An Overview of the Application of Multivariate Analysis to the Evaluation of Beer Sensory Quality and Shelf-Life Stability
Abstract
:1. Introduction
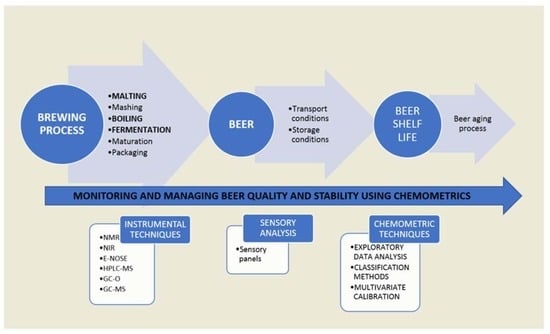
2. Beer Production Process
2.1. Malting
2.2. Boiling
2.3. Fermentation
3. Analysis of Beer Quality and Stability Using Chemometric Tools
3.1. Exploratory Analysis
3.2. Classification Techniques
3.3. Multivariate Calibration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Information System on Alcohol and Health World Health Organization. Available online: https://apps.who.int/gho/data/node.gisah.A1039?lang=en&showonly=GISAH (accessed on 31 August 2020).
- The Brewers of Europe. European Beer Trends—Beer Statistics 2021. Available online: https://brewersofeurope.org/uploads/mycms-files/documents/publications/2021/european-beer-statistics-2020.pdf (accessed on 10 April 2022).
- The Brewers of Europe. European Beer Trends—Beer Statistics 2019. Available online: https://brewersofeurope.org/uploads/mycms-files/documents/publications/2019/european-beer-trends-2019-web.pdf (accessed on 10 April 2022).
- The Brewers of Europe. European Beer Trends—Beer Statistics 2020. Available online: https://brewersofeurope.org/uploads/mycms-files/documents/publications/2020/european-beer-trends-2020.pdf (accessed on 10 April 2022).
- Carvalho, N.B.; Minim, L.A.; Nascimento, M.; de Castro Ferreira, G.H.; Minim, V.P.R. Characterization of the consumer market and motivations for the consumption of craft beer. Br. Food J. 2018, 120, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Bamforth, C.W. The microbiology of malting and brewing. Microbiol. Mol. Biol. Rev. 2013, 77, 157–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuberger, A.L.; Broeckling, C.D.; Sedin, D.; Holbrook, C.; Barr, L.; Kirkpatrick, K.; Prenni, J.E. Evaluation of non-volatile metabolites in beer stored at high temperature and utility as an accelerated method to predict flavour stability. Food Chem. 2016, 200, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Jaskula-Goiris, B.; De Causmaecker, B.; De Rouck, G.; Aerts, G.; Paternoster, A.; Braet, J.; De Cooman, L. Influence of transport and storage conditions on beer quality and flavour stability. J. Inst. Brew. 2019, 125, 60–68. [Google Scholar] [CrossRef]
- Mutz, Y.S.; Rosario, D.K.A.; Conte-Junior, C.A. Insights into chemical and sensorial aspects to understand and manage beer aging using chemometrics. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3774–3801. [Google Scholar] [CrossRef]
- Priest, F.G.; Stewart, G.G. Handbook of Brewing, Food Science and Technology, 2nd ed.; CRC Taylor & Francis: Boca Raton, FL, USA, 1995; ISBN 082472657X. [Google Scholar]
- Iimure, T.; Sato, K. Beer proteomics analysis for beer quality control and malting barley breeding. Food Res. Int. 2013, 54, 1013–1020. [Google Scholar] [CrossRef]
- Vivian, A.F.; Aoyagui, C.T.; de Oliveira, D.N.; Catharino, R.R. Mass spectrometry for the characterization of brewing process. Food Res. Int. 2016, 89, 281–288. [Google Scholar] [CrossRef]
- Silva, F.; Nogueira, L.C.; Gonçalves, C.; Ferreira, A.A.; Ferreira, I.M.P.L.V.O.; Teixeira, N. Electrophoretic and HPLC methods for comparative study of the protein fractions of malts, worts and beers produced from scarlett and prestige barley (Hordeum vulgare L.) varieties. Food Chem. 2008, 106, 820–829. [Google Scholar] [CrossRef]
- Briggs, D.E.; Brookes, P.A.; Stevens, R.B.C.A.; Boulton, C.A. Brewing Science and Practice; Woodhead Publishing: Cambridge, UK, 2004; ISBN 0849325471. [Google Scholar]
- MacLeod, L.; Evans, E. Barley: Malting. Encycl. Food Grains Second Ed. 2015, 3, 423–433. [Google Scholar] [CrossRef]
- Prestes, D.N.; Spessato, A.; Talhamento, A.; Gularte, M.A.; Schirmer, M.A.; Vanier, N.L.; Rombaldi, C.V. The addition of defatted rice bran to malted rice improves the quality of rice beer. LWT—Food Sci. Technol. 2019, 112, 108262. [Google Scholar] [CrossRef]
- Esslinger, H.M. Handbook of Brewing: Processes, Technology, Markets; Eßlinger, H., Ed.; Wiley-VCH: Weinhein, Germany, 2009; Volume 1, ISBN 9788578110796. [Google Scholar]
- Mallet, J. Malt: A Practical Guide from field to brewhouse; Brewers Publications: Boulder, CO, USA, 2014; ISBN 9788578110796. [Google Scholar]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley protein properties, extraction and applications, with a focus on brewers’ spent grain protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Błazewicz, J.; Kawa-Rygielska, J.; Leszczynska, D.; Grabinski, J.; Gasinski, A. Influence of variety and nitrogen fertilization on the technological parameters of special malts prepared from naked and hulled oat varieties. Agronomy 2021, 11, 2566. [Google Scholar] [CrossRef]
- Schönberger, C.; Kostelecky, T. The role of hops in brewing—125th anniversary review. J. Inst. Brew. 2011, 117, 259–267. [Google Scholar] [CrossRef]
- Onishi, A.; Proudlove, M.O. Isolation of beer foam polypeptides by hydrophobic interaction chromatography and their partial characterisation. J. Sci. Food Agric. 1994, 65, 233–240. [Google Scholar] [CrossRef]
- Bettenhausen, H.M.; Barr, L.; Broeckling, C.D.; Chaparro, J.M.; Holbrook, C.; Sedin, D.; Heuberger, A.L. Influence of malt source on beer chemistry, flavor, and flavor stability. Food Res. Int. 2018, 113, 487–504. [Google Scholar] [CrossRef]
- Mayolle, J.E.; Lullien-Pellerin, V.; Corbineau, F.; Boivin, P.; Guillard, V. Water diffusion and enzyme activities during malting of barley grains: A relationship assessment. J. Food Eng. 2012, 109, 358–365. [Google Scholar] [CrossRef]
- Mosher, M.; Trantham, K. Brewing Science: A Multidisciplinary Approach; Spronger, Ed.; Springer: Cham, Switzerland, 2017; ISBN 9783319463933. [Google Scholar]
- Boeira, L.S.; Bryce, J.H.; Stewart, G.G.; Flannigan, B. Inhibitory effect of Fusarium mycotoxins on growth of brewing yeasts. Deoxynivalenol and Nivalenol. J. Inst. Brew. 1999, 105, 366–375. [Google Scholar] [CrossRef]
- Müller, C.; Methner, F.J. An accelerated malting procedure-influences on malt quality and cost savings by reduced energy consumption and malting losses. J. Inst. Brew. 2015, 121, 181–192. [Google Scholar] [CrossRef]
- Bamforth, C.; Russell, I.; Stewart, G. Handbook of Alcoholic Beverages. Beer: A Quality Perspective.; Bamforth, C., Ed.; Elsevier: Amsterdam, The Nederlands, 2009; ISBN 9780080926094. [Google Scholar]
- Castro, L.F.; Affonso, A.D.; Lehman, R.M. Impact of Specialty Malts on Wort and Beer Characteristics. Fermentation 2021, 7, 137. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef] [Green Version]
- De Keukeleirc, D. Fundamentals of beer and hop chemistry. Quim. Nova 1999, 23, 108–112. [Google Scholar] [CrossRef]
- Kornysova, O.; Stanius, Z.; Obelevicius, K.; Ragazinskiene, O.; Skrzydlewska, E.; Maruska, A. Capillary zone electrophoresis method for determination of bitter (alpha- and beta-) acids in hop (Humulus lupulus L.) cone extracts. Adv. Med. Sci. 2009, 54, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Caballero, I.; Blanco, C.A.; Porras, M. Iso-α-acids, bitterness and loss of beer quality during storage. Trends Food Sci. Technol. 2012, 26, 21–30. [Google Scholar] [CrossRef]
- Sturm, B.; Raut, S.; Kulig, B.; Münsterer, J.; Kammhuber, K.; Hensel, O.; Crichton, S.O.J. In-process investigation of the dynamics in drying behavior and quality development of hops using visual and environmental sensors combined with chemometrics. Comput. Electron. Agric. 2020, 175, 105547. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Jorge, K.; Nogueira, L.C.; Silva, F.; Trugo, L.C. Effects of the combination of hydrophobic polypeptides, iso-α acids, and malto-oligosaccharides on beer foam stability. J. Agric. Food Chem. 2005, 53, 4976–4981. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [Green Version]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Boulton, C.A.; Box, W.G.; Graham, N.S.; Linforth, R.S.T.; Smart, K.A. The oxidative stress response of a lager brewing yeast strain during industrial propagation and fermentation. FEMS Yeast Res. 2008, 8, 574–585. [Google Scholar] [CrossRef] [Green Version]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.R.; Powell, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef] [Green Version]
- Paternoster, A.; Jaskula-Goiris, B.; Perkisas, T.; Springael, J.; De Rouck, G.; De Cooman, L.; Braet, J. A model to simulate the overall ageing score impact of temperature and time on the sensorial quality of lager. J. Inst. Brew. 2019. [Google Scholar] [CrossRef]
- Siebert, K.J. Chemometrics in Brewing—A Review. J. Am. Soc. Brew. Chem. 2001, 59, 147–156. [Google Scholar] [CrossRef]
- Lehnhardt, F.; Gastl, M.; Becker, T. Forced into aging: Analytical prediction of the flavor-stability of lager beer. A review. Crit. Rev. Food Sci. Nutr. 2018, 0, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baert, J.J.; De Clippeleer, J.; Hughes, P.S.; De Cooman, L.; Aerts, G. On the origin of free and bound staling aldehydes in beer. J. Agric. Food Chem. 2012, 60, 11449–11472. [Google Scholar] [CrossRef] [PubMed]
- Schieberle, P.; Komarek, D. Changes in key aroma compounds during natural beer aging. ACS Symp. Ser. 2002, 836, 70–79. [Google Scholar] [CrossRef]
- da Silva, L.A.; Flumignan, D.L.; Tininis, A.G.; Pezza, H.R.; Pezza, L. Discrimination of Brazilian lager beer by 1H NMR spectroscopy combined with chemometrics. Food Chem. 2019, 272, 488–493. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Barros, A.S.; Carvalho, B.; Brandão, T.; Gil, A.M. Probing beer aging chemistry by nuclear magnetic resonance and multivariate analysis. Anal. Chim. Acta 2011, 702, 178–187. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Forina, M. NIR spectroscopy coupled with multivariate computational tools for qualitative characterization of the aging of beer. Comput. Electron. Agric. 2014, 100, 34–40. [Google Scholar] [CrossRef]
- Sileoni, V.; Marconi, O.; Perretti, G. Near-infrared spectroscopy in the brewing industry. Crit. Rev. Food Sci. Nutr. 2015, 55, 1771–1791. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic nose as a tool for monitoring the authenticity of food—A review. Food Anal. Methods 2017, 10, 1800–1816. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Siadat, M.; Lozano, J.; Ahmadi, H.; Razavi, S.H.; Dicko, A. Aging fingerprint characterization of beer using electronic nose. Sensors Actuators B Chem. 2011, 159, 51–59. [Google Scholar] [CrossRef]
- Santos, J.R.; Carneiro, J.R.; Guido, L.F.; Almeida, P.J.; Rodrigues, J.A.; Barros, A.A. Determination of E-2-nonenal by high-performance liquid chromatography with UV detection: Assay for the evaluation of beer ageing. J. Chromatogr. A 2003, 985, 395–402. [Google Scholar] [CrossRef]
- Dong, L.; Hou, Y.; Li, F.; Piao, Y.; Zhang, X.; Zhang, X.; Li, C.; Zhao, C. Characterization of volatile aroma compounds in different brewing barley cultivars. J. Sci. Food Agric. 2015, 95, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Rendall, R.; Reis, M.S.; Pereira, A.C.; Pestana, C.; Pereira, V.; Marques, J.C. Chemometric analysis of the volatile fraction evolution of Portuguese beer under shelf storage conditions. Chemom. Intell. Lab. Syst. 2015, 142, 131–142. [Google Scholar] [CrossRef]
- Murakami, A.A.; Goldstein, H.; Navarro, A.; Seabrooks, J.R.; Ryder, D.S. Investigation of beer flavor by gas chromatography-olfactometry. J. Am. Soc. Brew. Chem. 2003, 61, 23–32. [Google Scholar] [CrossRef]
- Bravo, A.; Herrera, J.C.; Scherer, E.; Ju-Nam, Y.; Rübsam, H.; Madrid, J.; Zufall, C.; Rangel-Aldao, R. Formation of α-dicarbonyl compounds in beer during storage of pilsner. J. Agric. Food Chem. 2008, 56, 4134–4144. [Google Scholar] [CrossRef]
- Hempel, A.; O’Sullivan, M.G.; Papkovsky, D.B.; Kerry, J.P. Use of optical oxygen sensors to monitor residual oxygen in pre- and post-pasteurised bottled beer and its effect on sensory attributes and product acceptability during simulated commercial storage. LWT—Food Sci. Technol. 2013, 50, 226–231. [Google Scholar] [CrossRef]
- Cavallini, N.; Savorani, F.; Bro, R.; Cocchi, M. A Metabolomic Approach to Beer Characterization. Molecules 2021, 26, 1472. [Google Scholar] [CrossRef]
- Krebs, G.; Gastl, M.; Becker, T. Chemometric modeling of palate fullness in lager beers. Food Chem. 2021, 342, 128253. [Google Scholar] [CrossRef]
- Palmioli, A.; Alberici, D.; Ciaramelli, C.; Airoldi, C. Metabolomic profiling of beers: Combining 1H NMR spectroscopy and chemometric approaches to discriminate craft and industrial products. Food Chem. 2020, 327, 127025. [Google Scholar] [CrossRef]
- Giannetti, V.; Boccacci Mariani, M.; Torrelli, P.; Marini, F. Flavour component analysis by HS-SPME/GC–MS and chemometric modeling to characterize Pilsner-style Lager craft beers. Microchem. J. 2019, 149, 103991. [Google Scholar] [CrossRef]
- Coelho, E.; Magalhães, J.; Pereira, F.B.; Macieira, F.; Domingues, L.; Oliveira, J.M. Volatile fingerprinting differentiates diverse-aged craft beers. LWT—Food Sci. Technol. 2019, 108, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Lehnhardt, F.; Steiner, J.; Gastl, M.; Becker, T. Prediction power and accuracy of forced ageing—Matching sensory and analytical results for lager beer. BrewingScience 2018, 71, 39–48. [Google Scholar] [CrossRef]
- Martins, C.; Brandão, T.; Almeida, A.; Rocha, S.M. Unveiling the lager beer volatile terpenic compounds. Food Res. Int. 2018, 114, 199–207. [Google Scholar] [CrossRef]
- Gordon, R.; Chapman, J.; Power, A.; Chandra, S.; Roberts, J.; Cozzolino, D. Unfrazzled by Fizziness: Identification of beers using attenuated total reflectance mid-infrared spectroscopy and multivariate analysis. Food Anal. Methods 2018, 11, 2360–2367. [Google Scholar] [CrossRef]
- Stefanuto, P.H.; Perrault, K.A.; Dubois, L.M.; L’Homme, B.; Allen, C.; Loughnane, C.; Ochiai, N.; Focant, J.F. Advanced method optimization for volatile aroma profiling of beer using two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2017, 1507, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.V.; Amador, V.S.; Sena, M.M.; Augusti, R.; Piccin, E. Paper spray mass spectrometry and PLS-DA improved by variable selection for the forensic discrimination of beers. Anal. Chim. Acta 2016, 940, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gagula, G.; Magdić, D.; Horvat, D. PLSR modelling of quality changes of lager and malt beer during storage. J. Inst. Brew. 2016, 122, 116–125. [Google Scholar] [CrossRef]
- Tan, J.; Li, R.; Jiang, Z.T. Chemometric classification of Chinese lager beers according to manufacturer based on data fusion of fluorescence, UV and visible spectroscopies. Food Chem. 2015, 184, 30–36. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Figueira, J.A.; Rodrigues, F.P.; Ornelas, L.P.; Branco, R.N.; Silva, C.L.; Câmara, J.S. A powerful methodological approach combining headspace solid phase microextraction, mass spectrometry and multivariate analysis for profiling the volatile metabolomic pattern of beer starting raw materials. Food Chem. 2014, 160, 266–280. [Google Scholar] [CrossRef]
- Inui, T.; Tsuchiya, F.; Ishimaru, M.; Oka, K.; Komura, H. Different beers with different hops. Relevant compounds for their aroma characteristics. J. Agric. Food Chem. 2013, 61, 4758–4764. [Google Scholar] [CrossRef]
- Čejka, P.; Čulík, J.; Horák, T.; Jurková, M.; Olšovská, J. Use of chemical indicators of beer aging for ex-post checking of storage conditions and prediction of the sensory stability of beer. J. Agric. Food Chem. 2013, 61, 12670–12675. [Google Scholar] [CrossRef] [PubMed]
| Malt Types | Color SRM 1 | Color Description | Organoleptic Characteristics |
|---|---|---|---|
| Base Malts | |||
| Pilsner | 1.2–2 | Very Pale | Little green, with the smell and taste of fresh wort. |
| Pale | 1.6–2.8 | Light colored | Deeper malt aroma than Pilsner. |
| Pale Ale | 2.7–3.8 | Darker than standards pale malts | Not excessively pronounced malt aroma, with notes of biscuit or toast. |
| Vienna Malt | 2.5–4.0 | Imparts a rich orange color to beer | Slightly toasty and nutty |
| Melanoidin Malt | 17–25 | Sweet honey-like flavor. | |
| Munich | 3–20 | Covers a broad range of colors | Malty profile. |
| Specialty Malts | |||
| Caramel Malts | |||
| Special Glassy (Carapils) | 1–12 | Add body and impart sweetness to beer | |
| Caramel/Crystal | 10–200 | Can imply significant color differences depending on the method of manufacture. | Can imply significant aroma differences depending on the method of manufacture. |
| Roasted Malts | |||
| Biscuit | 20–30 | Bread crust, nutty, and toasted aromas. Dry finish. | |
| Amber | 20–36 | Nutty, biscuit, toffee taste. | |
| Brown | 40–150 | Darker than Amber. | Nutty, biscuit, toffee taste. |
| Chocolate | 350–500 | Dark color. | Treacle and chocolate aromas. Present dray and ashy aspects. |
| Black | 435–550 | Bitter, dry, and burnt aromas. | |
| Roasted | 300–650 | Smoky, coffee, chocolate, and roast aromas. | |
| Aim of the Study | Year | Analytical Techniques | Chemometric Techniques | Reference |
|---|---|---|---|---|
| Proposal of a methodology fast non-destructive metabolomic characterization of beer exploring the compositional profile of the product. | 2021 | NMR spectroscopy | PCA MCR (Multivariate Curve resolution) | [57] |
| Evaluation of the factors that influence the perception of the intensity of palate fullness and selected descriptors of mouthfeel in fresh lager beer. | 2021 | Physical chemical parameters Macromolecular characterization Sensory panel | HCA PLS | [58] |
| Understand the changes during the drying process to optimize the process, improving the process performance and the quality of the product. | 2020 | Hyperspectral imaging | PLSR | [34] |
| Metabolomic profiling of beers to discriminate craft and industrial products. | 2020 | NMR spectroscopy | PCA PLS-DA | [59] |
| Build and test a model capable of estimating the quality of beer. | 2019 | Sensory panel | The model was created using Curve Fitting Toolbox in Matlab | [40] |
| Differentiate Brazilian lager beers by styles employing NMR spectroscopy combined with chemometric approach. | 2019 | H NMR | PCA PLSDA SIMCA | [45] |
| Characterize the craft beers to differentiate them from the other competing and lower-quality products. | 2019 | GC-MS | PLS-DA LDA | [60] |
| Multivariate analysis as a tool to discriminate and characterize differences in barrel diverse-aged beers using volatile fingerprinting. | 2019 | GC-MS | PCA | [61] |
| Understand if there would be metabolite differences among six commercial barley sources and if this difference is reflected in the chemistry and in the sensory attributes of beer. | 2018 | UHPLC-MS HILIC-MS GC-MS ICP-MS Sensory analysis | PCA | [23] |
| Compounds behavior in natural and forced aging—recommendations as to how prediction by forced aging should be used. | 2018 | GC-O GC-MS Sensory panel | PCA | [62] |
| Beer volatile terpenic compounds. | 2018 | HSPME-MS GC × GC-TOF-MS | HCA | [63] |
| Traceability, quality control, and food adulteration. | 2018 | Mir spectroscopy coupled with attenuated total reflectance (ATR) | PCA PLS-DA | [64] |
| Method optimization for volatile aroma profiling of beer. | 2017 | GC × GC-TOF-MS | PCA HCA | [65] |
| Characterization of brewing process—“Processomics”. | 2016 | Electro spray ionization-Mass Spectrometry (ESI-MS) | PLS-DA | [12] |
| Differentiation between beers according to their price market. | 2016 | Paper spray mass spectrometry (PS-MS) | PLS-DA | [66] |
| Create mathematical models that can be used during the measurement of beer shelf life. | 2016 | Physical chemical parameters Haze | PLSR-PR PLSR-RSM | [67] |
| Developing accelerated model to evaluate brewing techniques that affect flavor stability using metabolomics on non-volatile compounds in beer. | 2016 | UPLC-MS | PCA | [7] |
| Study volatile profiles and characterize odor-active compounds of brewing barley in order to determine the variability of the aroma composition among different brewing barley cultivars. | 2015 | GC-MS | PCA Hierarchical Clustering | [52] |
| Propose a methodology for determining the start of the period of time in which beer fresh features start to change. | 2015 | GC-MS | PCA | [53] |
| Using data fusion to establish a model to classify Chinese lager beer according to the manufacturer. | 2015 | Fluorescence/UV/Visible spectroscopies | PCA LDA | [68] |
| Monitoring the aging process in alcoholic and non-alcoholic beers. | 2014 | NIR | PCA KNN LDA StepLDA GA SELECT | [47] |
| Investigate the volatile metabolomic profile of raw materials used in beer. | 2014 | HS-SPME GC-qMS | PCA SLDA | [69] |
| Determine the effectiveness of incorporating an oxygen sensor into lager beer bottles and predicting the sensory quality of the beer with respect to the oxidation and staling. | 2013 | Optic oxygen sensors Sensory panel | PLSR | [56] |
| Clarify the aroma compounds affecting the various hop aroma characteristics, using beer prepared with different hop varieties. | 2013 | GC × GC-TOF-MS | PCA | [70] |
| Development of a method for retrospective determination of temperature conditions to which beer had been exposed | 2013 | GC-MS Sensory panel | MLR ANN | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, A.C.; Aceña, L.; Mestres, M.; Boqué, R. An Overview of the Application of Multivariate Analysis to the Evaluation of Beer Sensory Quality and Shelf-Life Stability. Foods 2022, 11, 2037. https://doi.org/10.3390/foods11142037
de Lima AC, Aceña L, Mestres M, Boqué R. An Overview of the Application of Multivariate Analysis to the Evaluation of Beer Sensory Quality and Shelf-Life Stability. Foods. 2022; 11(14):2037. https://doi.org/10.3390/foods11142037
Chicago/Turabian Stylede Lima, Ana Carolina, Laura Aceña, Montserrat Mestres, and Ricard Boqué. 2022. "An Overview of the Application of Multivariate Analysis to the Evaluation of Beer Sensory Quality and Shelf-Life Stability" Foods 11, no. 14: 2037. https://doi.org/10.3390/foods11142037
APA Stylede Lima, A. C., Aceña, L., Mestres, M., & Boqué, R. (2022). An Overview of the Application of Multivariate Analysis to the Evaluation of Beer Sensory Quality and Shelf-Life Stability. Foods, 11(14), 2037. https://doi.org/10.3390/foods11142037