Efficacy of 405 nm Light-Emitting Diode Illumination and Citral Used Alone and in Combination for Inactivation of Vibrio parahaemolyticus on Shrimp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strains and Culture Conditions
2.3. Light-Emitting Diode (LED) Illumination System
2.4. Temperature Control
2.5. Antimicrobial Activity of LED Combined with Citral against V. parahaemolyticus in PBS
2.6. Antibacterial Effect of LED Combined with Citral against V. parahaemolyticus on Shrimp
2.7. Sensory Evaluation for Fresh Shrimp by Trained Panel
2.8. Bacterial Morphology
2.9. Determination of Bacterial Outer Membrane Integrity
2.10. Comet Assay
2.11. DNA Fragmentation Analysis
2.12. Statistical Analysis
3. Results
3.1. Bacterial Inactivation by Combination of LED Illumination and Citral in PBS
3.2. Bacterial Inactivation by Combination of LED Illumination and Citral on Shrimp
3.3. Sensory Evaluation for Shrimp
3.4. FESEM Observations
3.5. Effect of 405 nm LED Illumination and Citral on Outer Membrane Integrity
3.6. Comet Assay
3.7. DNA Fragmentation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-Cholera Vibrios: The Microbial Barometer of Climate Change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Baker-Austin, C.; Oliver, J.; Alam, M.; Ali, A.; Waldor, M.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 8. [Google Scholar] [CrossRef]
- Kang, C.-H.; Shin, Y.; Yu, H.; Kim, S.; So, J.-S. Antibiotic and heavy-metal resistance of Vibrio parahaemolyticus isolated from oysters in Korea. Mar. Pollut. Bull. 2018, 135, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, J.; Li, H.; Tan, S.; Chen, Y.; Yu, H. Prevalence, Antibiotic Susceptibility and Diversity of Vibrio parahaemolyticus Isolates in Seafood from South China. Front. Microbiol. 2017, 8, 2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan Norhana, M.N.; Poole, S.E.; Deeth, H.C.; Dykes, G.A. Prevalence, persistence and control of Salmonella and Listeria in shrimp and shrimp products: A review. Food Control 2010, 21, 343–361. [Google Scholar] [CrossRef]
- Chaiyakosa, S.; Charernjiratragul, W.; Umsakul, K.; Vuddhakul, V. Comparing the efficiency of chitosan with chlorine for reducing Vibrio parahaemolyticus in shrimp. Food Control 2007, 18, 1031–1035. [Google Scholar] [CrossRef]
- Henneberger, P.K.; Olin, A.-C.; Andersson, E.; Hagberg, S.; Torén, K. The Incidence of Respiratory Symptoms and Diseases Among Pulp Mill Workers with Peak Exposures to Ozone and Other Irritant Gases. Chest 2005, 128, 3028–3037. [Google Scholar] [CrossRef]
- Kumar, A.; Ghate, V.; Kim, M.J.; Zhou, W.; Khoo, G.H.; Yuk, H.G. Inactivation and changes in metabolic profile of selected foodborne bacteria by 460 nm LED illumination. Food Microbiol. 2017, 63, 12–21. [Google Scholar] [CrossRef]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M.S. Applications of Light-Emitting Diodes (LEDs) in Food Processing and Water Treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.J.; Anderson, J.; Woolsey, G. Inactivation of Bacterial Pathogens following Exposure to Light from a 405-Nanometer Light-Emitting Diode Array. Appl. Environ. Microbiol. 2009, 75, 1932–1937. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, L.E.; Maclean, M.; MacGregor, S.J.; Anderson, J.G. Inactivation of Campylobacter jejuni by exposure to high-intensity 405-nm visible light. Foodborne Pathog. Dis. 2010, 7, 1211–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-J.; Mikš-Krajnik, M.; Kumar, A.; Yuk, H.-G. Inactivation by 405 ± 5 nm light emitting diode on Escherichia coli O157:H7, Salmonella Typhimurium, and Shigella sonnei under refrigerated condition might be due to the loss of membrane integrity. Food Control 2016, 59, 99–107. [Google Scholar] [CrossRef]
- Ghate, V.S.; Ng, K.S.; Zhou, W.; Yang, H.; Khoo, G.H.; Yoon, W.-B.; Yuk, H.-G. Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. Int. J. Food Microbiol. 2013, 166, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ghate, V.; Kim, M.J.; Zhou, W.; Khoo, G.H.; Yuk, H.G. Antibacterial efficacy of 405, 460 and 520 nm light emitting diodes on Lactobacillus plantarum, Staphylococcus aureus and Vibrio parahaemolyticus. J. Appl. Microbiol. 2016, 120, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-J.; Bang, W.S.; Yuk, H.-G. 405 ± 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol. 2017, 62, 124–132. [Google Scholar] [CrossRef]
- Kim, M.J.; Tang, C.H.; Bang, W.S.; Yuk, H.G. Antibacterial effect of 405 ± 5 nm light emitting diode illumination against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella on the surface of fresh-cut mango and its influence on fruit quality. Int. J. Food Microbiol. 2017, 244, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kim, M.-J.; Yuk, H.-G. Influence of 405 nm light-emitting diode illumination on the inactivation of Listeria monocytogenes and Salmonella spp. on ready-to-eat fresh salmon surface at chilling storage for 8 h and their susceptibility to simulated gastric fluid. Food Control 2018, 88, 61–68. [Google Scholar] [CrossRef]
- Kim, M.J.; Yuk, H.G. Antibacterial Mechanism of 405-Nanometer Light-Emitting Diode against Salmonella at Refrigeration Temperature. Appl. Env. Microbiol. 2017, 83, e02582-16. [Google Scholar] [CrossRef] [Green Version]
- Luksienė, Z.; Zukauskas, A. Prospects of photosensitization in control of pathogenic and harmful micro-organisms. J. Appl. Microbiol. 2009, 107, 1415–1424. [Google Scholar] [CrossRef]
- Kim, M.-J.; Mikš-Krajnik, M.; Kumar, A.; Ghate, V.; Yuk, H.-G. Antibacterial effect and mechanism of high-intensity 405 ± 5 nm light emitting diode on Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus under refrigerated condition. J. Photochem. Photobiol. B Biol. 2015, 153, 33–39. [Google Scholar] [CrossRef]
- Josewin, S.W.; Ghate, V.; Kim, M.-J.; Yuk, H.-G. Antibacterial effect of 460 nm light-emitting diode in combination with riboflavin against Listeria monocytogenes on smoked salmon. Food Control 2018, 84, 354–361. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Food Safety Standard for Uses of Food Additives (GB 2760-2011). Available online: www.nhc.gov.cn (accessed on 9 December 2020).
- Food and Drug Administration (2005). GRAS Notifications. Available online: www.fda.gov (accessed on 5 December 2020).
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus In Vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Natural Plant-Derived Chemical Compounds as Listeria monocytogenes Inhibitors In Vitro and in Food Model Systems. Pathogens 2021, 10, 12. [Google Scholar] [CrossRef]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef] [Green Version]
- Somolinos, M.; García, D.; Condón, S.; Mackey, B.; Pagán, R. Inactivation of Escherichia coli by citral. J. Appl. Microbiol. 2010, 108, 1928–1939. [Google Scholar] [CrossRef]
- Korenblum, E.; Regina de Vasconcelos Goulart, F.; de Almeida Rodrigues, I.; Abreu, F.; Lins, U.; Alves, P.B.; Blank, A.F.; Valoni, É.; Sebastián, G.V.; Alviano, D.S.; et al. Antimicrobial action and anti-corrosion effect against sulfate reducing bacteria by lemongrass (Cymbopogon citratus) essential oil and its major component, the citral. AMB Express 2013, 3, 44. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, M.I.; González-García, M.P.; Ponce-Monter, H.A.; Castañeda-Hernández, G.; Aguilar-Robles, P. Synergistic effect of the interaction between naproxen and citral on inflammation in rats. Phytomedicine Int. J. Phytother. Phytopharm. 2010, 18, 74–79. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, Y.; Ma, S.; Tu, J.; Li, J.; Liang, S.; Xu, Y.; Shi, C. Effect of 405-nm light-emitting diode on environmental tolerance of Cronobacter sakazakii in powdered infant formula. Food Res. Int. 2021, 144, 110343. [Google Scholar] [CrossRef] [PubMed]
- Jeyasekaran, G.; Ganesan, P.; Anandaraj, R.; Jeya Shakila, R.; Sukumar, D. Quantitative and qualitative studies on the bacteriological quality of Indian white shrimp (Penaeus indicus) stored in dry ice. Food Microbiol. 2006, 23, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; MacGregor, S.J.; Anderson, J.; Woolsey, G. High- intensity narrow-spectrum light inactivation and wavelength Sensitivity of Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 285, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdoch, L.E.; Maclean, M.; Endarko, E.; MacGregor, S.J.; Anderson, J.G. Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. Sci. World J. 2012, 2012, 137805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ma, S.; Guo, K.; Guo, D.; Li, J.; Wang, M.; Wang, Y.; Zhang, C.; Xia, X.; Shi, C. Efficacy of 405-nm LED illumination and citral used alone and in combination for the inactivation of Cronobacter sakazakii in reconstituted powdered infant formula. Food Res. Int. 2022, 154, 111027. [Google Scholar] [CrossRef]
- Guo, D.; Sun, H.; Sun, Z.; Xia, X.; Shi, C. Antimicrobial activity of citral against Vibrio parahaemolyticus. Food Sci. 2019, 40, 113–120. [Google Scholar] [CrossRef]
- Endarko, E.; Maclean, M.; Timoshkin, I.V.; MacGregor, S.J.; Anderson, J.G. High-Intensity 405 nm Light Inactivation of Listeria monocytogenes. Photochem. Photobiol. 2012, 88, 1280–1286. [Google Scholar] [CrossRef]
- Guo, D.; Wang, S.; Li, J.; Bai, F.; Yang, Y.; Xu, Y.; Liang, S.; Xia, X.; Wang, X.; Shi, C. The antimicrobial activity of coenzyme Q(0) against planktonic and biofilm forms of Cronobacter sakazakii. Food Microbiol. 2020, 86, 103337. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2000, 3, 3–8. [Google Scholar]
- Nitzan, Y.; Ashkenazi, H. Photoinactivation of Acinetobacter baumannii and Escherichia coli B by a Cationic Hydrophilic Porphyrin at Various Light Wavelengths. Curr. Microbiol. 2001, 42, 408–414. [Google Scholar] [CrossRef] [PubMed]

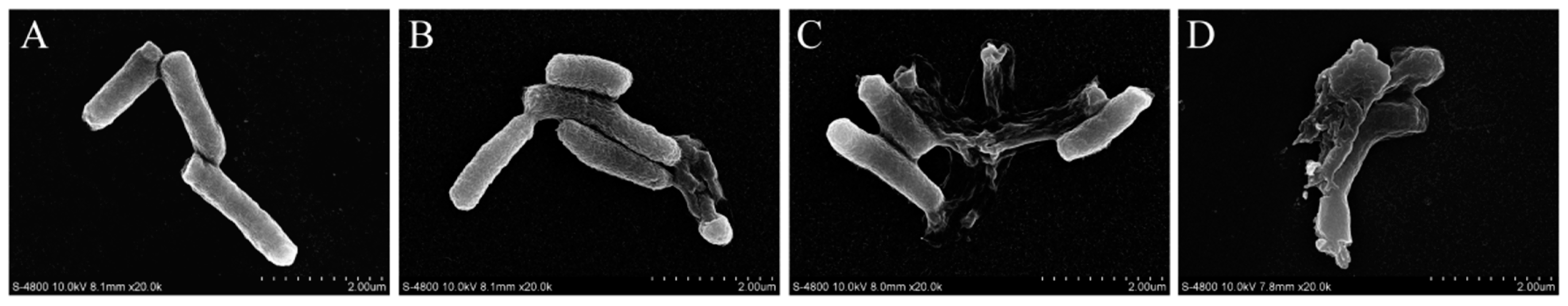
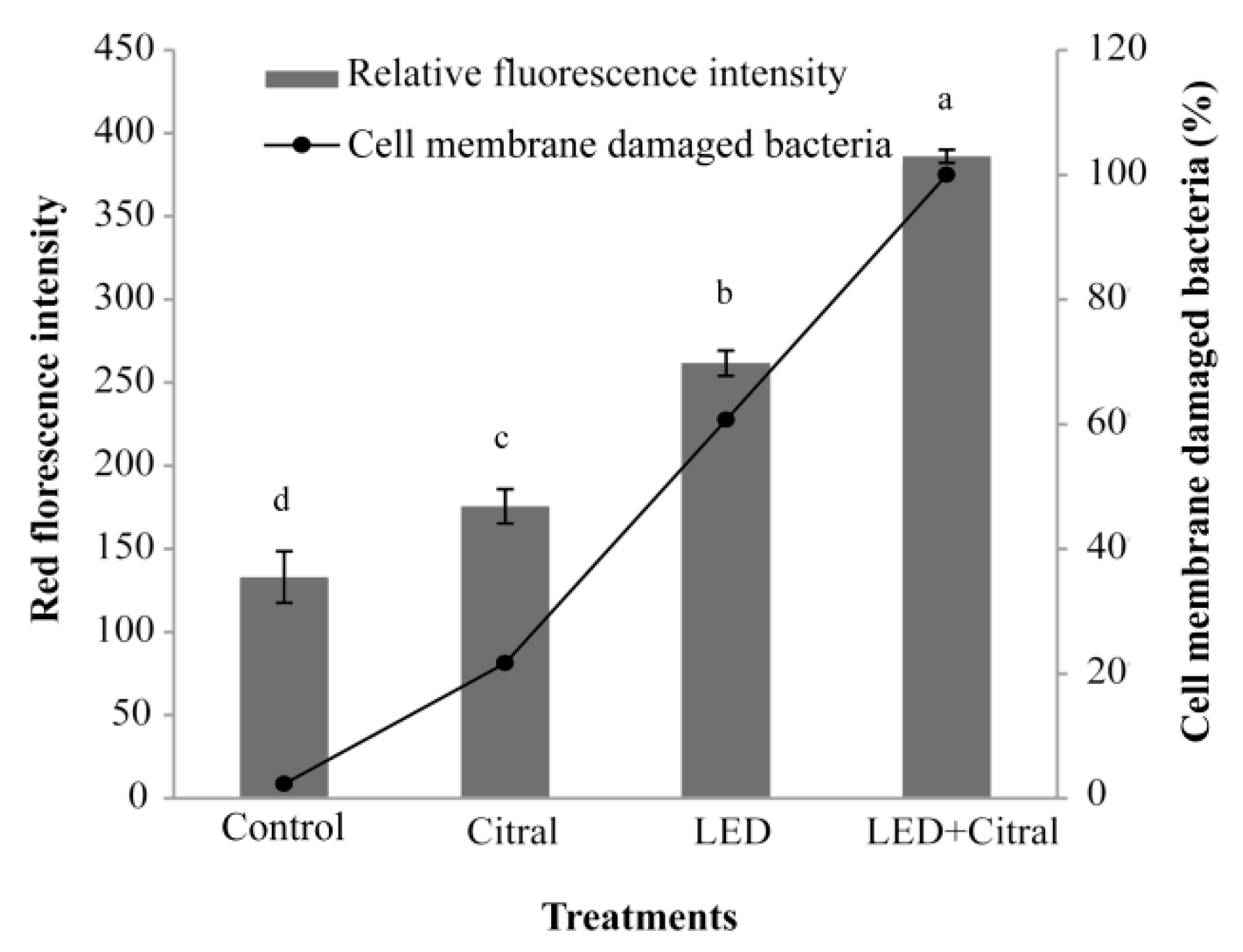
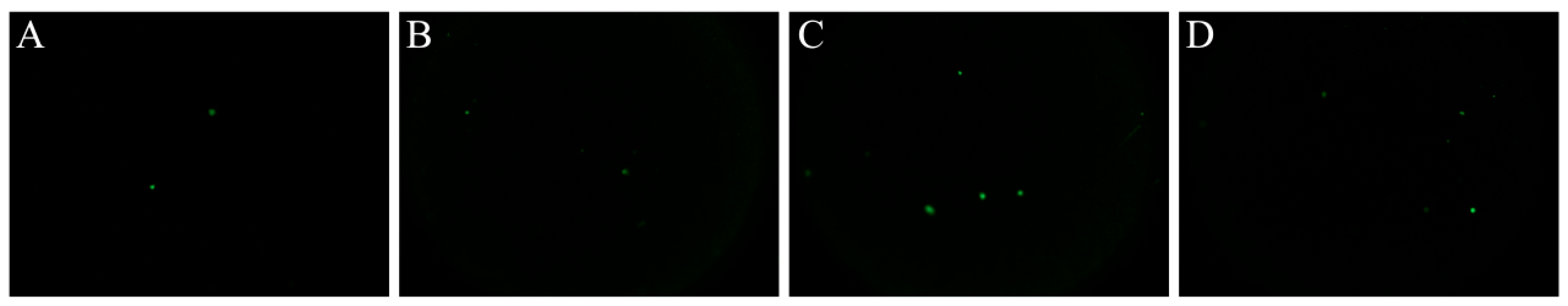
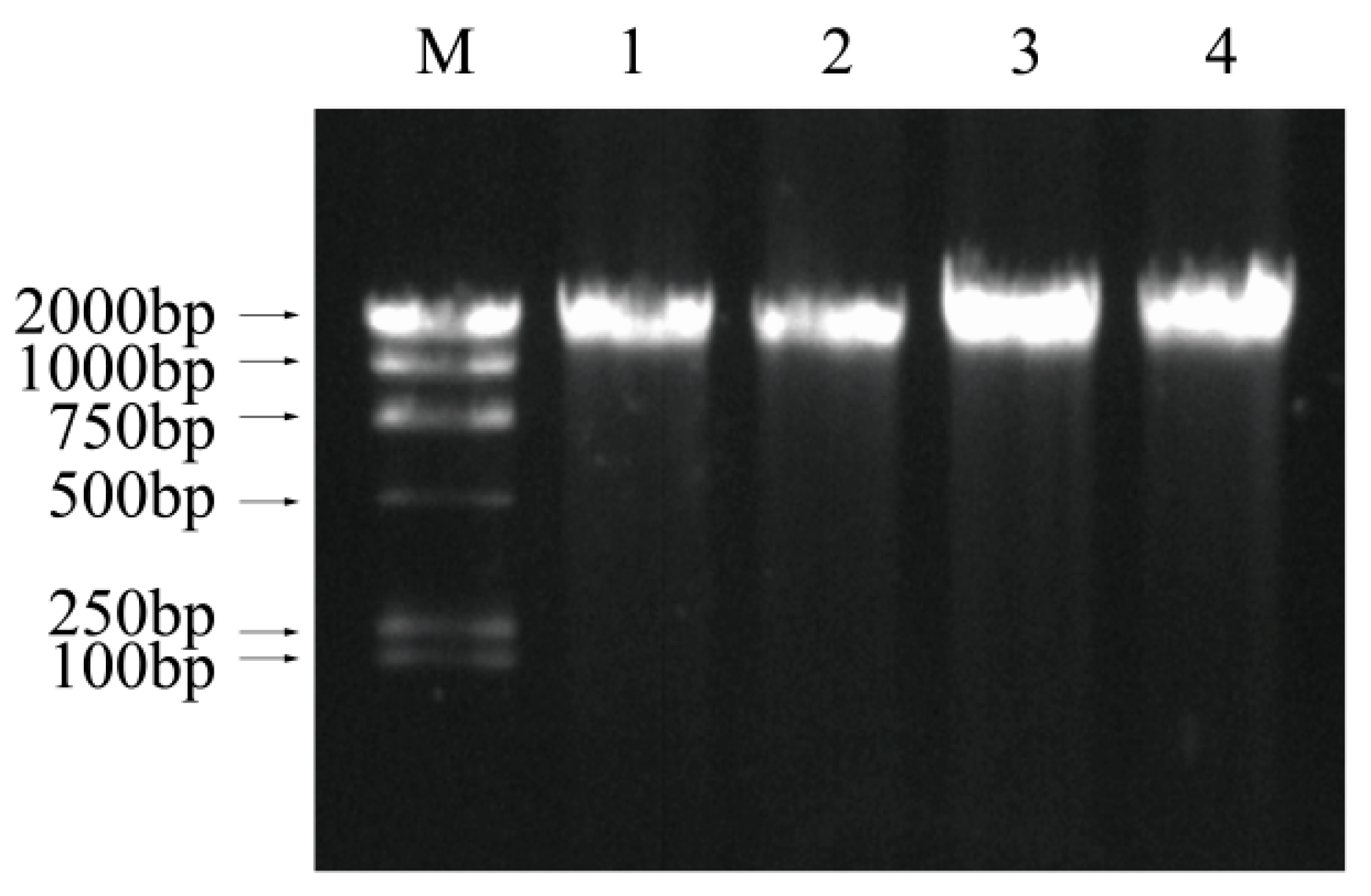
| Time (min) | Vibrio parahemolyticus (log CFU/mL) | |||
|---|---|---|---|---|
| Control | LED | Citral | LED + Citral | |
| 0 | 6.56 ± 0.08 a | 6.56 ± 0.08 a | 6.56 ± 0.08 a | 6.56 ± 0.08 a |
| 2 | 6.61 ± 0.10 a | 5.54 ± 0.12 a | 6.71 ± 0.10 a | 2.70 ± 0.01 a |
| 5 | 6.49 ± 0.23 a | 4.51 ± 0.19 b | 6.15 ± 0.33 a | ND c |
| 10 | 6.28 ± 0.27 a | ND c | 6.03 ± 0.25 b | ND c |
| 20 | 6.37 ± 0.12 a | ND c | 5.58 ± 0.22 b | ND c |
| 30 | 6.52 ± 0.41 a | ND b | 5.54 ± 0.27 a | ND b |
| 60 | 6.26 ± 0.22 a | ND c | 5.03 ± 0.30 b | ND c |
| Time (min) | Vibrio parahemolyticus (log CFU/mL) | |||
|---|---|---|---|---|
| Control | LED | Citral | LED + Citral | |
| 0 | 6.02 ± 0.18 a | 6.02 ± 0.18 a | 6.02 ± 0.18 a | 6.02 ± 0.18 a |
| 5 | 6.02 ± 0.18 a | 4.63 ± 0.25 ab | 5.83 ± 0.23 b | 4.65 ± 0.38 b |
| 10 | 5.84 ± 0.26 a | 4.43 ± 0.19 a | 5.76 ± 0.22 b | 4.30 ± 0.15 b |
| 20 | 5.81 ± 0.19 a | 3.12 ± 0.09 a | 5.60 ± 0.24 b | 2.45 ± 0.10 b |
| 60 | 6.07 ± 0.10 a | 2.75 ± 0.09 a | 5.28 ± 0.07 b | 1.78 ± 0.33 c |
| 120 | 5.90 ± 0.18 a | 2.53 ± 0.02 b | 4.35 ± 0.21 c | ND d |
| Treatments | Odor | Appearance | Texture | Overall Acceptance |
|---|---|---|---|---|
| Control | 5.3 ± 0.6 a | 5.3 ± 0.5 a | 5.3 ± 0.8 a | 15.9 ± 1.3 a |
| LED | 5.5 ± 0.5 a | 5.3 ± 0.6 a | 5.2 ± 0.7 a | 16.0 ± 1.3 a |
| Citral | 5.6 ± 0.5 a | 5.0 ± 0.8 a | 5.2 ± 0.6 a | 15.8 ± 1.4 a |
| LED + Citral | 5.0 ± 0.4 a | 5.3 ± 0.6 a | 5.6 ± 0.5 a | 15.9 ± 1.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, S.; Guo, D.; Liu, Z.; Gao, J.; Zhan, X.; Wang, Y.; Shi, C.; Xia, X. Efficacy of 405 nm Light-Emitting Diode Illumination and Citral Used Alone and in Combination for Inactivation of Vibrio parahaemolyticus on Shrimp. Foods 2022, 11, 2008. https://doi.org/10.3390/foods11142008
Zhang Y, Wang S, Guo D, Liu Z, Gao J, Zhan X, Wang Y, Shi C, Xia X. Efficacy of 405 nm Light-Emitting Diode Illumination and Citral Used Alone and in Combination for Inactivation of Vibrio parahaemolyticus on Shrimp. Foods. 2022; 11(14):2008. https://doi.org/10.3390/foods11142008
Chicago/Turabian StyleZhang, Yingying, Shuo Wang, Du Guo, Zhiyuan Liu, Jianxue Gao, Xiangjun Zhan, Yutang Wang, Chao Shi, and Xiaodong Xia. 2022. "Efficacy of 405 nm Light-Emitting Diode Illumination and Citral Used Alone and in Combination for Inactivation of Vibrio parahaemolyticus on Shrimp" Foods 11, no. 14: 2008. https://doi.org/10.3390/foods11142008
APA StyleZhang, Y., Wang, S., Guo, D., Liu, Z., Gao, J., Zhan, X., Wang, Y., Shi, C., & Xia, X. (2022). Efficacy of 405 nm Light-Emitting Diode Illumination and Citral Used Alone and in Combination for Inactivation of Vibrio parahaemolyticus on Shrimp. Foods, 11(14), 2008. https://doi.org/10.3390/foods11142008






