Effects of Germination and Popping on the Anti-Nutritional Compounds and the Digestibility of Amaranthus hypochondriacus Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Popping and Germination
2.4. Seeds Size and Weight
2.5. Proximate Analysis
2.6. GC-MS Determination of the Fatty Acid Profile
2.7. Mineral’s Determination
2.8. In Vitro Protein Digestibility
2.9. Anti-Nutritional Compounds
2.10. Statistical Analysis
3. Results
3.1. Seed Size and Weight
3.2. Proximate Analysis
3.3. GC-MS Determination of the Fatty Acid Profile
3.4. Mineral Content Determination
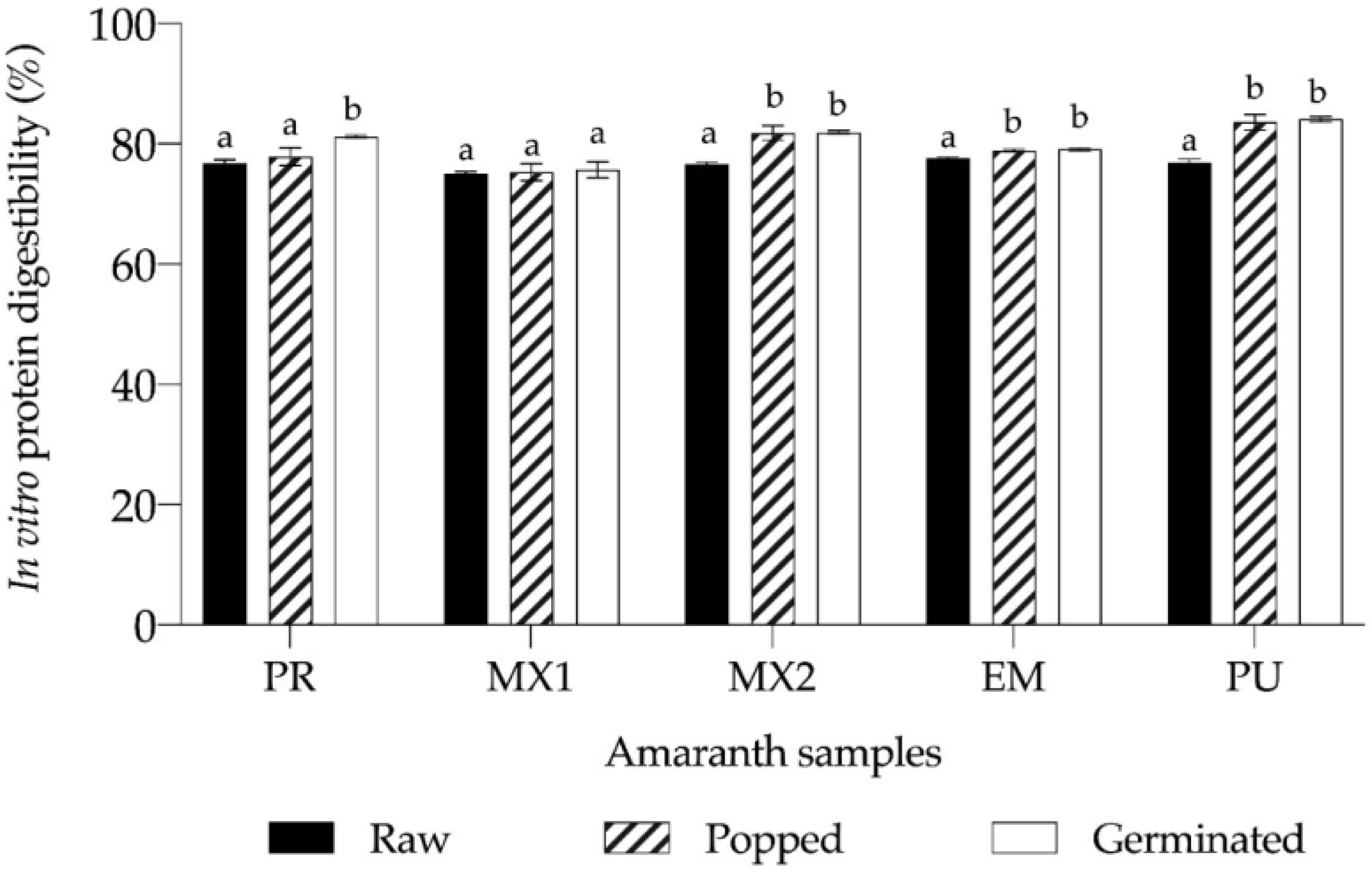
3.5. Digestibility
3.6. Anti-Nutritional Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Venskutonis, P.R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Nutrients and antinutrient constituents of Amaranthus caudatus L. Cultivated on different soils. Saudi J. Biol. Sci. 2020, 27, 3570–3580. [Google Scholar] [CrossRef]
- Alegbejo, J.O. Nutritional Value and Utilization of Amaranthus (Amaranthus spp.)–A Review. Bayero J. Pure Appl. Sci. 2014, 6, 136. [Google Scholar] [CrossRef]
- Gamel, T.H.; Linssen, J.P.; Mesallam, A.S.; Damir, A.A.; Shekib, L.A. Effect of seed treatments on the chemical composition of two amaranth species: Oil, sugars, fibres, minerals and vitamins. J. Sci. Food Agric. 2005, 86, 82–89. [Google Scholar] [CrossRef]
- Murakami, T.; Yutani, A.; Yamano, T.; Iyota, H.; Konishi, Y. Effects of Popping on Nutrient Contents of Amaranth Seed. Plant Foods Hum. Nutr. 2014, 69, 25–29. [Google Scholar] [CrossRef]
- El Anany, A.M. Nutritional Composition, Antinutritional Factors, Bioactive Compounds and Antioxidant Activity of Guava Seeds (Psidium Myrtaceae) as Affected by Roasting Processes. J. Food Sci. Technol. 2015, 52, 2175–2183. [Google Scholar] [CrossRef]
- Koch, W.; Czop, M.; Iłowiecka, K.; Nawrocka, A.; Wiącek, D. Dietary Intake of Toxic Heavy Metals with Major Groups of Food Products—Results of Analytical Determinations. Nutrients 2022, 14, 1626. [Google Scholar] [CrossRef]
- Akande, K.; Doma, U.; Agu, H.; Adamu, H. Major Antinutrients Found in Plant Protein Sources: Their Effect on Nutrition. Pak. J. Nutr. 2010, 9, 827–832. [Google Scholar] [CrossRef]
- Valadez-Vega, C.; Lugo-Magaña, O.; Morales-González, J.A.; Delgado-Olivares, L.; Izquierdo-Vega, J.A.; Sánchez-Gutiérrez, M.; López-Contreras, L.; Bautista, M.; Velázquez-González, C. Phytochemical, cytotoxic, and genotoxic evaluation of protein extract of Amaranthus hypochondriacus seeds. CyTA-J. Food 2021, 19, 701–709. [Google Scholar] [CrossRef]
- Ensminger, L.G. The Association of Official Analytical Chemists. Clin. Toxicol. 1976, 9, 471. [Google Scholar] [CrossRef]
- Amare, E.; Mouquet-Rivier, C.; Rochette, I.; Adish, A.; Haki, G.D. Effect of popping and fermentation on proximate composition, minerals and absorption inhibitors, and mineral bioavailability of Amaranthus caudatus grain cultivated in Ethiopia. J. Food Sci. Technol. 2016, 53, 2987–2994. [Google Scholar] [CrossRef]
- Crowley, J.F.; Goldstein, I.J. Datura Stramonium Lectin. Methods Enzymol. 1982, 83, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Martinez, T.F.; Moyano, F.J. Determination of Trypsin Inhibitor Activity of Soy Products: A Collaborative Analysis of an Improved Procedure. J. Sci. Food Agric. 2003, 83. [Google Scholar] [CrossRef]
- Giron-Martinez, M.C. Determinacion Semicuantitativa de Saponinas en Muestras Vegetales Aprovechando Su Capacidad Hemolitica; Universidad Nacional Autónoma de México: Mexico City, Mexico, 1992. [Google Scholar]
- Butler, G. The distribution of the cyanoglucosides linamarin and lotaustralin in higher plants. Phytochemistry 1965, 4, 127–131. [Google Scholar] [CrossRef]
- Gamel, T.H.; Linssen, J.P.; Mesallem, A.S.; Damir, A.A.; Shekib, L.A. Effect of seed treatments on the chemical composition and properties of two amaranth species: Starch and protein. J. Sci. Food Agric. 2005, 85, 319–327. [Google Scholar] [CrossRef]
- Abalone, R.; Cassinera, A.; Gastón, A.; Lara, M. Some Physical Properties of Amaranth Seeds. Biosyst. Eng. 2004, 89, 109–117. [Google Scholar] [CrossRef]
- De Bock, P.; Daelemans, L.; Selis, L.; Raes, K.; Vermeir, P.; Eeckhout, M.; Van Bockstaele, F. Comparison of the Chemical and Technological Characteristics of Wholemeal Flours Obtained from Amaranth (Amaranthus sp.), Quinoa (Chenopodium quinoa) and Buckwheat (Fagopyrum sp.) Seeds. Foods 2021, 10, 651. [Google Scholar] [CrossRef]
- Oteri, M.; Gresta, F.; Costale, A.; Lo Presti, V.; Meineri, G.; Chiofalo, B. Amaranthus Hypochondriacus l. As a Sustainable Source of Nutrients and Bioactive Compounds for Animal Feeding. Antioxidants 2021, 10, 876. [Google Scholar] [CrossRef]
- Szabóová, M.; Záhorský, M.; Gažo, J.; Geuens, J.; Vermoesen, A.; D’hondt, E.; Hricová, A. Differences in Seed Weight, Amino Acid, Fatty Acid, Oil, and Squalene Content in γ-Irradiation-Developed and Commercial Amaranth Varieties (Amaranthus spp.). Plants 2020, 9, 1412. [Google Scholar] [CrossRef]
- Valdez, G.; Uscanga, E.; Kohashi, J.; García, R.; Martínez, D.; Torres, J.; García, A. Tamaño De Semilla, Granulometría Del Sustrato Y Profundidad De Siembra En El Vigor De Semilla Y Plántula De Dos Malezas. Agrociencia 2015, 49, 899–915. [Google Scholar]
- Capriles, V.D.; Coelho, K.D.; Guerra-Matias, A.C.; Arêas, J.A.G. Effects of Processing Methods on Amaranth Starch Digestibility and Predicted Glycemic Index. J. Food Sci. 2008, 73, H160–H164. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, V. The Revival of Amaranth as a Third-Millennium Food. Neuroendocrinol. Lett. 2012, 33 (Suppl. 3), 3–7. [Google Scholar]
- Aguilar, E.G.; de Jesús Albarracín, G.; Uñates, M.A.; Piola, H.D.; Camiña, J.M.; Escudero, N.L. Evaluation of the Nutritional Quality of the Grain Protein of New Amaranths Varieties. Plant Foods Hum. Nutr. 2015, 70, 21–26. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value of Pseudocereals and Their Increasing Use as Functional Gluten-Free Ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Krulj, J.; Brlek, T.; Pezo, L.; Brkljača, J.; Popović, S.; Zeković, Z.; Bodroža Solarov, M. Extraction Methods of Amaranthus Sp. Grain Oil Isolation. J. Sci. Food Agric. 2016, 96, 3552–3558. [Google Scholar] [CrossRef]
- Acar, N.; Vohra, P.; Becker, R.; Hanners, G.D.; Saunders, R.M. Nutritional Evaluation of Grain Amaranth for Growing Chickens. Poult. Sci. 1988, 67, 1166–1173. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and Health Benefits of Soy Proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in Quinoa and Amaranth Grains and Their Antioxidant, Anti-Inflammatory, and Potential Health Beneficial Effects: A Review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Srivastava, J.; Singh, N.; Singh, S.P. Mineral Profile and Variability in Vegetable Amaranth (Amaranthus Tricolor). Plant Foods Hum. Nutr. 2006, 61, 23–28. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Nutrients, Minerals, Pigments, Phytochemicals, and Radical Scavenging Activity in Amaranthus Blitum Leafy Vegetables. Sci. Rep. 2020, 10, 3868. [Google Scholar] [CrossRef]
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Gohara, A.K.; Souza, A.H.P.; de Souza, N.E.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Evaluation of Nutritional Compounds in New Amaranth and Quinoa Cultivars. Food Sci. Technol. 2013, 33, 339–344. [Google Scholar] [CrossRef]
- Arêas, J.A.G.; Carlos-Menezes, A.C.C.C.; Soares, R.A.M. Amaranth. Encycl. Food Health 2015, 135–140. [Google Scholar] [CrossRef]
- Bozorov, S.S.; Berdiev, N.S.; Ishimov, U.J.; Olimjonov, S.S.; Ziyavitdinov, J.F.; Asrorov, A.M.; Salikhov, S.I. Chemical Composition and Biological Activity of Seed Oil of Amaranth Varieties. Nov. Biotechnol. Chim. 2018, 17, 66–73. [Google Scholar] [CrossRef]
- Martirosyan, D.M.; Miroshnichenko, L.A.; Kulakova, S.N.; Pogojeva, A.V.; Zoloedov, V.I. Amaranth Oil Application for Coronary Heart Disease and Hypertension. Lipids Health Dis. 2007, 6, 1. [Google Scholar] [CrossRef][Green Version]
- Gupta, C.; Sehgal, S. Development, Acceptability and Nutritional Value of Weaning Mixtures. Plant Foods Hum. Nutr. 1991, 41, 107–116. [Google Scholar] [CrossRef]
- Guzmán-Maldonado, H.; Paredes-López, O. Production of High-Protein Flour and Maltodextrins from Amaranth Grain. Process Biochem. 1994, 29, 289–293. [Google Scholar] [CrossRef]
- Muyonga, J.; Cole, C.G.; Duodu, K. Extraction and Physico-Chemical Characterisation of Nile Perch (Lates Niloticus) Skin and Bone Gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Ramesh, D.; Prakash, J. Nutritional and Functional Properties of Amaranth Grain Flour Fractions Obtained by Differential Sieving. Prog. Chem. Biochem. Res. 2020, 2020. [Google Scholar] [CrossRef]
- Najdi Hejazi, S.; Orsat, V.; Azadi, B.; Kubow, S. Improvement of the in Vitro Protein Digestibility of Amaranth Grain through Optimization of the Malting Process. J. Cereal Sci. 2016, 68, 59–65. [Google Scholar] [CrossRef]
- Bressani, R.; Kalinowski, L.S.; Ortiz, M.A.; Elias, L.G. Nutritional Evaluation of Roasted, Flaked and Popped. Arch. Latinoam. Nutr. 1987, 37, 525–531. [Google Scholar] [PubMed]
- Grundy, M.M.L.; Momanyi, D.K.; Holland, C.; Kawaka, F.; Tan, S.; Salim, M.; Boyd, B.J.; Bajka, B.; Mulet-Cabero, A.I.; Bishop, J.; et al. Effects of Grain Source and Processing Methods on the Nutritional Profile and Digestibility of Grain Amaranth. J. Funct. Foods 2020, 72, 104065. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Andabati, B.; Ssepuuya, G. Effect of Heat Processing on Selected Grain Amaranth Physicochemical Properties. Food Sci. Nutr. 2014, 2, 9–16. [Google Scholar] [CrossRef]
- Lara, N.; Ruales, J. Popping of Amaranth Grain (Amaranthus Caudatus) and Its Effect on the Functional, Nutritional and Sensory Properties. J. Sci. Food Agric. 2002, 82, 797–805. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Chemical Modifications of Peptides and Their Impact on Food Properties. Chem. Rev. 2011, 111, 7876–7903. [Google Scholar] [CrossRef] [PubMed]
- Olawoye, B.T.; Gbadamosi, S.O. Effect of Different Treatments on in Vitro Protein Digestibility, Antinutrients, Antioxidant Properties and Mineral Composition of Amaranthus Viridis Seed. Cogent Food Agric. 2017, 3, 1296402. [Google Scholar] [CrossRef]
- Cornejo, F.; Novillo, G.; Villacrés, E.; Rosell, C.M. Evaluation of the Physicochemical and Nutritional Changes in Two Amaranth Species (Amaranthus Quitensis and Amaranthus Caudatus) after Germination. Food Res. Int. 2019, 121, 933–939. [Google Scholar] [CrossRef]
- Chaparro Rojas, D.C.; Pismag Portilla, R.Y.; Elizalde Correa, A.; Vivas Quila, N.J.; Erazo Caicedo, C.A. Efecto de la germinación sobre el contenido y digestibilidad de proteína en semillas de amaranto, quinua, soya y guandul. Rev. Bio Agro 2010, 8, 35–42. [Google Scholar]
- Mengs, U. Toxic Effects of Sennosides in Laboratory Animals and in Vitro. Pharmacology 1988, 36 (Suppl. 1), 180–187. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D. Antinutrients in Plant-Based Foods: A Review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef]
- Ozeki, M.; Kamemura, K.; Moriyama, K.; Itoh, Y.; Furuichi, Y.; Umekawa, H.; Takahashi, T. Purification and Characterization of a Lectin from Amaranthus Hypochondriacus Var. Mexico Seeds. Biosci. Biotechnol. Biochem. 1996, 60, 2048–2051. [Google Scholar] [CrossRef] [PubMed]
- Santaella-Verdejo, A.; Gallegos, B.; Pérez-Campos, E.; Hernández, P.; Zenteno, E. Use of Amaranthus Leucocarpus Lectin to Differentiate Cervical Dysplasia (CIN). Prep. Biochem. Biotechnol. 2007, 37, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gamel, T.H.; Linssen, J.P.; Mesallam, A.S.; Damir, A.A.; Shekib, L.A. Seed Treatments Affect Functional and Antinutritional Properties of Amaranth Flours. J. Sci. Food Agric. 2006, 86, 1095–1102. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; González De Mejía, E.; Loarca-Piña, G. Purification and Acute Toxicity of a Lectin Extracted from Tepary Bean (Phaseolus Acutifolius). Food Chem. Toxicol. 2003, 41, 21–27. [Google Scholar] [CrossRef]
- Valadez-Vega, C.; Guzmán-Partida, A.M.; Soto-Cordova, F.J.; Álvarez-Manilla, G.; Morales-González, J.A.; Madrigal-Santillán, E.; Villagómez-Ibarra, J.R.; Zúniga-Pérez, C.; Gutiérrez-Salinas, J.; Becerril-Flores, M.A. Purification, Biochemical Characterization, and Bioactive Properties of a Lectin Purified from the Seeds of White Tepary Bean (Phaseolus Acutifolius Variety Latifolius). Molecules 2011, 16, 2561–2582. [Google Scholar] [CrossRef]
- Peumans, W.J.; Van Damme, E.J.M. Plant Lectins: Versatile Proteins with Important Perspectives in Biotechnology. Biotechnol. Genet. Eng. Rev. 1998, 15, 199–228. [Google Scholar] [CrossRef]
- Koeppe, S.J.; Rupnow, J.H.; Walker, C.E.; Davis, A. Isolation and Heat Stability of Trypsin Inhibitors in Amaranth (Amaranthus Hypochondriacus). J. Food Sci. 1985, 50, 1519–1521. [Google Scholar] [CrossRef]
- Marcone, M.F. First Report of the Characterization of the Threatened Plant Species Amaranthus Pumilus (Seabeach Amaranth). J. Agric. Food Chem. 2000, 48, 378–382. [Google Scholar] [CrossRef]
- Escudero, N.L.; Albarracín, G.; Fernández, S.; De Arellano, L.M.; Mucciarelli, S. Nutrient and Antinutrient Composition of Amaranthus Muricatus. Plant Foods Hum. Nutr. 1999, 54, 327–336. [Google Scholar] [CrossRef]
- Bau, H.M.; Villaume, C.; Nicolas, J.P.; Méjean, L. Effect of Germination on Chemical Composition, Biochemical Constituents and Antinutritional Factors of Soya Bean (Glycine Max) Seeds. J. Sci. Food Agric. 1997, 73, 1–9. [Google Scholar] [CrossRef]
- Mojib, N.; Amad, M.; Thimma, M.; Aldanondo, N.; Kumaran, M.; Irigoien, X. Carotenoid Metabolic Profiling and Transcriptome-Genome Mining Reveal Functional Equivalence among Blue-Pigmented Copepods and Appendicularia. Mol. Ecol. 2014, 23, 2740–2756. [Google Scholar] [CrossRef]
- Jalgaonkar, K.; Jha, S.K.; Sharma, D.K. Effect of Thermal Treatments on the Storage Life of Pearl Millet (Pennisetum Glaucum) Flour. Indian J. Agric. Sci. 2016, 86, 762–767. [Google Scholar]
- Oleszek, W.; Junkuszew, M.; Stochmal, A. Determination and Toxicity of Saponins from Amaranthus Cruentus Seeds. J. Agric. Food Chem. 1999, 47, 3685–3687. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Oke, M.O.; Olaniyan, S.A.; Ajala, A.S. A Review of Cyanogenic Glycosides in Edible Plants. In Toxicology—New Aspects to This Scientific Conundrum; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total Phenolic, Flavonoid and Tannin Contents and Antioxidant and Antimicrobial Activities of Organic Extracts of Shoots of the Plant Limonium Delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Francisco, I.A.; Pinotti, M.H.P. Cyanogenic Glycosides in Plants. Brazilian Arch. Biol. Technol. 2000, 43, 487–492. [Google Scholar] [CrossRef]
| Samples | Dimensions (mm) | Weight (g/100 Seeds) | ||
|---|---|---|---|---|
| Width | Length | Thickness | ||
| PR | 0.94 ± 0.069 b | 1.21 ± 0.128 b | 0.790 ± 0.034 a | 0.69 ± 0.157 a |
| MX1 | 0.95 ± 0.07 b | 1.17 ± 0.048 ab | 0.792 ± 0.023 a | 1.03 ± 0.17 c |
| MX2 | 0.87 ± 0.054 a | 1.11 ± 0.031 a | 0.791 ± 0.013 a | 0.62 ± 0.063 d |
| EM | 0.92 ± 0.064 a | 1.160 ± 0.107 ab | 0.821 ± 0.015 b | 0.69 ± 0.128 d |
| PU | 0.94 ± 0.064 b | 1.180 ± 0.079 ab | 0.822 ± 0.019 b | 0.95 ± 0.195 b |
| Samples (%) | Protein | Ether Extract | Crude Fiber | Moisture | Ash | Carbohydrates |
|---|---|---|---|---|---|---|
| PR | 12.29 ± 1.31 a | 2.98 ± 0.04 a | 7.17 ± 0.41 a | 10.55 ± 0.07 a | 3.34 ± 0.1 a | 63.65 ± 1.287 a |
| MX1 | 18.80 ± 2.2 b | 4.67 ± 0.014 b | 7.35 ± 0.03 a | 10.37 ± 0.15 a | 3.29 ± 0.04 a | 55.50 ± 1.822 b |
| MX2 | 18.63 ± 1.99 b | 13.39 ± 2.03 c | 7.01 ± 0.07 a | 10.53 ± 0.06 a | 2.96 ± 0.13 b | 47.45 ± 0.507 b |
| EM | 11.35 ± 1.05 a | 0.26 ± 0.01 d | 8.09 ± 1.58 a | 10.48 ± 0.1 a | 2.73 ± 0.15 b | 67.06 ± 0.595 d |
| PU | 13.55 ± 1.17 a | 0.27 ± 0.3 d | 10.85 ± 0.78 a | 10.50 ± 0.41 a | 2.70 ± 0.23 b | 62.10 ± 0.422 a |
| Fatty Acid Concentrations % | Amaranth Samples | ||||
|---|---|---|---|---|---|
| PR | MX1 | MX2 | EM | PU | |
| Myristic (14:0) | ND | 0.63 ± 0.06 b | 1.14 ± 0.14 a | 0.38±0.07c | 0.19 ± 0.01 c |
| Myristoleic (14:1) | ND | ND | ND | 0.16±0.01a | 0.16 ± 0.04 a |
| Palmitic (16:0) | 17.15 ± 0.95 a | 18.61 ± 1.44 a | 15.45 ± 1.45 a | 17.00±2.47a | 16.01 ± 2.58 a |
| Palmitoleic (16:2) | ND | ND | ND | 0.8 ±0.06a | 0.89 ± 0.02 a |
| Palmitolenic (16:3) | ND | ND | ND | 0.85±0.12a | 0.92 ± 0.06 a |
| Stearic (18:0) | 11.9 ± 1.61 a | 3.47 ± 0.16 b | 1.81 ± 0.11 b | 2.80±0.40b | 3.08 ± 1.02 b |
| Oleic (18:1) | 31.60 ± 0.35 a | 29.27 ± 1. 98 a | 31.51 ± 1.49 a | 29.41±0.66a | 29.60 ± 1.29 a |
| Linoleic (18:2) | 45.96 ± 0.32 a | 45.33 ± 0.39 a | 46.05 ± 1.79 a | 45.78±1.09a | 46.45 ± 2.30 a |
| Linolenic (18:3) | 1.56 ± 0.41 a | 1.14 ± 0.03 a | 1.43 ± 0.05 a | 1.22±0.20a | 1.16 ± 0.22 a |
| Arachidonic (20:4) | 1.02 ± 0.12 a | 0.51 ± 0.19 b | 0.87 ± 0.07 c | 1.19±0.36b | 0.83 ± 0.06 c |
| Minerals (ppm) | Amaranth Samples | LOD (mg L−1 ) | Slopes of the Calibration Curves | ||||
|---|---|---|---|---|---|---|---|
| PR | MX1 | MX2 | EM | PU | |||
| B3+ | 5.30 ± 1.07 a | 5.39 ± 1.16 a | 5.99 ± 1.76 ab | 6.61 ± 2.38 bc | 7.33 ± 3.1 c | 0.970 | 0.9999 |
| Ca2+ | 12.26 ± 0.012 b | 16.40 ± 0.006 c | 11.80 ± 0.014 b | 10.06 ± 0.016 a | 11.65 ± 0.004 b | 0.087 | 0.9998 |
| Fe2+ | 0.34 ± 0.021 a | 0.70 ± 0.043 b | 0.33 ± 0.03 a | 0.36 ±0.088 a | 0.38 ± 0.014 a | 0.015 | 0.9998 |
| K+ | 43.10 ± 4.2 b | 49.13 ± 3.88 c | 44.96 ± 2.4 bc | 32.66 ± 0.006 a | 32.98 ± 0.005 a | 0.025 | 0.9999 |
| Mg2+ | 21.23 ± 1.38 ab | 25.03 ± 1.88 c | 23.23 ± 1.31 bc | 20.40 ± 0.043 a | 20.25 ± 0.065 a | 0.034 | 0.9999 |
| Mn2+ | 0.01 ± 0.11 a | 0.06 ± 0.017 ab | 0.11 ± 0.08 b | 0.31 ± 0.04 c | 0.36 ± 0.066 c | 0.011 | 0.9998 |
| Na+ | 2.28 ± 1.2 a | 1.75 ± 0.028 a | 1.49 ± 0.023 a | 1.49 ± 0.055 a | 1.46 ± 0.005 a | 0.045 | 0.9997 |
| Zn2+ | 0.09 ± 0.011 a | 0.06 ± 0.017 a | 0.03 ± 0.08 a | 0.02 ± 0.04 a | 0.07 ± 0.006 a | 0.023 | 0.9997 |
| Units | Treatment | Amaranthus hypochondriacus Resources | ||||
|---|---|---|---|---|---|---|
| PR | MX1 | MX2 | EM | PU | ||
| Lectins concentration Erythrocytes Type A(HAU/mg protein) | Raw | 91.03 ± 0.99 a | 11.24 ± 0.14 b | 31.50 ± 0.14 c | 59.19 ± 0.81 d | 65.19 ± 2.45 e |
| Popped | 1.71 ± 0.012 b | 3.32 ± 0.013 d | 1.74 ± 0.0.32 bc | 1.66 ± 0.025 a | 1.78 ± 0.017 c | |
| Germinated | 6.51 ± 0.011 bc | 3.25 ± 0.005 a | 6.52 ± 0.013 c | 6.49 ± 0.016 b | 6.52 ± 0.015 bc | |
| Lectins concentration Erythrocytes Type O(HAU/mg protein) | Raw | 22.77 ± 0.25 a | 22.48 ± 0.29 a | 31.51 ± 0.14 b | 118.38 ± 1.62 c | 130.58 ± 1.09 d |
| Popped | 1.72 ± 0.012 b | 3.33 ± 0.014 d | 1.77 ± 0.032 bc | 1.63 ± 0.025 a | 1.79 ± 0.0177 c | |
| Germinated | 6.51 ± 0.01 bc | 3.26 ± 0.006 a | 6.25 ± 0.014 c | 6.49 ± 0.014 b | 6.52 ± 0.015 bc | |
| Tannins concentration (mg catechin/g) | Raw | 0.105 ± 0.005 ab | 0.086 ± 0.004 a | 0.0933 ± 0.038 ab | 0.210 ± 0.033 c | 0.111 ± 0.001 b |
| Popped | 1.671 ± 0.051 bc | 1.517 ± 0.034 a | 1.5967 ± 0.070 ab | 1.802 ± 0.051 c | 1.969 ± 0.032 d | |
| Germinated | 1.554 ± 0.036 b | 1.907 ± 0.065 c | 1.9592 ± 0.008 c | 1.407 ± 0.006 a | 1.382 ± 0.083 a | |
| Trypsin inhibitors (UTI/mg) | Raw | 0.831 ± 0.123 ab | 0.868 ± 0.01 ab | 1.016 ± 0.43 b | 0.848 ± 0.05 ab | 0.519 ± 0.08 a |
| Popped | ND | ND | ND | ND | ND | |
| Germinated | ND | ND | ND | ND | ND | |
| Saponins (HU/mg) | Raw | 5.33 ± 0 a | 10.66 ± 0 b | 10.66 ± 0 b | 10.66 ± 0 b | 10.66 ± 0 b |
| Popped | ND | ND | ND | ND | ND | |
| Germinated | ND | ND | ND | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valadez-Vega, C.; Lugo-Magaña, O.; Figueroa-Hernández, C.; Bautista, M.; Betanzos-Cabrera, G.; Bernardino-Nicanor, A.; González-Amaro, R.M.; Alonso-Villegas, R.; Morales-González, J.A.; González-Cruz, L. Effects of Germination and Popping on the Anti-Nutritional Compounds and the Digestibility of Amaranthus hypochondriacus Seeds. Foods 2022, 11, 2075. https://doi.org/10.3390/foods11142075
Valadez-Vega C, Lugo-Magaña O, Figueroa-Hernández C, Bautista M, Betanzos-Cabrera G, Bernardino-Nicanor A, González-Amaro RM, Alonso-Villegas R, Morales-González JA, González-Cruz L. Effects of Germination and Popping on the Anti-Nutritional Compounds and the Digestibility of Amaranthus hypochondriacus Seeds. Foods. 2022; 11(14):2075. https://doi.org/10.3390/foods11142075
Chicago/Turabian StyleValadez-Vega, Carmen, Olivia Lugo-Magaña, Claudia Figueroa-Hernández, Mirandeli Bautista, Gabriel Betanzos-Cabrera, Aurea Bernardino-Nicanor, Rosa María González-Amaro, Rodrigo Alonso-Villegas, José A. Morales-González, and Leopoldo González-Cruz. 2022. "Effects of Germination and Popping on the Anti-Nutritional Compounds and the Digestibility of Amaranthus hypochondriacus Seeds" Foods 11, no. 14: 2075. https://doi.org/10.3390/foods11142075
APA StyleValadez-Vega, C., Lugo-Magaña, O., Figueroa-Hernández, C., Bautista, M., Betanzos-Cabrera, G., Bernardino-Nicanor, A., González-Amaro, R. M., Alonso-Villegas, R., Morales-González, J. A., & González-Cruz, L. (2022). Effects of Germination and Popping on the Anti-Nutritional Compounds and the Digestibility of Amaranthus hypochondriacus Seeds. Foods, 11(14), 2075. https://doi.org/10.3390/foods11142075










