Flower and Calyces
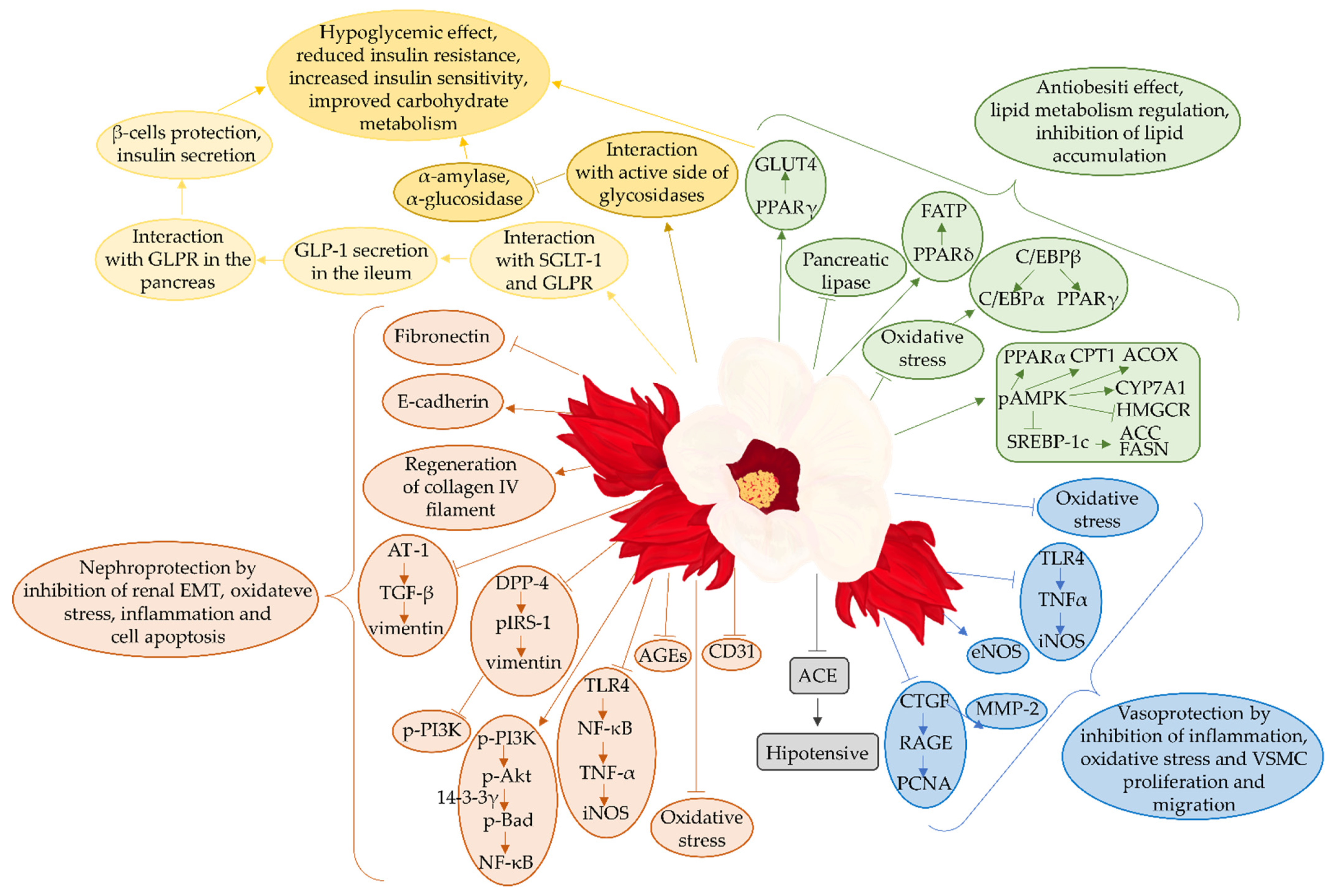
4.10. Molecular Mechanisms Underlying Antidiabetic Effect of Hibiscus sabdariffa
5. Hibiscus sabdariffa in Diabetic and Prediabetic Patients
5.1. Effect of Hibiscus sabdariffa on Blood Pressure in Diabetic Patients
5.2. Effect of Hibiscus sabdariffa on the Lipid Profile in Diabetic and Pre-Diabetic Patients
6. Hibiscus sabdariffa as a Prospect in Creating a New Generation of Drugs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, S. Hibiscus Sabdariffa: An Ideal yet under-Exploited Candidate for Nutraceutical Applications. Biomed. Prev. Nutr. 2014, 4, 23–27. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A Review on Phytochemistry and Therapeutic Uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Amos, A.; Khiatah, B. Mechanisms of Action of Nutritionally Rich Hibiscus Sabdariffa’s Therapeutic Uses in Major Common Chronic Diseases: A Literature Review. J. Am. Coll. Nutr. 2020, 41, 116–124. [Google Scholar] [CrossRef]
- Cid-Ortega, S.; Guerrero-Beltrán, J.A. Roselle Calyces (Hibiscus sabdariffa), an Alternative to the Food and Beverages Industries: A Review. J. Food Sci. Technol. 2015, 52, 6859–6869. [Google Scholar] [CrossRef]
- Salem, M.A.; Zayed, A.; Beshay, M.E.; Abdel Mesih, M.M.; Ben Khayal, R.F.; George, F.A.; Ezzat, S.M. Hibiscus sabdariffa L.: Phytoconstituents, Nutritive, and Pharmacological Applications. Adv. Tradit. Med. 2021. [Google Scholar] [CrossRef]
- Sankaralingam, B.; Balan, L.; Chandrasekaran, S.; Muthu Selvam, A. Anthocyanin: A Natural Dye Extracted from Hibiscus sabdariffa (L.) for Textile and Dye Industries. Appl. Biochem. Biotechnol. 2022, 1–18. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mariod, A.A.; Mahunu, G.K.; Abdualrahman, M.A.Y.; Tchabo, W. Assessment of Antioxidant Properties, Instrumental and Sensory Aroma Profile of Red and White Karkade/Roselle (Hibiscus sabdariffa L.). J. Food Meas. Charact. 2017, 11, 1559–1568. [Google Scholar] [CrossRef]
- Nerdy, N.; Barus, B.R.; El-Matury, H.J.; Ginting, S.; Zebua, N.F.; Bakri, T.K. Comparison of Flavonoid Content and Antioxidant Activity in Calyces of Two Roselle Varieties (Hibiscus sabdariffa L.). IOP Conf. Ser. Earth Environ. Sci. 2022, 956, 012001. [Google Scholar] [CrossRef]
- Salami, S.O.; Afolayan, A.J. Suitability of Roselle-Hibiscus sabdariffa L. as Raw Material for Soft Drink Production. J. Food Qual. 2020, 2020, 8864142. [Google Scholar] [CrossRef]
- Shruthi, V.H.; Ramachandra, C.T.; Nidoni, U.; Hiregoudar, S.; Naik, N.; Kurubar, A.R. Roselle (Hibiscus sabdariffa L.) as a Source of Natural Colour: A Review. Plant Arch. 2016, 16, 515–522. [Google Scholar]
- Islam, M. Food and Medicinal Values of Roselle (Hibiscus sabdariffa L. Linne Malvaceae) Plant Parts: A Review. Open J. Nutr. Food Sci. Rev. 2019, 1, 14–20. [Google Scholar]
- Singh, R.; Ashish, A.; Shah, A.; Shekhar Pandey, S. Interaction between Oxidative Stress and Diabetes: A Mini-Review. J. Diabetes Metab. Disord. Control 2020, 7, 58–61. [Google Scholar] [CrossRef]
- Anel, T.C.; Thokchom, R.; Subapriya, M.S.; Thokchom, J.; Singh, S.S. Hibiscus sabdariffa —A Natural Micro Nutrient Source. Int. J. Adv. Res. Biol. Sci. 2016, 3, 243–248. [Google Scholar]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef]
- Banerjee, P. Functional Food: A Brief Overview. Int. J. Bioresour. Sci. 2019, 6, 57–60. [Google Scholar] [CrossRef]
- Islam, A.K.M.A.; Jamini, T.S.; Islam, A.K.M.M.; Yeasmin, S. Roselle: A Functional Food with High Nutritional and Medicinal Values. Fundam. Appl. Agric. 2016, 1, 44–49. [Google Scholar]
- Bedi, P.S.; Bekele, M.; Gure, G. Phyto-Chemistry and Pharmacological Activities of Hibiscus sabdariffa Linn.—A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 41–54. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chen, J.-H.; Wang, C.-J. Chemopreventive Properties and Molecular Mechanisms of the Bioactive Compounds in Hibiscus sabdariffa Linne. Curr. Med. Chem. 2011, 18, 1245–1254. [Google Scholar] [CrossRef]
- Perez-Torres, I.; Ruiz-Ramirez, A.; Banos, G.; El-Hafidi, M. Hibiscus sabdariffa Linnaeus (Malvaceae), Curcumin and Resveratrol as Alternative Medicinal Agents Against Metabolic Syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 25–37. [Google Scholar] [CrossRef]
- Koala, M.; Ramde-Tiendrebeogo, A.; Ouedraogo, N.; Ilboudo, S.; Kaboré, B.; Kini, F.B.; Ouedraogo, S. HPTLC Phytochemical Screening and Hydrophilic Antioxidant Activities of Apium graveolens L., Cleome gynandra L., and Hibiscus sabdariffa L. Used for Diabetes Management. Am. J. Anal. Chem. 2021, 12, 15–28. [Google Scholar] [CrossRef]
- Guardiola, S.; Mach, N. Therapeutic Potential of Hibiscus Sabdariffa: A Review of the Scientific Evidence. Endocrinol. Nutr. 2014, 61, 274–295. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Rui, L.; Gonzalez De Mejia, E. Hibiscus sabdariffa L.: Phytochemical Composition and Nutraceutical Properties. ACS Symp. Ser. 2012, 1109, 289–305. [Google Scholar] [CrossRef]
- Giacoman-Martínez, A.; Alarcón-Aguilar, F.J.; Zamilpa, A.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; García-Macedo, R.; Román-Ramos, R.; Almanza-Pérez, J.C. Triterpenoids from Hibiscus sabdariffa L. with PPARδ/γ Dual Agonist Action: In Vivo, In Vitro and In Silico Studies Authors. Planta Med. 2019, 85, 412–423. [Google Scholar] [CrossRef]
- Ifie, I.; Ifie, B.E.; Ibitoye, D.O.; Marshall, L.J.; Williamson, G. Seasonal Variation in Hibiscus sabdariffa (Roselle) Calyx Phytochemical Profile, Soluble Solids and α-Glucosidase Inhibition. Food Chem. 2018, 261, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, I.F.; Castaño-Tostado, E.; Ramírez-De León, J.A.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Effect of Stevia and Citric Acid on the Stability of Phenolic Compounds and in Vitro Antioxidant and Antidiabetic Capacity of a Roselle (Hibiscus sabdariffa L.) Beverage. Food Chem. 2015, 172, 885–892. [Google Scholar] [CrossRef]
- Hapsari, B.W.; Manikharda; Setyaningsih, W. Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health. Horticulturae 2021, 7, 35. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yang, W.K.; Kim, H.Y.; Min, B.; Caturla, N.; Jones, J.; Park, Y.C.; Lee, Y.C.; Kim, S.H. Metabolaid® Combination of Lemon Verbena and Hibiscus Flower Extract Prevents High-Fat Diet-Induced Obesity through AMP-Activated Protein Kinase Activation. Nutrients 2018, 10, 1204. [Google Scholar] [CrossRef]
- Lans, C.A. Ethnomedicines Used in Trinidad and Tobago for Urinary Problems and Diabetes Mellitus. J. Ethnobiol. Ethnomed. 2006, 2, 45. [Google Scholar] [CrossRef]
- Huang, C.N.; Chan, K.C.; Lin, W.T.; Su, S.L.; Wang, C.J.; Peng, C.H. Hibiscus sabdariffa Inhibits Vascular Smooth Muscle Cell Proliferation and Migration Induced by High Glucoses—A Mechanism Involves Connective Tissue Growth Factor Signals. J. Agric. Food Chem. 2009, 57, 3073–3079. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Teralı, K.; Olofinsan, K.A.; Surgun, S.; Ogbaga, C.C.; Ajiboye, T.O. Antidiabetic Activity-Guided Isolation of Gallic and Protocatechuic Acids from Hibiscus sabdariffa Calyxes. J. Food Biochem. 2019, 43, e12927. [Google Scholar] [CrossRef] [PubMed]
- Elkafrawy, N.; Younes, K.; Naguib, A.; Badr, H.; Zewain, S.K.; Kamel, M.; Raoof, G.F.A.; El-Desoky, A.M.; Mohamed, S. Antihypertensive Efficacy and Safety of a Standardized Herbal Medicinal Product of Hibiscus sabdariffa and Olea europaea Extracts (NW Roselle): A Phase-II, Randomized, Double-Blind, Captopril-Controlled Clinical Trial. Phyther. Res. 2020, 34, 3379–3387. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Aqueous Extracts of Roselle (Hibiscus sabdariffa Linn.) Varieties Inhibit α-Amylase and α-Glucosidase Activities in Vitro. J. Med. Food 2013, 16, 88–93. [Google Scholar] [CrossRef]
- Mardiah; Zakaria, F.R.; Prangdimurti, E.; Damanik, R. Anti-Inflammatory of Purple Roselle Extract in Diabetic Rats Induced by Streptozotocin. Procedia Food Sci. 2015, 3, 182–189. [Google Scholar] [CrossRef]
- Jeffery, T.D.; Richardson, M.L. A Review of the Effectiveness of Hibiscus for Treatment of Metabolic Syndrome. J. Ethnopharmacol. 2021, 270, 113762. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.M.; Dinneen, S.F. What Is Diabetes? Medicine 2019, 47, 1–4. [Google Scholar] [CrossRef]
- Li, W.; Huang, E.; Gao, S. Type 1 Diabetes Mellitus and Cognitive Impairments: A Systematic Review. J. Alzheimer’s Dis. 2017, 57, 29–36. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, A.; Khan, I. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Janež, A.; Guja, C.; Mitrakou, A.; Lalic, N.; Tankova, T.; Czupryniak, L.; Tabák, A.G.; Prazny, M.; Martinka, E.; Smircic-Duvnjak, L. Insulin Therapy in Adults with Type 1 Diabetes Mellitus: A Narrative Review. Diabetes Ther. 2020, 11, 387–409. [Google Scholar] [CrossRef]
- Tricco, A.C.; Ashoor, H.M.; Antony, J.; Bouck, Z.; Rodrigues, M.; Pham, B.; Khan, P.A.; Nincic, V.; Darvesh, N.; Yazdi, F.; et al. Comparative Efficacy and Safety of Ultra-Long-Acting, Long-Acting, Intermediate-Acting, and Biosimilar Insulins for Type 1 Diabetes Mellitus: A Systematic Review and Network Meta-Analysis. J. Gen. Intern. Med. 2021, 36, 2414–2426. [Google Scholar] [CrossRef]
- Ziegler, R.; Neu, A. Diabetes in Childhood and Adolescence—A Guideline-Based Approach to Diagnosis, Treatment, and Follow-Up. Dtsch. Arztebl. Int. 2018, 115, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 Diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Laakso, M. Biomarkers for Type 2 Diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- He, Z.X.; Zhou, Z.W.; Yang, Y.; Yang, T.; Pan, S.Y.; Qiu, J.X.; Zhou, S.F. Overview of Clinically Approved Oral Antidiabetic Agents for the Treatment of Type 2 Diabetes Mellitus. Clin. Exp. Pharmacol. Physiol. 2015, 42, 125–138. [Google Scholar] [CrossRef]
- Abdi, H.; Azizi, F.; Amouzegar, A. Insulin Monotherapy versus Insulin Combined with Other Glucose-Lowering Agents in Type 2 Diabetes: A Narrative Review. Int. J. Endocrinol. Metab. 2018, 16, e65600. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic Syndrome: A Closer Look at the Growing Epidemic and Its Associated Pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of Diabetes Mellitus and Diabetes Complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Parhofer, K.G. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Amin, A.R.; Kassab, R.B.; Abdel Moneim, A.E.; Amin, H.K. Comparison Among Garlic, Berberine, Resveratrol, Hibiscus Sabdariffa, Genus Zizyphus, Hesperidin, Red Beetroot, Catha edulis, Portulaca Oleracea, and Mulberry Leaves in the Treatment of Hypertension and Type 2 DM: A Comprehensive Review. Nat. Prod. Commun. 2020, 15, 1–24. [Google Scholar] [CrossRef]
- Shane-McWhorter, L. Dietary Supplements for Diabetes Are Decidedly Popular: Help Your Patients Decide. Diabetes Spectr. 2013, 26, 259–266. [Google Scholar] [CrossRef][Green Version]
- Salleh, N.H.; Zulkipli, I.N.; Mohd Yasin, H.; Ja’Afar, F.; Ahmad, N.; Wan Ahmad, W.A.N.; Ahmad, S.R. Systematic Review of Medicinal Plants Used for Treatment of Diabetes in Human Clinical Trials: An ASEAN Perspective. Evid.-Based Complement. Altern. Med. 2021, 2021, 5570939. [Google Scholar] [CrossRef]
- Sakhaei, R.; Nadjarzadeh, A.; Esmaeili, A.; Mohammadi, M.; Hemayati, R.; Reza, J.Z.; Mozaffari-Khosravi, H.; Ramezani-Jolfaie, N. Cardiovascular and Renal Effects of Hibiscus sabdariffa Linnaeus. in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Controlled Trial. J. Nutr. Food Secur. 2021, 6, 116–126. [Google Scholar] [CrossRef]
- Adefolalu, F.S.; Salawa, J.S.; Gara, T.Y.; Abubakar, A.N. Hypoglycemic and Hypolipidemic Effect of Methanol Extract of Hibiscus sabdariffa Seed in Alloxan Induced Diabetic Albino Rats. Niger. J. Basic Appl. Sci. 2020, 27, 151–156. [Google Scholar] [CrossRef]
- Bule, M.; Albelbeisi, A.H.; Nikfar, S.; Amini, M.; Abdollahi, M. The Antidiabetic and Antilipidemic Effects of Hibiscus Sabdariffa: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Food Res. Int. 2020, 130, 108980. [Google Scholar] [CrossRef]
- Zhang, B.; Yue, R.; Wang, Y.; Wang, L.; Chin, J.; Huang, X.; Jiang, Y. Effect of Hibiscus sabdariffa (Roselle) Supplementation in Regulating Blood Lipids among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis. Phyther. Res. 2020, 34, 1083–1095. [Google Scholar] [CrossRef]
- Mohanty, T.; Bhadra, P. In-Silico Analysis of Roselle (Hibiscus sabdariffa L.) for Antidiabetic. Int. Bimon. 2020, 10, 20764–20768. [Google Scholar]
- Abdul Wahab, R.; Al-obaidi, N.G.; Yahya, N.A.; Che Marzuki, N.H.; Mohd Bohari, S.P. Formulation of a Stable Water-in-Oil Nanoemulsion Rich in Anti-Diabetic Components of the Roselle Extract for Controlled Release. Chem. Pap. 2022, 76, 2341–2356. [Google Scholar] [CrossRef]
- Haidari, M.; Alami, K.; Hossaini, A.; Mousavi, S.Y. Effect of Afghan Hibiscus sabdariffa L. and Carum carvi L. Hydro-Alcoholic Extracts Either Alone or in Combination on Blood Glucose Level in Diabetic Rats. Int. J. Ayurvedic Med. 2020, 11, 759–764. [Google Scholar] [CrossRef]
- Ogo, O.A.; Gloria, O.; Bawa, I.; Emmanuel, E.U. Nutraceutical Properties of Hibiscus sabdariffa Stem and Leaf Extract as a Potential Alternative to Management of Clinical Manifestations Associated with Diabetes Mellitus Abstract. J. Nutraceuticals Food Sci. 2021, 6, 11. [Google Scholar]
- Ibrahim, K.G.; Bello, B.A.; Mainasara, A.S.; Abubakar, M.B. Effect of Co-Administration of Glibenclamide and Aqueous Calyx Extract of Hibiscus sabdariffa on Oxidative Stress Markers in Streptozotocin-Induced Diabetic Rats. Niger. J. Physiol. Sci. 2021, 36, 79–86. [Google Scholar]
- Shadhan, R.M.; Adam, Z.; Bohari, S.P.M. Hibiscus sabdariffa Linn Fruit Derivatives as Alternative Agents for Diabetes Mellitus Care: A Basic Insight. Asia-Pacific J. Mol. Biol. Biotechnol. 2021, 29, 73–83. [Google Scholar] [CrossRef]
- Oyakhire, F.; Emokpae, M.A.; Ogie, E.; Valentine, E.E. Effect of Diabetes Mellitus on the Excretory Function of the Liver. Med. Lab. Technol. J. 2021, 7, 155. [Google Scholar] [CrossRef]
- Niu, B.; Xie, X.; Xiong, X.; Jiang, J. Network Pharmacology-Based Analysis of the Anti-Hyperglycemic Active Ingredients of Roselle and Experimental Validation. Comput. Biol. Med. 2022, 141, 104636. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Ying, L.; Mohammed Yusof, N.L.; Budin, S.B. Potential of Hibiscus sabdariffa Linn. Polyphenol-Rich Extract in Improving Diabetes-Induced Vascular Functional and Structural Abnormalities in Rats. Sains Malays. 2021, 50, 1959–1970. [Google Scholar] [CrossRef]
- Indriawati, R.; Vinivera, V.; Wibowo, T. Hypoglycemic and Hypolipidemic Effects Red Rosella Flower Steeping on Diabetic Rats. Atl. Press 2021, 33, 114–118. [Google Scholar] [CrossRef]
- Rodríguez-Fierros, F.L.; Guarner-Lans, V.; Soto, M.E.; Manzano-Pech, L.; Díaz-Díaz, E.; Soria-Castro, E.; Rubio-Ruiz, M.E.; Jiménez-Trejo, F.; Pérez-Torres, I. Article Modulation of Renal Function in a Metabolic Syndrome Rat Model by Antioxidants in Hibiscus sabdariffa L. Molecules 2021, 26, 2074. [Google Scholar] [CrossRef]
- Benoite, T.; Vigasini, N. Antioxidant and Antidiabetic Activities of Ethanolic Extract of Hibiscus sabdariffa Calyx and Stevia rebaudiana Leaf. Asian J. Biol. Life Sci. 2021, 10, 217–224. [Google Scholar] [CrossRef]
- Khan, M. Nutritional and Health Importance of Hibiscus Sabdariffa: A Review and Indication for Research Needs. J. Nutr. Health Food Eng. 2017, 6, 125–128. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Lamm, M.G.; Funk, J.L.; Ritenbaugh, C. Hibiscus sabdariffa L. in the Treatment of Hypertension and Hyperlipidemia: A Comprehensive Review of Animal and Human Studies. Fitoterapia 2013, 85, 84–94. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. On Obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Olivares-Vicente, M.; Encinar, J.A.; Barrajón-Catalán, E.; Segura-Carretero, A.; Joven, J.; Micol, V. Multi-Targeted Molecular Effects of Hibiscus sabdariffa Polyphenols: An Opportunity for a Global Approach to Obesity. Nutrients 2017, 9, 907. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In Vitro Inhibitory Effects of Plant-Based Foods and Their Combinations on Intestinal α-Glucosidase and Pancreatic α-Amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as Alpha-Amylase and Alpha-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini-Reviews Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.K.; Hao, H.; Bian, Y.; Ge, Y.X.; Lu, S.; Xie, H.X.; Wang, K.M.; Tao, H.; Yuan, C.; Zhang, J.; et al. Discovery of New α-Glucosidase Inhibitors: Structure-Based Virtual Screening and Biological Evaluation. Front. Chem. 2021, 9, 639279. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-Amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.; Melzig, M.F. Medicinal Plants Traditionally Used for Treatment of Obesity and Diabetes Mellitus-Screening for Pancreatic Lipase and α-Amylase Inhibition. Phyther. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Asghari, B.; Esmaeili, M.A.; Dehghan, H.; Ghazi, I. α-Glucosidase and α-Amylase Inhibitory Effect and Antioxidant Activity of Ten Plant Extracts Traditionally Used in Iran for Diabetes. J. Med. Plants Res. 2013, 7, 257–266. [Google Scholar] [CrossRef]
- Hansawasdi, C.; Kawabata, J.; Kasai, T. Alpha-Amylase Inhibitors from Roselle (Hibiscus sabdariffa Linn.) Tea. Biosci. Biotechnol. Biochem. 2000, 64, 1041–1043. [Google Scholar] [CrossRef]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative Analysis of Hibiscus sabdariffa (Roselle) Hot and Cold Extracts in Respect to Their Potential for α-Glucosidase Inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef]
- Gondokesumo, M.E.; Kusuma, H.S.W.; Widowati, W. α-/β-Glucosidase and α-Amylase Inhibitory Activities of Roselle (Hibiscus sabdariffa L.) Ethanol Extract. Mol. Cell. Biomed. Sci. 2017, 1, 34. [Google Scholar] [CrossRef]
- Liu, T.T.; Liu, X.T.; Chen, Q.X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M. Highlighting Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e1–e8. [Google Scholar] [CrossRef]
- Siegień, J.; Buchholz, T.; Popowski, D.; Granica, S.; Osińska, E.; Melzig, M.F.; Czerwińska, M.E. Pancreatic Lipase and α-Amylase Inhibitory Activity of Extracts from Selected Plant Materials after Gastrointestinal Digestion in Vitro. Food Chem. 2021, 355, 129414. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, Z.M.R.; Khan, N.A.K.; Ali, I.M.; Dayoob, M.; Hussein, S.S. Hibiscus sabdariffa Extract as Anti-Aging Supplement through Its Antioxidant and Anti-Obesity Activities. Biomed. Res. Ther. 2020, 7, 3572–3578. [Google Scholar] [CrossRef]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. Ppars-Orchestrated Metabolic Homeostasis in the Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the Roles of PPARγ in Adipocytes via Dynamic Change of Transcription Complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Amoroso, R. Inhibition of PPARγ by Natural Compounds as a Promising Strategy in Obesity and Diabetes. Open Med. Chem. J. 2019, 13, 7–15. [Google Scholar] [CrossRef]
- Janson, B.; Prasomthong, J.; Malakul, W.; Boonsong, T.; Tunsophon, S. Hibiscus sabdariffa L. Calyx Extract Prevents the Adipogenesis of 3T3-L1 Adipocytes, and Obesity-Related Insulin Resistance in High-Fat Diet-Induced Obese Rats. Biomed. Pharmacother. 2021, 138, 111438. [Google Scholar] [CrossRef]
- Yang, M.Y.; Peng, C.H.; Chan, K.C.; Yang, Y.I.S.; Huang, C.N.; Wang, C.J. The Hypolipidemic Effect of Hibiscus sabdariffa Polyphenols via Inhibiting Lipogenesis and Promoting Hepatic Lipid Clearance. J. Agric. Food Chem. 2010, 58, 850–859. [Google Scholar] [CrossRef]
- Long, Q.; Chen, H.; Yang, W.; Yang, L.; Zhang, L. Delphinidin-3-Sambubioside from Hibiscus Sabdariffa. L Attenuates Hyperlipidemia in High Fat Diet-Induced Obese Rats and Oleic Acid-Induced Steatosis in HepG2 Cells. Bioengineered 2021, 12, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Cai, G.Y.; Chen, X.M. Clinical and Pathological Factors Associated with Progression of Diabetic Nephropathy. Nephrology 2017, 22, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Wang, C.J.; Huang, C.N.; Chen, M.L.; Chen, M.J.; Peng, C.H. Polyphenols of Hibiscus sabdariffa Improved Diabetic Nephropathy via Attenuating Renal Epithelial Mesenchymal Transition. J. Agric. Food Chem. 2013, 61, 7545–7551. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Yang, Y.S.; Chan, K.C.; Wang, C.J.; Chen, M.L.; Huang, C.N. Hibiscus sabdariffa Polyphenols Alleviate Insulin Resistance and Renal Epithelial to Mesenchymal Transition: A Novel Action Mechanism Mediated by Type 4 Dipeptidyl Peptidase. J. Agric. Food Chem. 2014, 62, 9736–9743. [Google Scholar] [CrossRef]
- Huang, C.N.; Wang, C.J.; Yang, Y.S.; Lin, C.L.; Peng, C.H. Hibiscus sabdariffa Polyphenols Prevent Palmitate-Induced Renal Epithelial Mesenchymal Transition by Alleviating Dipeptidyl Peptidase-4-Mediated Insulin Resistance. Food Funct. 2016, 7, 475–482. [Google Scholar] [CrossRef]
- Bandyk, D.F. The Diabetic Foot: Pathophysiology, Evaluation, and Treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef]
- Abbas, H.A.; Abdo, I.M.; Moustafa, M.Z. In Vitro Antibacterial and Antibiofilm Activities of Hibiscus sabdariffa L. Extract and Apple Vinegar against Bacteria Isolated from Diabetic Foot Infections. J. Pharm. Technol. 2014, 7, 131–136. [Google Scholar]
- Shadhan, R.M.; Bohari, S.P.M. Effects of Hibiscus sabdariffa Linn. Fruit Extracts on α-Glucosidase Enzyme, Glucose Diffusion and Wound Healing Activities. Asian Pac. J. Trop. Biomed. 2017, 7, 466–472. [Google Scholar] [CrossRef]
- Shadhan, R.M.; Bohari, S.P.M.; Adam, Z.; Jamaluddin, H. Hibiscus sabdariffa Linn Fruits Methanolic Extract and Fractions Mediated Glucose Uptake Stimulation and Glucose Transporter 4 Regulation. J. Pharm. Negat. Results 2018, 9, 39–43. [Google Scholar] [CrossRef]
- Sunmonu, T.O.; Lewu, F.B. Phytochemical Analysis, in Vitro Antioxidant Activity and Inhibition of Key Diabetic Enzymes by Selected Nigerian Medicinal Plants with Antidiabetic Potential. Indian J. Pharm. Educ. Res. 2019, 53, 250–260. [Google Scholar] [CrossRef]
- Nazratun Nafizah, A.H.; Budin, S.B.; Zaryantey, A.H.; Mariati, A.R.; Santhana, R.L.; Osman, M.; Muhd Hanis, M.I.; Jamaludin, M. Aqueous Calyxes Extract of Roselle or Hibiscus sabdariffa Linn Supplementation Improves Liver Morphology in Streptozotocin Induced Diabetic Rats. Arab J. Gastroenterol. 2017, 18, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.H.M.; Budin, S.B.; Osman, M.; Mohamed, J. Protective Role of Hibiscus sabdariffa Calyx Extract against Streptozotocin Induced Sperm Damage in Diabetic Rats. EXCLI J. 2012, 11, 659–669. [Google Scholar] [PubMed]
- Aba, P.E.; Nwaigwe, C.U.; Okwuagwu, F.O.; Udem, S.C.; Asuzu, I.U. Effect of Aqueous Extract of Hibiscus sabdariffa on Some Biochemical Parameters in Alloxan-Induced Diabetic Rats. Comp. Clin. Path. 2014, 23, 1675–1680. [Google Scholar] [CrossRef]
- El- Hady, A.M.; Mabrouk Gabr, N.; Ali Abbas, M. Effect of Red Hibiscus Aqueous Extract on Alloxan-Induced Diabetes in Adult Male Albino Rats. Al-Azhar Med. J. 2015, 44, 237–246. [Google Scholar] [CrossRef]
- Ajani, E.O.; Bamisaye, F.A.; Amusa, T.O.; Atolani, O.; Kola-Mustapha, A.T.; Njinga, N.S.; Quadri, L.A.; Bakare-Odunola, M.T.; Oladiji, A.T.; Kambizi, L. Roselle Hibiscus Sabdarrifa Calyces Extracts Modulates Cardiovascular Disease Risk and Kidney Dysfunctions in Diabetic Rats. Plant Arch. 2021, 21, 1350–1359. [Google Scholar] [CrossRef]
- Andraini, T.; Yolanda, S. Prevention of Insulin Resistance with Hibiscus sabdariffa Linn. Extract in High-Fructose Fed Rat. Med. J. Indones. 2014, 23, 192–196. [Google Scholar] [CrossRef]
- Bunbupha, S.; Pakdeechote, P.; Kukongviriyapan, U.; Pannangpetch, P.; Prachaney, P.; Itharat, A.; Berkban, T. Hibiscus sabdariffa extract improves insulin resistance and oxidative stress status in insulin resistant rats induced by a high fructose diet. In Proceedings of the 2012 International and National Conference for The Sustainable Community Development of “Local Community: The Foundation of Development in the ASEAN Economic Community (AEC)”, 16–19 February 2012; pp. 125–130. [Google Scholar]
- Ajiboye, T.O.; Raji, H.O.; Adeleye, A.O.; Adigun, N.S.; Giwa, O.B.; Ojewuyi, O.B.; Oladiji, A.T. Hibiscus sabdariffa Calyx Palliates Insulin Resistance, Hyperglycemia, Dyslipidemia and Oxidative Rout in Fructose-Induced Metabolic Syndrome Rats. J. Sci. Food Agric. 2016, 96, 1522–1531. [Google Scholar] [CrossRef]
- Farombi, E.O.; Ige, O.O. Hypolipidemic and Antioxidant Effects of Ethanolic Extract from Dried Calyx of Hibiscus sabdariffa in Alloxan-Induced Diabetic Rats. Fundam. Clin. Pharmacol. 2007, 21, 601–609. [Google Scholar] [CrossRef]
- Yusof, N.L.M.; Zainalabidin, S.; Fauzi, N.M.; Budin, S.B. Hibiscus sabdariffa (Roselle) Polyphenol-Rich Extract Averts Cardiac Functional and Structural Abnormalities in Type 1 Diabetic Rats. Appl. Physiol. Nutr. Metab. 2018, 43, 1224–1232. [Google Scholar] [CrossRef]
- Al-Qahtani, A.M.; Shaikh, I.A.; Habeeb, M.S. Hibiscus sabdariffa L. Extract Ameliorates the Diabetic Late Complications: Cardioprotective and Nephroprotective Effect in Streptozotocin-Induced Diabetic Rats. Int. J. Green Pharm. 2017, 11, S896–S904. [Google Scholar]
- Rosemary, R.; Haro, G. Antidiabetic Effect of Roselle Calyces Extract (Hibiscus sabdariffa L.) in Streptozotocin Induced Mice. Int. J. PharmTech Res. 2014, 6, 1703–1711. [Google Scholar]
- Seung, T.W.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Park, S.H.; Kwon, B.S.; Lee, C.J.; Kang, J.E.; Kim, D.O.; Lee, U.; et al. Ethyl Acetate Fraction from Hibiscus sabdariffa L. Attenuates Diabetes-Associated Cognitive Impairment in Mice. Food Res. Int. 2018, 105, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, D.O.; Adewole, O.S. Hibiscus sabdariffa Renews Pancreatic β-Cells in Experimental Type 1 Diabetic Model Rats. Morphologie 2019, 103, 80–93. [Google Scholar] [CrossRef]
- Kasim, R.M.; Jubaidi, F.F.; Taib, I.S.; Budin, S.B. Testicular Damage and Abnormal Sperm Characteristic Due to Chronic Hyperglycemia Exposure Restored by Polyphenol Rich Extract of Hibiscus sabdariffa Linn. J. Adv. Res. Appl. Sci. Eng. Technol. 2021, 1, 43–55. [Google Scholar]
- Lee, W.C.; Wang, C.J.; Chen, Y.H.; Hsu, J.D.; Cheng, S.Y.; Chen, H.C.; Lee, H.J. Polyphenol Extracts from Hibiscus sabdariffa Linnaeus Attenuate Nephropathy in Experimental Type 1 Diabetes. J. Agric. Food Chem. 2009, 57, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Chyau, C.C.; Chan, K.C.; Chan, T.H.; Wang, C.J.; Huang, C.N. Hibiscus sabdariffa Polyphenolic Extract Inhibits Hyperglycemia, Hyperlipidemia, and Glycation-Oxidative Stress While Improving Insulin Resistance. J. Agric. Food Chem. 2011, 59, 9901–9909. [Google Scholar] [CrossRef]
- Zainalabidin, S.; Budin, S.B.; Anuar, N.N.M.; Yusoff, N.A.; Yusof, N.L.M. Hibiscus sabdariffa Linn. Improves the Aortic Damage in Diabetic Rats by Acting as Antioxidant. J. Appl. Pharm. Sci. 2018, 8, 108–114. [Google Scholar] [CrossRef]
- Budin, S.B.; Abdul Rahman, W.Z.; Jubaidi, F.F.; Mohammed Yusof, N.L.; Taib, I.S.; Zainalabidin, S. Roselle (Hibiscus sabdiriffa) Polyphenol-Rich Extract Prevents Testicular Damage of Diabetic Rats. J. Appl. Pharm. Sci. 2018, 8, 065–070. [Google Scholar] [CrossRef]
- Yusof, N.L.M.; Jubaidi, F.F.; Nasir, S.N.M.; Yusoff, N.A.; Fauzi, N.M.; Zainalabidin, S.; Budin, S.B. Hibiscus sabdariffa (Roselle) Polyphenol-Rich Extract Prevents the Aortic Oxidative Damage in Type 1 Diabetic Rats. J. Teknol. 2018, 80, 1–8. [Google Scholar] [CrossRef]
- Yusof, N.L.M.; Tengku Affendi, T.N.T.; Jubaidi, F.F.; Abidin, S.Z.; Budin, S.B. Hibiscus sabdariffa Linn. (Roselle) Polyphenols-Rich Extract Prevents Hyperglycemia-Induced Cardiac Oxidative Stress and Mitochondrial Damage in Diabetic Rats. Sains Malays. 2020, 49, 2499–2506. [Google Scholar] [CrossRef]
- Ojewumi, A.W.; Kadiri, M. Physiological Evaluation of the Anti-Diabetic Properties of Hibiscus sabdariffa on Rats. J. Nat. Sci. Eng. Technol. 2013, 12, 50–61. [Google Scholar]
- Agoreyo, F.O.; Agoreyo, B.O.; Onuorah, M. Effect of Aqueous Extracts of Hibiscus sabdariffa and Zingiber Officinale on Blood Cholesterol and Glucose Levels of Rats. Afr. J. Biotechnol. 2008, 7, 3949–3951. [Google Scholar]
- Sunaryo, H.; Hikmawanti, N.P.E.; Listyaningrum, H.A. Study in Activity Combination of Physalis Angulata and Hibiscus sabdariffa in 70% Ethanol Extract to Decrease Blood Sugar Levels and Histopathology of Pancreas Langerhans Island in Alloxan Induced Diabetic Rats. In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), East Jakarta, Indonesia, 10–11 August 2018; pp. 117–122. [Google Scholar] [CrossRef]
- Su, N.; Li, J.; Yang, L.; Hou, G.; Ye, M. Hypoglycemic and Hypolipidemic Effects of Fermented Milks with Added Roselle (Hibiscus sabdariffa L.) Extract. J. Funct. Foods 2018, 43, 234–241. [Google Scholar] [CrossRef]
- Wihansah, R.R.S.; Arief, I.I.; Batubara, I. Anti-Diabetic Potency and Characteristics of Probiotic Goat-Milk Yogurt Supplemented with Roselle Extract during Cold Storage. Trop. Anim. Sci. J. 2018, 41, 191–199. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Całyniuk, B.; Grochowska-Niedworok, E.; Walkiewicz, K.; Kawecka, S.; Popiołek, E.; Fatyga, E. Malondialdehyde (MDA)—Product of Lipid Peroxidation as Marker of Homeostasis Disorders and Aging. Ann. Acad. Med. Silesiensis 2016, 70, 224–228. [Google Scholar] [CrossRef]
- Herdiani, N.; Wikurendra, E.A. Effect of Roselle Petal Extract on Decreased Levels of MDA In Rats with Type 2 Diabetes. J. Health Sci. 2021, 14, 48–52. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular Pathways Associated with Oxidative Stress in Diabetes Mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Sini, J.M.; Umar, I.A.; Inuwa, H.M. The Beneficial Effects of Extracts of Hibiscus sabdariffa Calyces in Alloxan-Diabetic Rats: Reduction of Free-Radical Load and Enhancement of Antioxidant Status. J. Pharmacogn. Phyther. 2011, 3, 141–149. [Google Scholar] [CrossRef]
- Saberzadeh-Ardestani, B.; Karamzadeh, R.; Basiri, M.; Hajizadeh-Saffar, E.; Farhadi, A.; Shapiro, A.M.J.; Tahamtani, Y.; Baharvand, H. Type 1 Diabetes Mellitus: Cellular and Molecular Pathophysiology at a Glance. Cell J. 2018, 20, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Gaglia, J.; Bonner-Weir Joslin, S. Inadequate β-Cell Mass Is Essential for the Pathogenesis of Type 2 Diabetes. Lancet Diabetes Endocrinol. 2020, 8, 249–256. [Google Scholar] [CrossRef]
- Marselli, L.; Suleiman, M.; Masini, M.; Campani, D.; Bugliani, M.; Syed, F.; Martino, L.; Focosi, D.; Scatena, F.; Olimpico, F.; et al. Are We Overestimating the Loss of Beta Cells in Type 2 Diabetes? Diabetologia 2014, 57, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Wisetmuen, E.; Pannangpetch, P.; Kongyingyoes, B.; Kukongviriyapan, U.; Yutanawiboonchai, W.; Itharat, A. Insulin Secretion Enhancing Activity of Roselle Calyx Extract in Normal and Streptozotocin-Induced Diabetic Rats. Pharmacogn. Res. 2013, 5, 65–70. [Google Scholar] [CrossRef]
- Kartinah, N.T.; Fadilah, F.; Ibrahim, E.I.; Suryati, Y. The Potential of Hibiscus sabdariffa Linn in Inducing Glucagon-Like Peptide-1 via SGLT-1 and GLPR in DM Rats. Biomed Res. Int. 2019, 2019, 8724824. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Bahiru, E.; Hsiao, R.; Phillipson, D.; Watson, K.E. Mechanisms and Treatment of Dyslipidemia in Diabetes. Curr. Cardiol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef]
- Lazarte, J.; Hegele, R.A. Dyslipidemia Management in Adults with Diabetes. Can. J. Diabetes 2020, 44, 53–60. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative Stress, Insulin Resistance, Dyslipidemia and Type 2 Diabetes Mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef]
- Wang, S.C.; Lee, S.F.; Wang, C.J.; Lee, C.H.; Lee, W.C.; Lee, H.J. Aqueous Extract from Hibiscus sabdariffa Linnaeus Ameliorate Diabetic Nephropathy via Regulating Oxidative Status and Akt/Bad/14-3-3γ in an Experimental Animal Model. Evid.-Based Complement. Altern. Med. 2011, 2011, 938126. [Google Scholar] [CrossRef]
- Gamel, A.M.I.E.; Aboraya, A.O. Effect of Hibiscus sabdariffa and Ceratonia Silique Aqueous Extracts on the Reproductive Hormones of Diabetic Male Rats. Home Econ. J. 2020, 36, 1–20. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver Glucose Metabolism in Humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Dentin, R.; Girard, J. Role of the Liver in the Control of Carbohydrate and Lipid Homeostasis. Diabetes Metab. 2004, 30, 398–408. [Google Scholar] [CrossRef]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The Role of Oxidative Stress and Inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Biology, Pathophysiology, and Therapeutics. Clin. Liver Dis. 2020, 15, 91–94. [Google Scholar] [CrossRef]
- Adeyemi, D.O.; Ukwenya, V.O.; Obuotor, E.M.; Adewole, S.O. Anti-Hepatotoxic Activities of Hibiscus sabdariffa L. In Animal Model of Streptozotocin Diabetes-Induced Liver Damage. BMC Complementary Altern. Med. 2014, 14, 277. [Google Scholar] [CrossRef]
- Warren, A.M.; Knudsen, S.T.; Cooper, M.E. Diabetic Nephropathy: An Insight into Molecular Mechanisms and Emerging Therapies. Expert Opin. Ther. Targets 2019, 23, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Wahba, N.; Mahmoud, M.; Zakaria, M. Hibiscus sabdariffa Extract Augments the Renoprotective Effect of Lisinopril against Streptozotocin-Induced Diabetic Nephropathy in Rats. Zagazig J. Pharm. Sci. 2016, 25, 47–67. [Google Scholar] [CrossRef]
- Yang, Y.S.; Huang, C.N.; Wang, C.J.; Lee, Y.J.; Chen, M.L.; Peng, C.H. Polyphenols of Hibiscus sabdariffa Improved Diabetic Nephropathy via Regulating the Pathogenic Markers and Kidney Functions of Type 2 Diabetic Rats. J. Funct. Foods 2013, 5, 810–819. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Luis-Rodríguez, D.; Martín-Núñez, E.; Tagua, V.G.; Hernández-Carballo, C.; Ferri, C.; Rodríguez-Rodríguez, A.E.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory Targets in Diabetic Nephropathy. J. Clin. Med. 2020, 9, 458. [Google Scholar] [CrossRef]
- Leon, B.M. Diabetes and Cardiovascular Disease: Epidemiology, Biological Mechanisms, Treatment Recommendations and Future Research. World J. Diabetes 2015, 6, 1246. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldánius, P.M. Diabetes, Cardiovascular Disease and the Microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of Angiotensin Convertin Enzyme (ACE) Activity by the Anthocyanins Delphinidin- and Cyanidin-3-O-Sambubiosides from Hibiscus Sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Wahba, N.; Mahmoud, A.A.A.; Mahmoud, M.F.; Zakaria, M. Hibiscus sabdariffa Extract Alleviates Vascular Complications in Streptozotocin-Induced Diabetic Rats. Zagazig J. Pharm. Sci. 2016, 25, 12–29. [Google Scholar] [CrossRef]
- Mohamed, J.; Shing, S.W.; Md Idris, M.H.; Budin, S.B.; Zainalabidin, S. The Protective Effect of Aqueous Extracts of Roselle (Hibiscus sabdariffa L. UKMR-2) against Red Blood Cell Membrane Oxidative Stress in Rats with Streptozotocin-Induced Diabetes. Clinics 2013, 68, 1358–1363. [Google Scholar] [CrossRef]
- Huaysrichan, W.; Prachaney, P.; Kongyingyoes, B.; Kukongviriyapan, U.; Itharat, A.; Pannangpetch, P. Hibiscus sabdariffa Linn. Calyx Extraction and Gallic Acid Improving Cardiac Diastolic Dysfunction in High Fat Diet-STZ-Induced Type 2 Diabetic Rats. Asia-Pacific J. Sci. Technol. 2017, 22, APST-22-02-09. [Google Scholar]
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; de Kretser, D.M.; Linn, T. Diabetes-Induced Hyperglycemia Impairs Male Reproductive Function: A Systematic Review. Hum. Reprod. Update 2018, 24, 86–105. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Vignera, S.L.; Mongioì, L.M.; Alamo, A.; Calogero, A.E. Diabetes Mellitus and Infertility: Different Pathophysiological Effects in Type 1 and Type 2 on Sperm Function. Front. Endocrinol. 2018, 9, 268. [Google Scholar] [CrossRef]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving It: A Review. J. Clin. Diagnostic Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Silva, A.A.N.; Silva, V.A. Phytotherapy in Reducing Glycemic Index and Testicular Oxidative Stress Resulting from Induced Diabetes: A Review. Braz. J. Biol. 2017, 77, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Puchai, C.; Kokmas, W.; Kruevaisayawan, H.; Sakara, T.; Onrawee, K. Effect of Hibiscus sabdariffa on Sperm Quality and Testicular Oxidative Stress in Rats Fed with High Fat Diet. J. Physiol. Biomed. Sci. Available 2019, 32, 36–41. [Google Scholar]
- Carreau, A.M.; Baillargeon, J.P. PCOS in Adolescence and Type 2 Diabetes. Curr. Diab. Rep. 2015, 15, 564. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, J.; Goodarzi, M.O. Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes 2021, 70, 627–637. [Google Scholar] [CrossRef]
- Nolan, C.J.; Prentki, M. Insulin Resistance and Insulin Hypersecretion in the Metabolic Syndrome and Type 2 Diabetes: Time for a Conceptual Framework Shift. Diabetes Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diab. Rep. 2016, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of Gut Microbiota on Diabetes Mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Burcelin, R.; Serino, M.; Chabo, C.; Blasco-Baque, V.; Amar, J. Gut Microbiota and Diabetes: From Pathogenesis to Therapeutic Perspective. Acta Diabetol. 2011, 48, 257–273. [Google Scholar] [CrossRef]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of Gut Microbiota in Adult Patients with Type 2 Diabetes and Healthy Individuals. Microb. Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Vezza, T.; Rodríguez-Nogales, A.; Ruiz-Malagón, A.J.; Hidalgo-García, L.; Garrido-Mesa, J.; Molina-Tijeras, J.A.; Romero, M.; Robles-Vera, I.; Pimentel-Moral, S.; et al. The Prebiotic Properties of Hibiscus sabdariffa Extract Contribute to the Beneficial Effects in Diet-Induced Obesity in Mice. Food Res. Int. 2020, 127, 108722. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Potter, V.J.; Karamichos, D.; Lee, D.J. Ocular Complications of Diabetes and Therapeutic Approaches. Biomed. Res. Int. 2016, 2016, 3801570. [Google Scholar] [CrossRef] [PubMed]
- Ozkol, H.U.; Koyuncu, I.; Tuluce, Y.; Dilsiz, N.; Soral, S.; Ozkol, H. Anthocyanin-Rich Extract from Hibiscus sabdariffa Calyx Counteracts UVC-Caused Impairments in Rats. Pharm. Biol. 2015, 53, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.A.; Shda, A.-M.H.; Gamal, E.-H.A.; Raied, F.; Masmal, A.M. Consumption of Hibiscus Reduces the Quality of Ocular Tear Film in Normal Eye Subjects X-Ray Crystallography View Project Organolithiums View Project. EC Ophthalmol. 2021, 12, 10–19. [Google Scholar]
- Tsado, A.N.; Onukogu, S.C.; Suleiman, A.; Mustapha, A.; Osuigwe, E.C.; Dannana, L.W.; Alawode, R.A.; Lawal, B.; Berinyuy, B.E. Phytochemicals, Hypoglycemic and Hypolipidemic Effects of Methanol Leaf Extract of Hibiscus sabdariffa in Alloxan Induced Diabetic Rats. GSC Biol. Pharm. Sci. 2019, 8, 070–078. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Ahadi, Z.; Barzegar, K. The Effect of Green Tea and Sour Tea on Blood Pressure of Patients with Type 2 Diabetes: A Randomized Clinical Trial. J. Diet. Suppl. 2013, 10, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Gurrola-Díaz, C.M.; García-López, P.M.; Sánchez-Enríquez, S.; Troyo-Sanromán, R.; Andrade-González, I.; Gómez-Leyva, J.F. Effects of Hibiscus sabdariffa Extract Powder and Preventive Treatment (Diet) on the Lipid Profiles of Patients with Metabolic Syndrome (MeSy). Phytomedicine 2010, 17, 500–505. [Google Scholar] [CrossRef]
- Harrison, A.P.; Cooper, R.G.; Suliman, M.A.; Alalami, U. The Efficacy of Karkadeh Tea in Controlling Post-Prandial Blood Glucose Levels. Am. J. Pharmacol. Toxicol. 2009, 4, 151–157. [Google Scholar] [CrossRef][Green Version]
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B.; Afkhami-Ardekani, M.; Fatehi, F. Effects of Sour Tea (Hibiscus sabdariffa) on Lipid Profile and Lipoproteins in Patients with Type II Diabetes. J. Altern. Complement. Med. 2009, 15, 899–903. [Google Scholar] [CrossRef]
- Yusni, Y.; Meutia, F. Action Mechanism of Rosella (Hibiscus sabdariffa L.) Used to Treat Metabolic Syndrome in Elderly Women. Evid.-Based Complement. Altern. Med. 2020, 2020, 5351318. [Google Scholar] [CrossRef]
- Sarbini, D.W.I.; Huriyati, E.M.Y.; Sadewa, H.; Wahyuningsih, M.S.H. The Effect of Rosella (Hibiscus sabdariffa Linn) on Insulin Resistance in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Int. J. Pharm. Res. 2019, 11, 547–557. [Google Scholar]
- Mozaffari-Khosravi, H.; Ahadi, Z.; Tafti, M.F. The Effect of Green Tea versus Sour Tea on Insulin Resistance, Lipids Profiles and Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Iran. J. Med. Sci. 2014, 39, 424–432. [Google Scholar]
- Tsabang, N.; Yedjou, C.; Tsambang, L.; Tchinda, A.; Donfagsiteli, N.; Agbor, G.; Tchounwou, P.; Nkongmeneck, B. Treatment of Diabetes and/or Hypertension Using Medicinal Plants in Cameroon. Med. Aromat. Plants 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Asgary, S.; Soltani, R.; Zolghadr, M.; Keshvari, M.; Sarrafzadegan, N. Evaluation of the Effects of Roselle (Hibiscus sabdariffa L.) on Oxidative Stress and Serum Levels of Lipids, Insulin and Hs-CRP in Adult Patients with Metabolic Syndrome: A Double-Blind Placebo-Controlled Clinical Trial. J. Complementary Integr. Med. 2016, 13, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Mayasari, N.R.; Susetyowati; Wahyuningsih, M.S.H.; Probosuseno. Antidiabetic Effect of Rosella-Stevia Tea on Prediabetic Women in Yogyakarta, Indonesia. J. Am. Coll. Nutr. 2018, 37, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, A.R.; Mardiah; Taniwiryono, D. Formulation of Ready to Drink (Rtd) Made from Roselle (Hibiscus sabdariffa. L.) Tea and Stevia (Stevia sebaudiana) Leaf Safe for Diabetics. Indones. J. Appl. Res. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Abubakar, S.M.; Ukeyima, M.T.; Spencer, J.P.E.; Lovegrove, J.A. Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans. Nutrients 2019, 11, 341. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B.A.; Afkhami-Ardekani, M.; Fatehi, F.; Noori-Shadkam, M. The Effects of Sour Tea (Hibiscus sabdariffa) on Hypertension in Patients with Type II Diabetes. J. Hum. Hypertens. 2009, 23, 48–54. [Google Scholar] [CrossRef]
- Serna, A.; Marhuenda, J.; Arcusa, R.; Pérez-Piñero, S.; Sánchez-Macarro, M.; García-Muñoz, A.M.; Victoria-Montesinos, D.; Cánovas, F.; López-Román, F.J. Effectiveness of a Polyphenolic Extract (Lippia citriodora and Hibiscus sabdariffa) on Appetite Regulation in Overweight and Obese Grade I Population: An 8-Week Randomized, Double-Blind, Cross-over, Placebo-Controlled Trial. Eur. J. Nutr. 2022, 61, 825–841. [Google Scholar] [CrossRef]
- Fan, D.; Li, L.; Li, Z.; Zhang, Y.; Ma, X.; Wu, L.; Zhang, H.; Guo, F. Biosynthesis of Selenium Nanoparticles and Their Protective, Antioxidative Effects in Streptozotocin Induced Diabetic Rats. Sci. Technol. Adv. Mater. 2020, 21, 505–514. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green Synthesis of Zinc Oxide Nanoparticles Using Hibiscus Subdariffa Leaf Extract: Effect of Temperature on Synthesis, Anti-Bacterial Activity and Anti-Diabetic Activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
| Chemical Groups | Active Compounds | References |
|---|---|---|
| Anthocyanins 1 | delphinidin-3-sambubioside cyanidin-3-sambubioside | [2,7,18] |
| Flavonoids 1 | quercetin, hibiscetin (hibiscetin-3-glucoside), sabdaritrin, gossypitrin, and other gossypetin glycosides, luteolin | [2,7,18,23] |
| Phenolic acids 1 | chlorogenic acid, protocatechuic acid, caffeic acid | [2,7,18,23] |
| Tannins 1 | no specific name indicated | [18,21] |
| Non-phenolic organic acids | hibiscus acid, hydroxy citric acid, malic acid, ascorbic acid, oxalic acid, succinic acid, tartaric acid, arachidic acid, citric acid | [2,7,18,22] |
| Triterpenoids | α-amyrin, lupeol | [24] |
| Polysaccharides (Sugars) | galactose, galacturonic acid, rhamnose, arabinose, glucose, mannose, xylose, pectins | [7,23] |
| Others | calcium, magnesium, iron, trace elements, and vitamins | [4,5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamrozik, D.; Borymska, W.; Kaczmarczyk-Żebrowska, I. Hibiscus sabdariffa in Diabetes Prevention and Treatment—Does It Work? An Evidence-Based Review. Foods 2022, 11, 2134. https://doi.org/10.3390/foods11142134
Jamrozik D, Borymska W, Kaczmarczyk-Żebrowska I. Hibiscus sabdariffa in Diabetes Prevention and Treatment—Does It Work? An Evidence-Based Review. Foods. 2022; 11(14):2134. https://doi.org/10.3390/foods11142134
Chicago/Turabian StyleJamrozik, Daniel, Weronika Borymska, and Ilona Kaczmarczyk-Żebrowska. 2022. "Hibiscus sabdariffa in Diabetes Prevention and Treatment—Does It Work? An Evidence-Based Review" Foods 11, no. 14: 2134. https://doi.org/10.3390/foods11142134
APA StyleJamrozik, D., Borymska, W., & Kaczmarczyk-Żebrowska, I. (2022). Hibiscus sabdariffa in Diabetes Prevention and Treatment—Does It Work? An Evidence-Based Review. Foods, 11(14), 2134. https://doi.org/10.3390/foods11142134






