Food-Grade Microencapsulation Systems to Improve Protection of the Epigallocatechin Gallate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions Used in the Microencapsulation Process
2.2. Spray-Drying Process: Microparticles Production
2.3. Microparticles Characterization
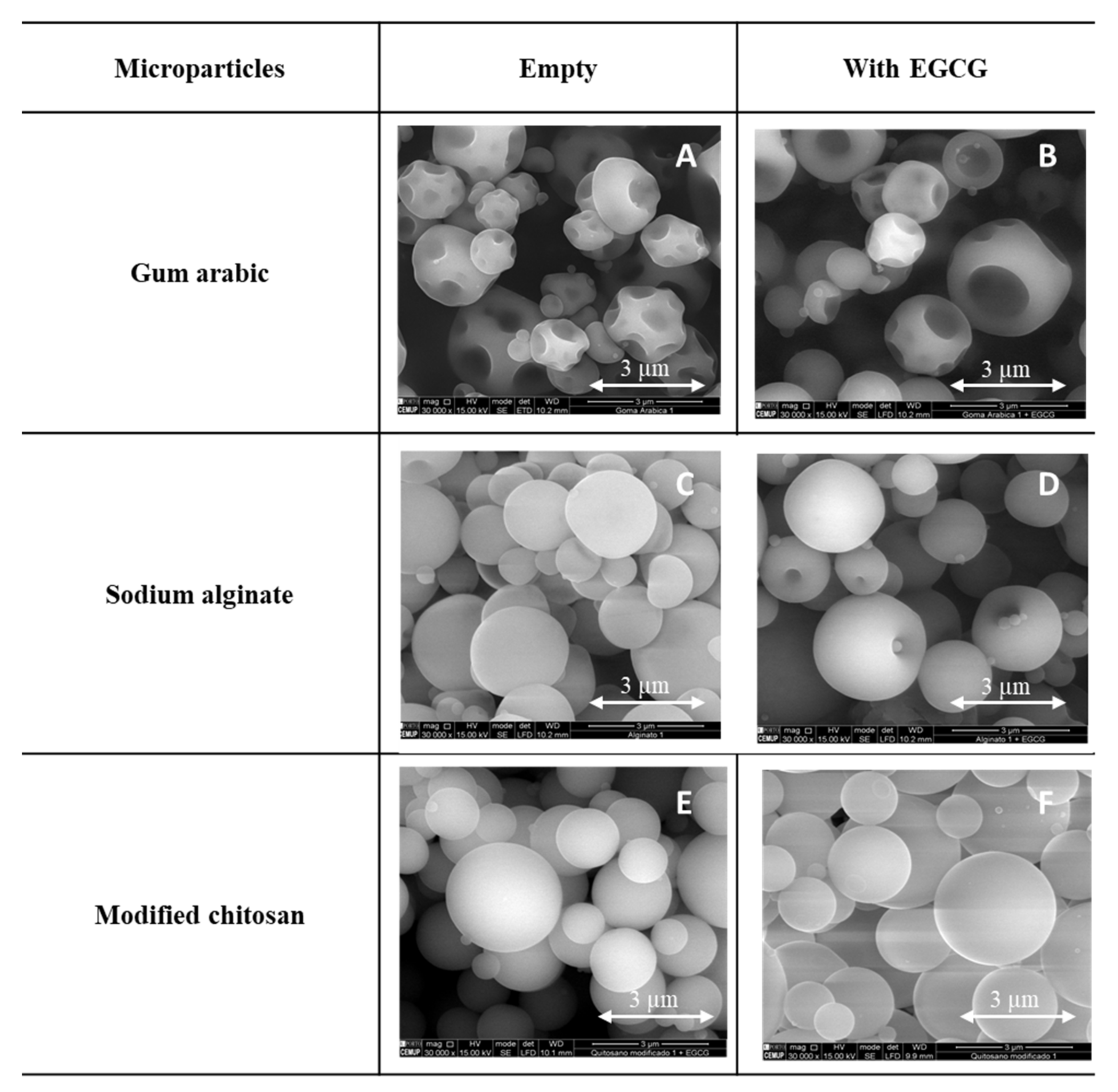
2.3.1. Scanning Electron Microscopy (SEM)
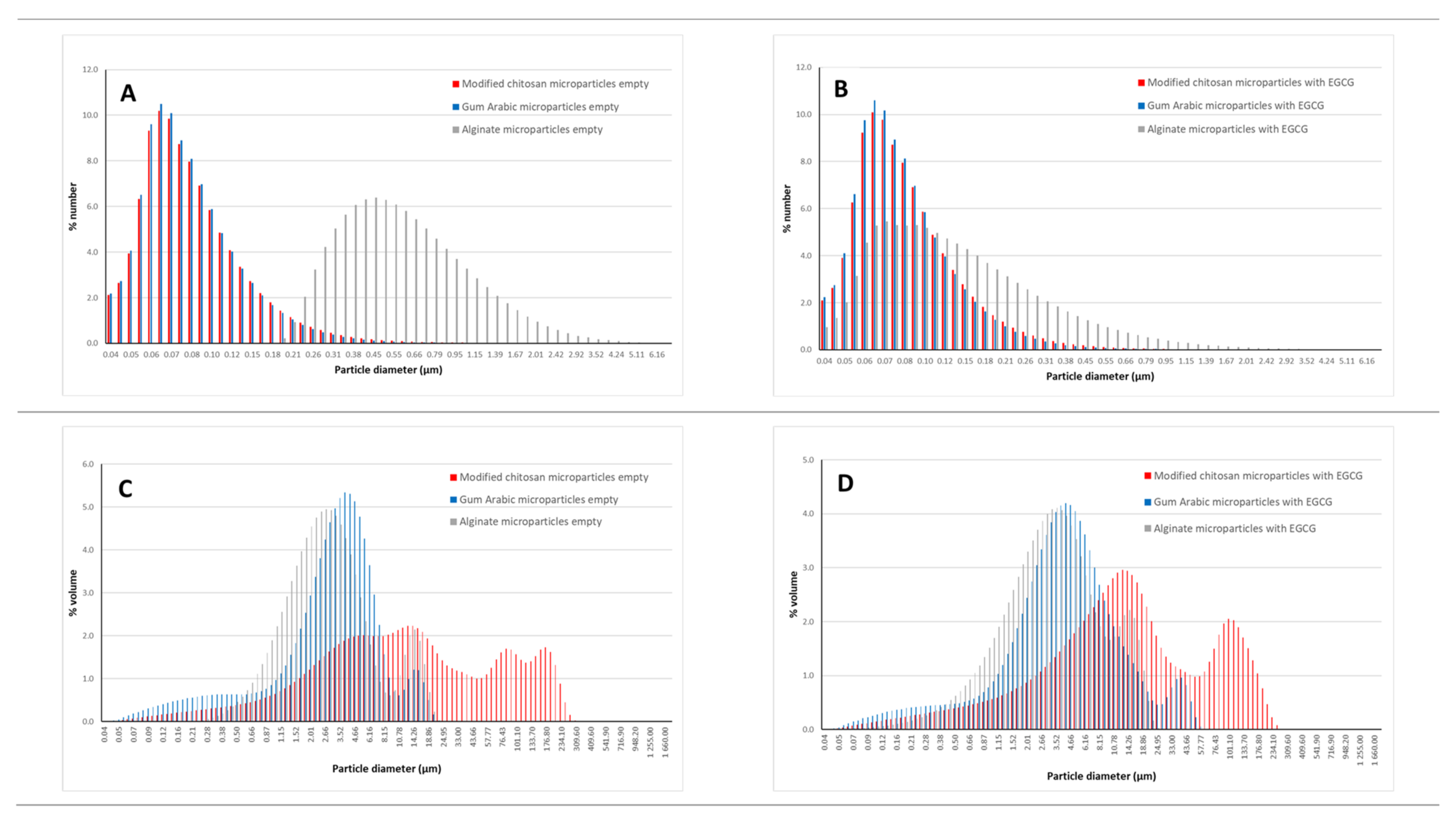
2.3.2. Particle Size—Laser Granulometry Analysis
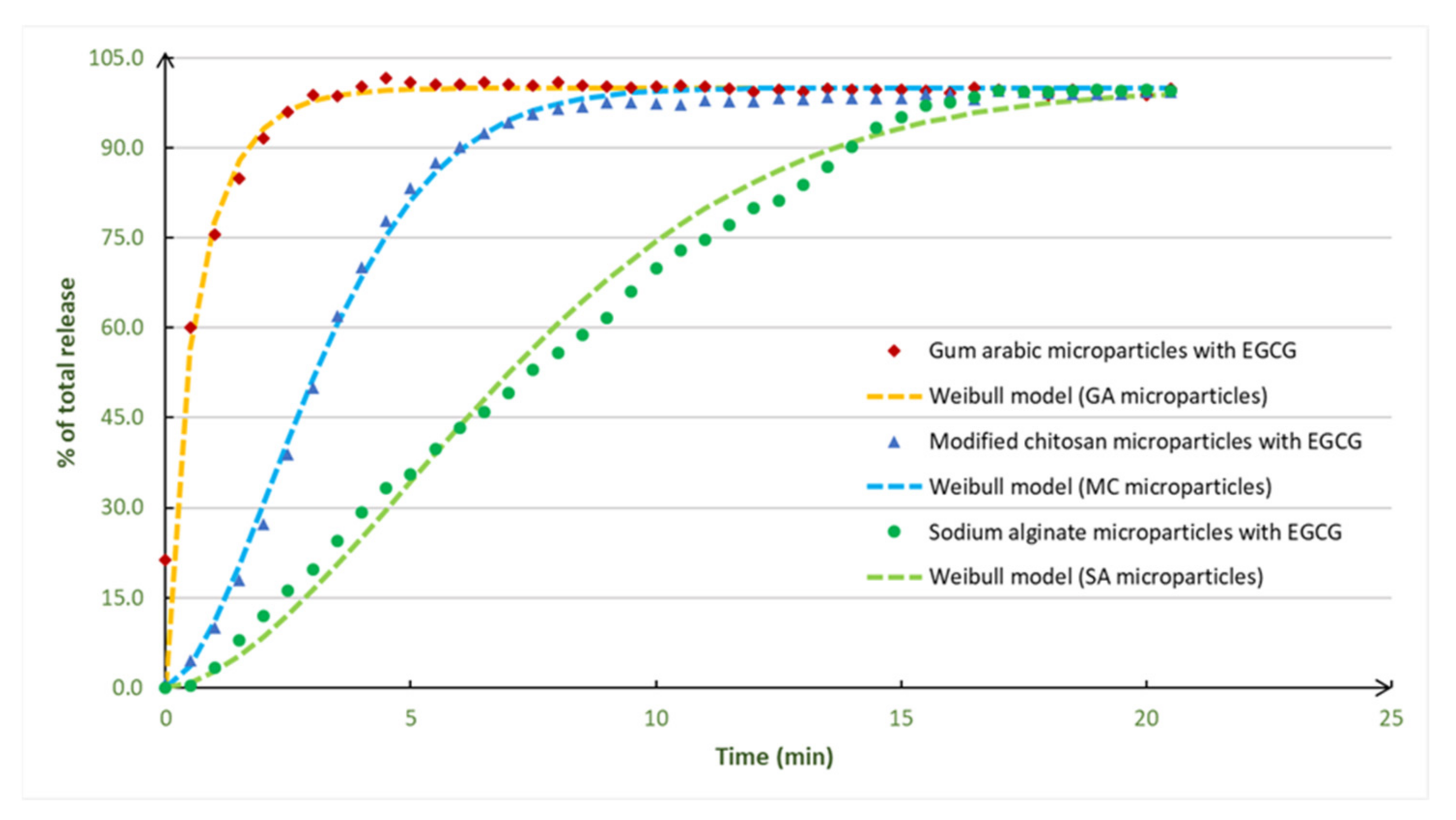
2.4. EGCG Controlled Release Studies
2.4.1. Determination of EGCG Calibration Curve
2.4.2. Encapsulation Efficiency and Loading Capacity
2.4.3. EGCG Release Studies
2.4.4. Kinetic Models
- Korsmeyer–Peppas model
- Weibull model
- Baker–Lonsdale model
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preparation of EGCG Microparticles: Determination of the Product Yield of the Spray-Drying Process
3.2. Morphology of the EGCG Microparticles: SEM
3.3. Microparticles Size: Laser Granulometry Analysis
3.4. EGCG Release Profiles and Controlled Release Studies
3.5. Food Application and Safety Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goëlo, V.; Chaumun, M.; Gonçalves, A.; Estevinho, B.N.; Rocha, F. Polysaccharide-based delivery systems for curcumin and turmeric powder encapsulation using a spray-drying process. Powder Technol. 2020, 370, 137–146. [Google Scholar] [CrossRef]
- Chaumun, M.; Goëlo, V.; Ribeiro, M.; Rocha, F.; Estevinho, B.N. In vitro evaluation of microparticles with Laurus nobilis L. extract prepared by spray-drying for application in food and pharmaceutical products. Food Bioprod. Process. 2020, 122, 124–135. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Horciu, I.-L.; Blaga, A.-C.; Rocha, F. Development of Controlled Delivery Functional Systems by Microencapsulation of Different Extracts of Plants: Hypericum perforatum L., Salvia officinalis L. and Syzygium aromaticum. Food Bioprocess Technol. 2021, 14, 1503–1517. [Google Scholar] [CrossRef]
- Cardoso, T.; Gonçalves, A.; Estevinho, B.N.; Rocha, F. Potential food application of resveratrol microparticles: Characterization and controlled release studies. Powder Technol. 2019, 355, 593–601. [Google Scholar] [CrossRef]
- He, A.; Guan, X.; Song, H.; Li, S.; Huang, K. Encapsulation of (−)-epigallocatechin-gallate (EGCG) in hordein nanoparticles. Food Biosci. 2020, 37, 100727. [Google Scholar] [CrossRef]
- Almatrood, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydh, F.A.; Alsahl, M.A.; Rahmani, A.H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Wu, Y.W.; Hung, J.I.; Chen, M.C. Epigallocatechin gallate/L-ascorbic acid–loaded poly-γ-glutamate microneedles with antioxidant, anti-inflammatory, and immunomodulatory effects for the treatment of atopic dermatitis. Acta Biomater. 2021, 130, 223–233. [Google Scholar] [CrossRef]
- Paximada, P.; Echegoyen, Y.; Koutinas, A.A.; Mandala, I.G.; Lagaron, J.M. Encapsulation of hydrophilic and lipophilized catechin into nanoparticles through emulsion electrospraying. Food Hydrocoll. 2017, 64, 123–132. [Google Scholar] [CrossRef]
- Gomes, J.F.P.S.; Rocha, S.; do Carmo Pereira, M.; Peres, I.; Moreno, S.; Toca-Herrera, J.; Coelho, M.A.N. Lipid/particle assemblies based on maltodextrin-gum arabic core as bio-carriers. Colloids Surf. B Biointerfaces 2010, 76, 449–455. [Google Scholar] [CrossRef]
- Gao, J.; Mao, Y.; Xiang, C.; Cao, M.; Ren, G.; Wang, K.; Ma, X.; Wu, D.; Xie, H. Preparation of β-lactoglobulin/gum arabic complex nanoparticles for encapsulation and controlled release of EGCG in simulated gastrointestinal digestion model. Food Chem. 2021, 354, 129516. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schrader, K.; Schwarz, K. Encapsulation of (−)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2019, 244, 91–100. [Google Scholar] [CrossRef]
- Chen, J.; Wei, N.; Lopez-Garcia, M.; Ambrose, D.; Lee, J.; Annelin, C.; Peterson, T. Development and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur. J. Pharm. Biopharm. 2017, 117, 286–291. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, J.; Liu, P.; Cui, B.; Abd El-Aty, A.M. Preparation and characterization of octenyl succinylated starch microgels via a water-in-oil (W/O) inverse microemulsion process for loading and releasing epigallocatechin gallate. Food Chem. 2021, 355, 129661. [Google Scholar] [CrossRef]
- Echeverri-Cuartas, C.E.; Agudelo, N.A.; Gartner, C. Chitosan-PEG-folate-Fe(III) complexes as nanocarriers of epigallocatechin–3–gallate. Int. J. Biol. Macromol. 2020, 165, 2909–2919. [Google Scholar] [CrossRef]
- Ding, J.; Xu, Z.; Qi, B.; Cui, S.; Wang, T.; Jiang, L.; Zhang, Y.; Sui, X. Fabrication and characterization of soybean oil bodies encapsulated in maltodextrin and chitosan-EGCG conjugates: An in vitro digestibility study. Food Hydrocoll. 2019, 94, 519–527. [Google Scholar] [CrossRef]
- Donsì, F.; Voudouris, P.; Veen, S.J.; Velikov, K.P. Zein-based colloidal particles for encapsulation and delivery of epigallocatechin gallate. Food Hydrocoll. 2017, 63, 508–517. [Google Scholar] [CrossRef]
- Fu, N.; Zhou, Z.; Jones, T.B.; Tan, T.T.Y.; Wu, W.D.; Lin, S.X.; Chen, X.D.; Chan, P.P.Y. Production of monodisperse epigallocatechin gallate (EGCG) microparticles by spray drying for high antioxidant activity retention. Int. J. Pharm. 2011, 413, 155–166. [Google Scholar] [CrossRef]
- Shi, M.; Shi, Y.L.; Li, X.M.; Yang, R.; Cai, Z.Y.; Li, Q.S.; Ma, S.C.; Ye, J.H.; Lu, J.L.; Liang, Y.R.; et al. Food-Grade Encapsulation Systems for (−)-Epigallocatechin Gallate. Molecules 2018, 23, 445. [Google Scholar] [CrossRef] [Green Version]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res. Int. 2021, 142, 110186. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of retinoic acid by atomization into biopolymeric matrices: Binary and ternary blends of alginic acid sodium, xanthan gum and modified chitosan. Food Hydrocoll. 2022, 124, 107310. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Shahgol, M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A by spray-drying, using binary and ternary blends of gum arabic, starch and maltodextrin. Food Hydrocoll. 2020, 108, 106029. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Mota, R.; Leite, J.P.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019, 343, 644–651. [Google Scholar] [CrossRef]
- Estevinho, B.M.A.N.; Rocha, F.A.N.; Santos, L.M.D.S.; Alves, M.A.C. Using water-soluble chitosan for flavour microencapsulation in food industry. J. Microencapsul. 2013, 30, 571–579. [Google Scholar] [CrossRef]
- Peres, I.; Rocha, S.; Gomes, J.; Morais, S.; Pereira, M.C.; Coelho, M. Preservation of catechin antioxidant properties loaded in carbohydrate nanoparticles. Carbohydr. Polym. 2011, 86, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Mao, L.; Yang, W.; Sun, C.; Dai, L.; Gao, Y. Evaluation of non-covalent ternary aggregates of lactoferrin, high methylated pectin, EGCG in stabilizing β-carotene emulsions. Food Chem. 2018, 240, 1063–1071. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, Y.; Xu, N.; Mu, H.; Zhang, H.; Duan, J. Improved viability of Akkermansia muciniphila by encapsulation in spray dried succinate-grafted alginate doped with epigallocatechin-3-gallate. Int. J. Biol. Macromol. 2020, 159, 373–382. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Spray-drying of oil-in-water emulsions for encapsulation of retinoic acid: Polysaccharide- and protein-based microparticles characterization and controlled release studies. Food Hydrocoll. 2022, 124, 107193. [Google Scholar] [CrossRef]
- Lucas, J.; Ralaivao, M.; Estevinho, B.N.; Rocha, F. A new approach for the microencapsulation of curcumin by a spray drying method, in order to value food products. Powder Technol. 2020, 362, 428–435. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Damas, A.M.; Martins, P.; Rocha, F. Study of the Inhibition Effect on the Microencapsulated Enzyme β-galactosidase. Environ. Eng. Manag. J. 2012, 11, 1923–1930. [Google Scholar] [CrossRef]
- Palacio, J.; Monsalve, Y.; Ramírez-Rodríguez, F.; López, B. Study of encapsulation of polyphenols on succinyl-chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101610. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F. Kinetic models applied to soluble vitamins delivery systems prepared by spray drying. Dry. Technol. 2017, 35, 1249–1257. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Lazar, R.; Blaga, A.; Rocha, F. Preliminary evaluation and studies on the preparation, characterization and in vitro release studies of different biopolymer microparticles for controlled release of folic acid. Powder Technol. 2020, 369, 279–288. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F. A Key for the Future of the Flavors in Food Industry: Nanoencapsulation and Microencapsulation. In Nanotechnology Applications in Food Flavor, Stability, Nutrition and Safety; Oprea, A.E., Grumezescu, A.M., Eds.; Elsevier Inc.: Oxford, UK, 2017; pp. 1–16. [Google Scholar]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Ramteke, K.H.; Dighe, P.; Kharat, A.R.; Patil, S. Mathematical Models of Drug Dissolution: A Review. Sch. Acad. J. Pharm. 2014, 3, 388–396. [Google Scholar] [CrossRef]
- Terada, T.; Tagami, M.; Ohtsubo, T.; Iwao, Y.; Noguchi, S.; Itai, S. Sustained-release microsphere formulation containing an agrochemical by polyurethane polymerization during an agitation granulation process. Int. J. Pharm. 2016, 509, 328–337. [Google Scholar] [CrossRef]
- Coelho, S.C.; Laget, S.; Benaut, P.; Rocha, F.; Estevinho, B.N. A new approach to the production of zein microstructures with vitamin B12, by electrospinning and spray drying techniques. Powder Technol. 2021, 392, 47–57. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Lević, S.; Kalušević, A.; Spoljarić, I.; Dordević, V.; Komes, D.; Mršić, G.; Nedovic, V. Efficiency Assessment of Natural Biopolymers as Encapsulants of Green Tea (Camellia sinensis L.) Bioactive Compounds by Spray Drying. Food Bioprocess Technol. 2015, 8, 2444–2460. [Google Scholar] [CrossRef]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Innovation and improvement in food fortification: Microencapsulation of vitamin B2 and B3 by a spray-drying method and evaluation of the simulated release profiles. J. Dispers. Sci. Technol. 2021, 1–13. [Google Scholar] [CrossRef]
- Khounvilay, K.; Estevinho, B.N.; Rocha, F.A.; Oliveira, J.M.; Vicente, A.; Sittikijyothin, W. Microencapsulation of citronella oil with carboxymethylated tamarind gum. Walailak J. Sci. Technol. 2018, 15, 515–527. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Souza, C.R.F.; Oliveira, W.P. Encapsulation of eugenol rich clove extract in solid lipid carriers. J. Food Eng. 2014, 127, 34–42. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Spray drying encapsulation of elderberry extract and evaluating the release and stability of phenolic compounds in encapsulated powders. Food Bioprocess Technol. 2019, 12, 1381–1394. [Google Scholar] [CrossRef]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Production of vitamin B1 microparticles by a spray drying process using different biopolymers as wall materials. Can. J. Chem. Eng. 2020, 98, 1682–1695. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Damas, A.M.; Martins, P.; Rocha, F. Microencapsulation of β-galactosidase with different biopolymers by a spray-drying process. Food Res. Int. 2014, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Davidov-Pardo, G.; Joye, I.J.; McClements, D.J. Food-Grade Protein-Based Nanoparticles and Microparticles for Bioactive Delivery. In Nature Protocols; Nature Publishing Group: London, UK, 2015; Volume 14, pp. 293–325. [Google Scholar]
- Aguiar, J.; Costa, R.; Rocha, F.; Estevinho, B.N.; Santos, L. Design of microparticles containing natural antioxidants: Preparation, characterization and controlled release studies. Powder Technol. 2017, 313, 287–292. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [Green Version]

| Microparticles | Mean Size (µm) | |
|---|---|---|
| Differential Number | Differential Volume | |
| Gum arabic | 0.10 ± 0.00 | 4.22 ± 0.02 |
| Gum arabic—EGCG | 0.10 ± 0.00 | 7.22 ± 0.03 |
| Modified chitosan | 0.11 ± 0.00 | 41.55 ± 9.31 |
| Modified chitosan—EGCG | 0.11 ± 0.00 | 36.12 ± 5.73 |
| Sodium alginate | 0.71 ± 0.00 | 4.31 ± 0.06 |
| Sodium alginate—EGCG | 0.21 ± 0.10 | 5.09 ± 0.10 |
| Biopolymer Used | Encapsulation Efficiency (%) | Loading Capacity (%) (w/w) | Total Release Time (min) |
|---|---|---|---|
| Gum arabic | 78.5 ± 5.5 | 0.79 ± 0.06 | 4 ± 1 |
| Sodium alginate | 100.0 ± 1.0 | 1.00 ± 0.01 | 21 ± 1 |
| Modified chitosan | 99.3 ± 1.0 | 0.99 ± 0.01 | 17 ± 1 |
| Microparticles with EGCG | Korsmeyer–Peppas Model | Weibull Model | Baker–Lonsdale Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of the Adjust (min) | KK (min-n) | n | Main Mechanism Associated to the Release | R2 | τdexperimental (min) | τdcalculated (min) | β | R2 | k | R2 | |
| Gum arabic | 2.0 | 0.744 | 0.18 | Fickian diffusion | 0.990 | 1.0 | 0.6 | 0.9 | 0.963 | 0.155 | 0.993 |
| Modified chitosan | 4.0 | 0.178 | 0.52 | Anomalous transport | 0.864 | 4.0 | 3.6 | 1.7 | 0.994 | 0.027 | 0.746 |
| Sodium alginate | 9.0 | 0.045 | 1.09 | Super case-II transport | 0.739 | 9.0 | 8.3 | 1.7 | 0.973 | 0.011 | 0.815 |
| Doses of EGCG (mg/Day) | EGCG Microparticles (g/Day) | |||
|---|---|---|---|---|
| Gum Arabic | Sodium Alginate | Modified Chitosan | ||
| Normal doses—consumption of green tea infusions | 90–300 | 11.4–38.1 | 9.0–30.0 | 9.1–30.2 |
| Food supplements | 5–1000 | 0.6–126.9 | 0.5–100.0 | 0.5–100.7 |
| “Safety” dose | <800 | <101.5 | <80 | <80.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralaivao, M.; Lucas, J.; Rocha, F.; Estevinho, B.N. Food-Grade Microencapsulation Systems to Improve Protection of the Epigallocatechin Gallate. Foods 2022, 11, 1990. https://doi.org/10.3390/foods11131990
Ralaivao M, Lucas J, Rocha F, Estevinho BN. Food-Grade Microencapsulation Systems to Improve Protection of the Epigallocatechin Gallate. Foods. 2022; 11(13):1990. https://doi.org/10.3390/foods11131990
Chicago/Turabian StyleRalaivao, Mathis, Jade Lucas, Fernando Rocha, and Berta N. Estevinho. 2022. "Food-Grade Microencapsulation Systems to Improve Protection of the Epigallocatechin Gallate" Foods 11, no. 13: 1990. https://doi.org/10.3390/foods11131990
APA StyleRalaivao, M., Lucas, J., Rocha, F., & Estevinho, B. N. (2022). Food-Grade Microencapsulation Systems to Improve Protection of the Epigallocatechin Gallate. Foods, 11(13), 1990. https://doi.org/10.3390/foods11131990







