The Regulation of Micro-Organisms’ Extra-Cellular Polysaccharides on Immunity: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Process
2.4. Quality Assessment
2.5. Data Organization and Analysis
3. Results
3.1. Basic Characteristics of Literature Search and Included Studies
3.2. Quality Assessment of Included Studies
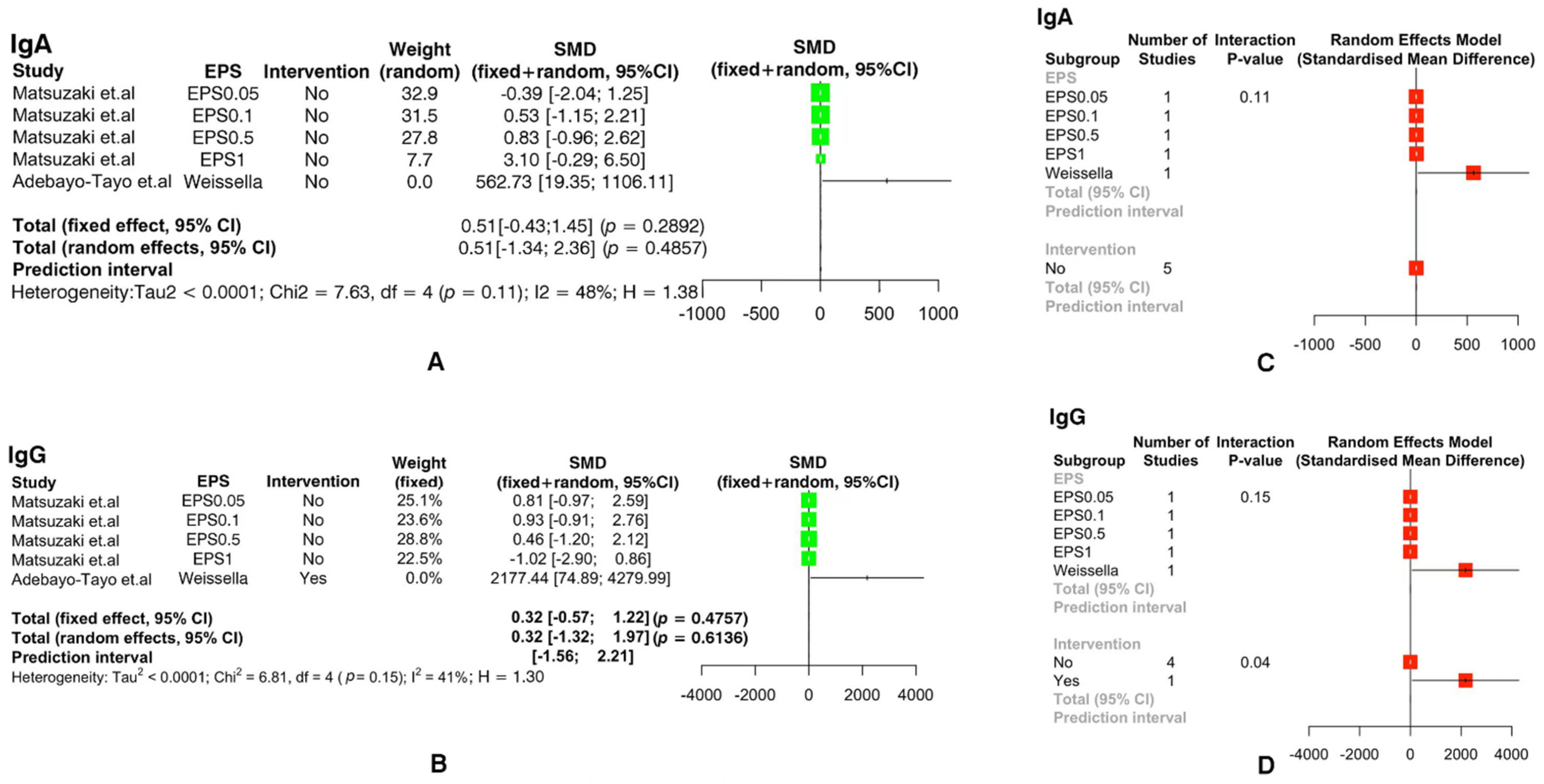
3.3. Meta-Analysis of Immune Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biot. 2016, 100, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- London, L.; Chaurin, V.; Auty, M.; Fenelon, M.A.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Use of Lactobacillus mucosae DPC 6426, an exopolysaccharide-producing strain, positively influences the techno-functional properties of yoghurt. Int. Dairy J. 2015, 40, 33–38. [Google Scholar] [CrossRef]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002, 87, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Carboni, E.; Tschudi, K.; Nam, J.; Lu, X.; Ma, A.W. Particle margination and its implications on intravenous anticancer drug delivery. AAPS PharmSciTech 2014, 15, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T.; Murakami, K.; Nishimura, I.; Nakano, T.; Obata, A. A sulfated polysaccharide, fucoidan, enhances the immunomodulatory effects of lactic acid bacteria. Int. J. Mol. Med. 2012, 29, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, C.; Spanò, A.; Maugeri, T.L.; Poli, A.; Arena, A.; Nicolaus, B. Role of bacterial exopolysaccharides as agents in counteracting immune disorders induced by herpes virus. Microorganisms 2015, 3, 464–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisacher, W.R.; Davison, W.N.S. Immunotherapy for food allergy. Curr. Opin. Otolaryngol. Head Neck. Surg. 2017, 25, 1. [Google Scholar] [CrossRef]
- Luo, M.; Gan, M.; Yu, X.; Wu, X.; Xu, F. Study on the regulatory effects and mechanisms of action of bifidobacterial exopolysaccharides on anaphylaxes in mice. Int. J. Biol. Macromol. 2020, 165, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Górska, S.; Schwarzer, M.; Srutkova, D.; Hermanova, P.; Brzozowska, E.; Kozakova, H.; Gamian, A. Polysaccharides L900/2 and L900/3 isolated from Lactobacillus rhamnosus LOCK 0900 modulate allergic sensitization to ovalbumin in a mouse model. Microb. Biotechnol. 2017, 10, 586–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisht, V.; Lal, B. Exploration of performance kinetics and mechanism of action of a potential novel bioflocculant BF-VB2 on clay and dye wastewater flocculation. Front. Microbiol. 2019, 10, 1288. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Tahmourespour, A.; Ahmadi, A.; Fesharaki, M. The anti-tumor activity of exopolysaccharides from Pseudomonas strains against HT-29 colorectal cancer cell line. Int. J. Biol. Macromol. 2020, 149, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Nampoothiri, K. Exposition of antitumour activity of a chemically characterized exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510. Biologia 2013, 68, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, T.; Şimşek, Ö. Potential health benefits of ropy exopolysaccharides produced by Lactobacillus plantarum. Molecules 2020, 25, 3293. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Suo, C.; Wang, Q.; Qu, X. Structure, physicochemical characterization, and antioxidant activity of the highly arabinose-branched exopolysaccharide EPS-M2 from Streptococcus thermophilus CS6. Int. J. Biol. Macromol. 2021, 192, 716–727. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.; Li, T.; Zhu, Z. Structure and hypoglycemic activity of a novel exopolysaccharide of Cordyceps militaris. Int. J. Biol. Macromol. 2021, 166, 496–508. [Google Scholar] [CrossRef]
- Gong, G.; Dang, T.; Deng, Y.; Han, J.; Zou, Z.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.; Wang, Z. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.W.; Wang, S.M.; Yi, H.X.; Han, X.; Fan, R.B.; Zhang, Y.C. Physicochemical characterization and antitumour activity of exopolysaccharides produced by Lactobacillus casei SB27 from yak milk. Carbohydr. Polym. 2017, 171, 307–315. [Google Scholar] [CrossRef]
- Surayot, U.; Wang, J.; Seesuriyachan, P.; Kuntiya, A.; Tabarsa, M.; Lee, Y.; Kim, J.K.; Park, W.; You, S. Exopolysaccharides from lactic acid bacteria: Structural analysis, molecular weight effect on immunomodulation. Int. J. Biol. Macromol. 2014, 68, 233–240. [Google Scholar] [CrossRef]
- Xiao, L.; Han, S.; Zhou, J.; Xu, Q.; Dong, M.; Fan, X.; Rui, X.; Chen, X.; Zhang, Q.; Li, W. Preparation, characterization and antioxidant activities of derivatives of exopolysaccharide from Lactobacillus helveticus MB2-1. Int. J. Biol. Macromol. 2020, 145, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedges, L.; Olkin, I. Statistical Methods in Meta-Analysis, 1st ed.; Acaademic Press: Orlando, FL, USA, 1985; Volume 5, pp. 75–106. ISBN 0-12-336380-2. [Google Scholar]
- Gregory, P.T. Analysis of patterns of aggregation under cover objects in an assemblage of six species of snakes. Herpetologica 2004, 60, 178–186. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Hou, D.; Lu, J.; Zhu, L.; Zhang, P.; Zhou, N.; Chen, K. Anti-tumor activity of exopolysaccharide from Rhizopus nigricans Ehrenb on S180 tumor-bearing mice. Bioorg. Med. Chem. Lett. 2016, 26, 2098–2104. [Google Scholar] [CrossRef]
- Hu, T.; Jiang, C.; Huang, Q.; Sun, F. A comb-like branched β-D-glucan produced by a Cordyceps sinensis fungus and its protective effect against cyclophosphamide-induced immunosuppression in mice. Carbohydr. Polym. 2016, 142, 259–267. [Google Scholar] [CrossRef]
- Ciszek-Lenda, M.; Nowak, B.; Srottek, M.; Gamian, A.; Marcinkiewicz, J. Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37. effects on the production of inflammatory mediators by mouse macrophages. Int. J. Exp. Pathol. 2011, 92, 382–391. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Nikolic, M.; López, P.; Suárez, A.; Miljkovic, M.; Kojic, M.; Margolles, A.; Golic, N.; Ruas-Madiedo, P. Exopolysaccharide-producing Bifidobacterium animalis subsp. lactis strains and their polymers elicit different responses on immune cells from blood and gut associated lymphoid tissue. Anaerobe 2014, 26, 24–30. [Google Scholar] [CrossRef]
- Sheng, L.; Chen, J.; Li, J.; Zhang, W. An exopolysaccharide from cultivated Cordyceps sinensis and its effects on cytokine expressions of immunocytes. Appl. Biochem. Biotechnol. 2011, 163, 669–678. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.G.; Ye, H.; Sun, Y.; Lu, Z.X.; Zeng, X.X. In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2010, 82, 1278–1283. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, D.; Li, H.; Sun, Y.; Zeng, X. Antioxidant and immunomodulatory activity of selenium exopolysaccharide produced by Lactococcus lactis subsp. lacti. Food Chem. 2013, 138, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cao, J.F.; Chen, G.C.; Xu, Y.H.; Lu, J.B.; Fang, F.; Chen, K.S. Anti-tumor and immunomodulatory activities of an exopolysaccharide from Rhizopus nigricans on CT26 tumor-bearing mice. Int. Immunopharmacol. 2016, 36, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Abed, S.; Essa, R.H.; Alaraji, K.H.Y. Evaluation of the antibacterial activity and immunomodulatory effect of purified exopolysaccharides (EPSs) produced from vancomycin resistant Enterococcus faecalis. Int. J. Drug Deliv. Tec. 2020, 10, 523–529. [Google Scholar] [CrossRef]
- Arena, A.; Gugliandolo, C.; Stassi, G.; Pavone, B.; Iannello, D.; Bisignano, G.; Maugeri, T.L. An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: Antiviral activity on immunocompetent cells. Immunol. Lett. 2009, 123, 132–137. [Google Scholar] [CrossRef]
- Matsuzaki, C.; Hayakawa, A.; Matsumoto, K.; Katoh, T.; Yamamoto, K.; Hisa, K. Exopolysaccharides produced by Leuconostoc mesenteroides strain NTM048 as an immunostimulant to enhance the mucosal barrier and influence the systemic immune response. J. Agric. Food. Chem. 2015, 63, 7009–7015. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Fashogbon, R. In vitro antioxidant, antibacterial, in vivo immunomodulatory, antitumor and hematological potential of exopolysaccharide produced by wild type and mutant Lactobacillus delbureckii subsp. bulgaricus. Heliyon 2020, 6, e03268. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.B.; Zhao, J.W.; Lu, C.J.; Zhu, W. Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb. Carbohydr. Polym. 2017, 156, 390–402. [Google Scholar] [CrossRef]
- Lee, J.-B.; Tanikawa, T.; Hayashi, K.; Asagi, M.; Kasahara, Y.; Hayashi, T. Characterization and biological effects of two polysaccharides isolated from Acanthopanax sciadophylloides. Carbohydr. Polym. 2015, 116, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Tang, X.F.; Shuai, X.X.; Jiang, C.J.; Xie, M.Y. Mannose receptor mediates the immune response to Ganoderma atrum polysaccharides in macrophages. J. Agric. Food Chem. 2017, 65, 348–357. [Google Scholar] [CrossRef]
- He, J.; Zang, S.L.; Liu, N.; Ji, M.; Ma, D.X.; Ji, C.Y. Epimedium polysaccharides attenuates hematotoxicity by reducing oxidative stress and enhancing immune function in mice model of benzene-induced bone marrow failure. Biomed. Pharmacother. 2020, 125, 109908. [Google Scholar] [CrossRef]
- Ren, Q.; Tang, Y.; Zhang, L.; Xu, Y.; Liu, N.; Ren, H. Exopolysaccharide produced by Lactobacillus casei promotes the differentiation of CD4(+) T cells into Th17 Cells in BALB/c mouse peyer’s patches in vivo and in vitro. J. Agric. Food Chem. 2020, 68, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.; Zhang, H.C.; Hu, Z.P.; Liang, Y.C.; Guo, S.; Yang, M.; Du, R.P.; Wang, X. Immunostimulatory activity of exopolysaccharides from probiotic Lactobacillus casei WXD030 strain as a novel adjuvant in vitro and in vivo. Food Agric. Immunol. 2018, 29, 1086–1105. [Google Scholar] [CrossRef] [Green Version]
- Makino, S.; Sato, A.; Goto, A.; Nakamura, M.; Ogawa, M.; Chiba, Y.; Hemmi, J.; Kano, H.; Takeda, K.; Okumura, K.; et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2016, 99, 915–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.H.; Duan, W.W.; Li, D.J.; Tang, X.X.; Duan, Z.H. Effects of polysaccharides from Auricularia auricula on the immuno-stimulatory activity and gut microbiota in immunosuppressed mice induced by cyclophosphamide. Front. Immunol. 2020, 11, 595700. [Google Scholar] [CrossRef]
- Lin, M.H.; Yang, Y.L.; Chen, Y.P.; Hua, K.F.; Lu, C.P.; Sheu, F.; Lin, G.H.; Tsay, S.S.; Liang, S.M.; Wu, S.H. A novel exopolysaccharide from the biofilm of Thermus aquaticus YT-1 induces the immune response through Toll-like receptor 2. J. Biol. Chem. 2011, 286, 17736–17745. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, C.; Kamishima, K.; Matsumoto, K.; Koga, H.; Katayama, T.; Yamamoto, K.; Hisa, K. Immunomodulating activity of exopolysaccharide-producing Leuconostoc mesenteroides strain NTM048 from green peas. J. Appl. Microbiol. 2014, 116, 980–989. [Google Scholar] [CrossRef]
- Costa, C.R.L.D.; Menolli, R.A.; Osaku, E.F.; Tramontina, R.; de Melo, R.H.; do Amaral, A.E.; Duarte, P.A.D.; de Carvalho, M.M.; Smiderle, F.R.; Silva, J.L.D.; et al. Exopolysaccharides from Aspergillus terreus: Production, chemical elucidation and immunoactivity. Int. J. Biol. Macromol. 2019, 139, 654–664. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Men, Y.; Zhang, J.; Liu, H.; Sun, Y. Structural characterization and immunomodulatory activity of exopolysaccharides from submerged culture of Auricularia auricula-judae. Int. J. Biol. Macromol. 2018, 115, 978–984. [Google Scholar] [CrossRef]
- Kuang, J.H.; Huang, Y.Y.; Hu, J.S.; Yu, J.J.; Zhou, Q.Y.; Liu, D.M. Exopolysaccharides from Bacillus amyloliquefaciens DMBA-K4 ameliorate dextran sodium sulfate-induced colitis via gut microbiota modulation. J. Funct. Foods 2020, 75, 1–10. [Google Scholar] [CrossRef]
- van Gool, M.M.J.; van Egmond, M. IgA and FcαRI: Versatile players in homeostasis, infection, and autoimmunity. Immunotargets Ther. 2020, 9, 351–372. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Paul, W.E. History of interleukin-4. Cytokine 2015, 75, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide Attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press Res. 2016, 41, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef] [Green Version]
- Henríquez-Olguín, C.; Altamirano, F.; Valladares, D.; López, J.; Allen, P.D.; Jaimovich, E. Altered ROS production, NF-κB activation and Interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells. Biochim. Biophys. Acta 2015, 1852, 1410–1419. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Okin, D.; Medzhitov, R. Evolution of inflammatory diseases. Curr. Biol. 2012, 22, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.; Fabbri, A.; Casini, A. Mediterranean Diet: Dietary Guidelines and Impact on Health and Disease; Romagnolo, D.F., Selmin, O.I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 17, pp. 89–96. ISBN 978-3-319-27969-5. [Google Scholar]
- Libby, P.; Okamoto, Y.; Rocha, V.Z.; Folco, E. Inflammation in atherosclerosis: Transition from theory to practice. Circ. J. 2010, 74, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, K.; Dhingra, S.; Slezak, J.; Sharma, A.K.; Bajaj, A.; Singal, P.K. Biology of TNFalpha and IL-10, and their imbalance in heart failure. Heart Fail Rev. 2009, 14, 113–123. [Google Scholar] [CrossRef]
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, M.H.; Kim, J.Y.; Lee, J.H.; You, S.; Lee, S.J. Antioxidative, hypolipidemic, and anti-inflammatory activities of sulfated polysaccharides from Monostroma nitidum. Food Sci. Biotechnol. 2015, 24, 199–205. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Ibrahim, A.Y.; Asker, M.S.; Mahmoud, M.G.; El Awady, M.E. Production, characterization and biological activities of acidic exopolysaccharide from marine Bacillus amyloliquefaciens 3MS 2017. Asian Pac. J. Trop. Med. 2017, 10, 715–725. [Google Scholar] [CrossRef]
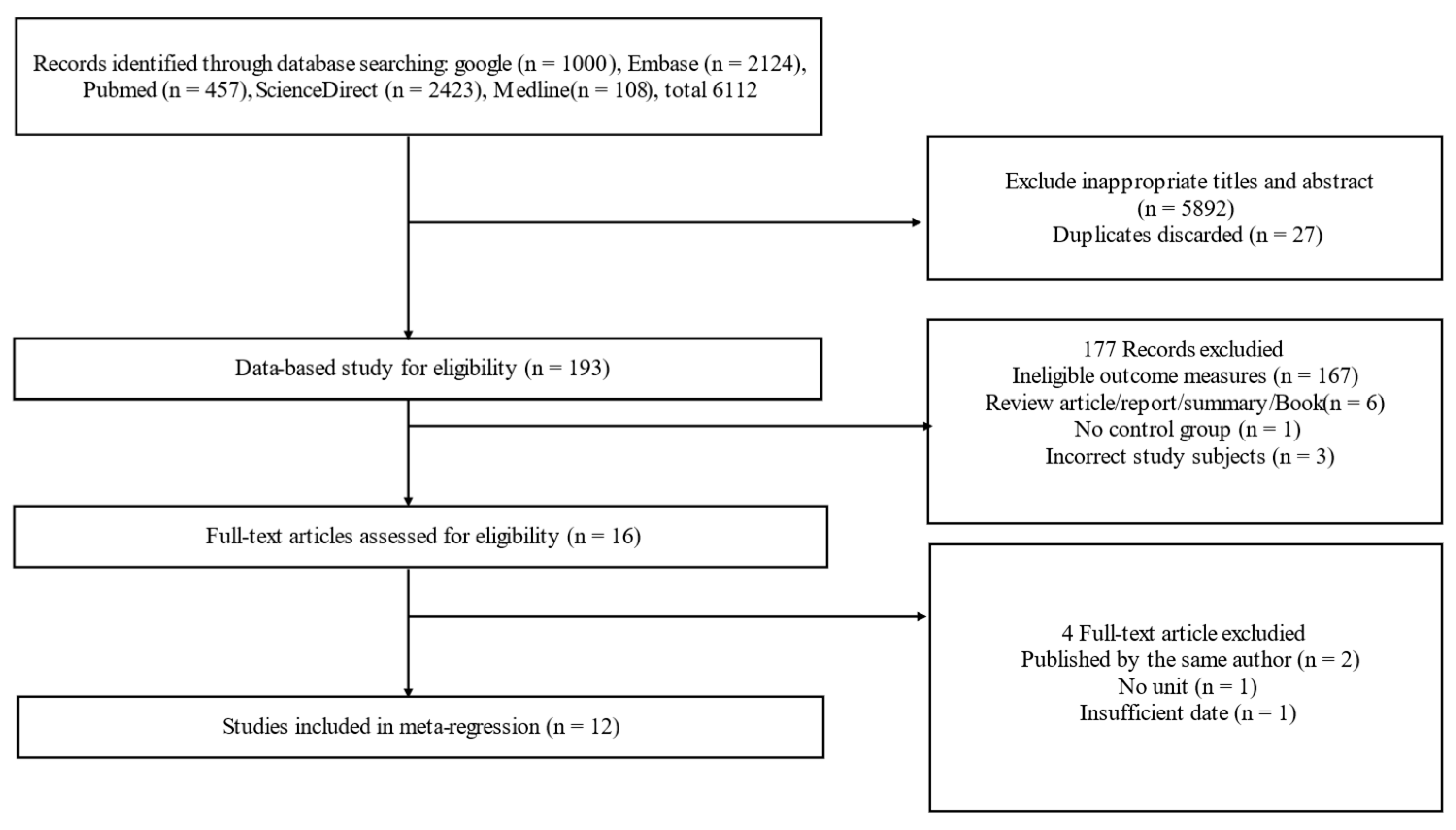
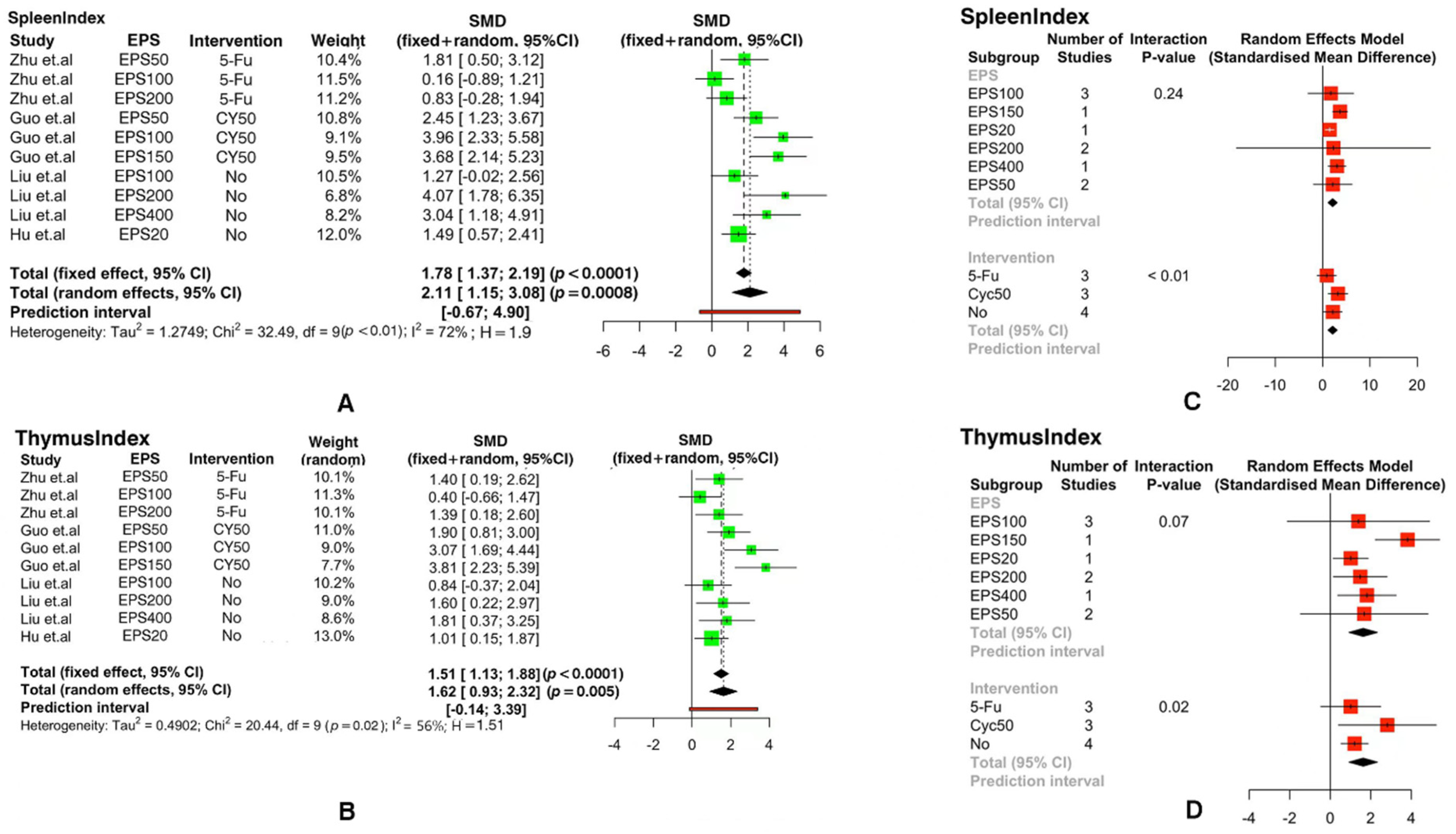
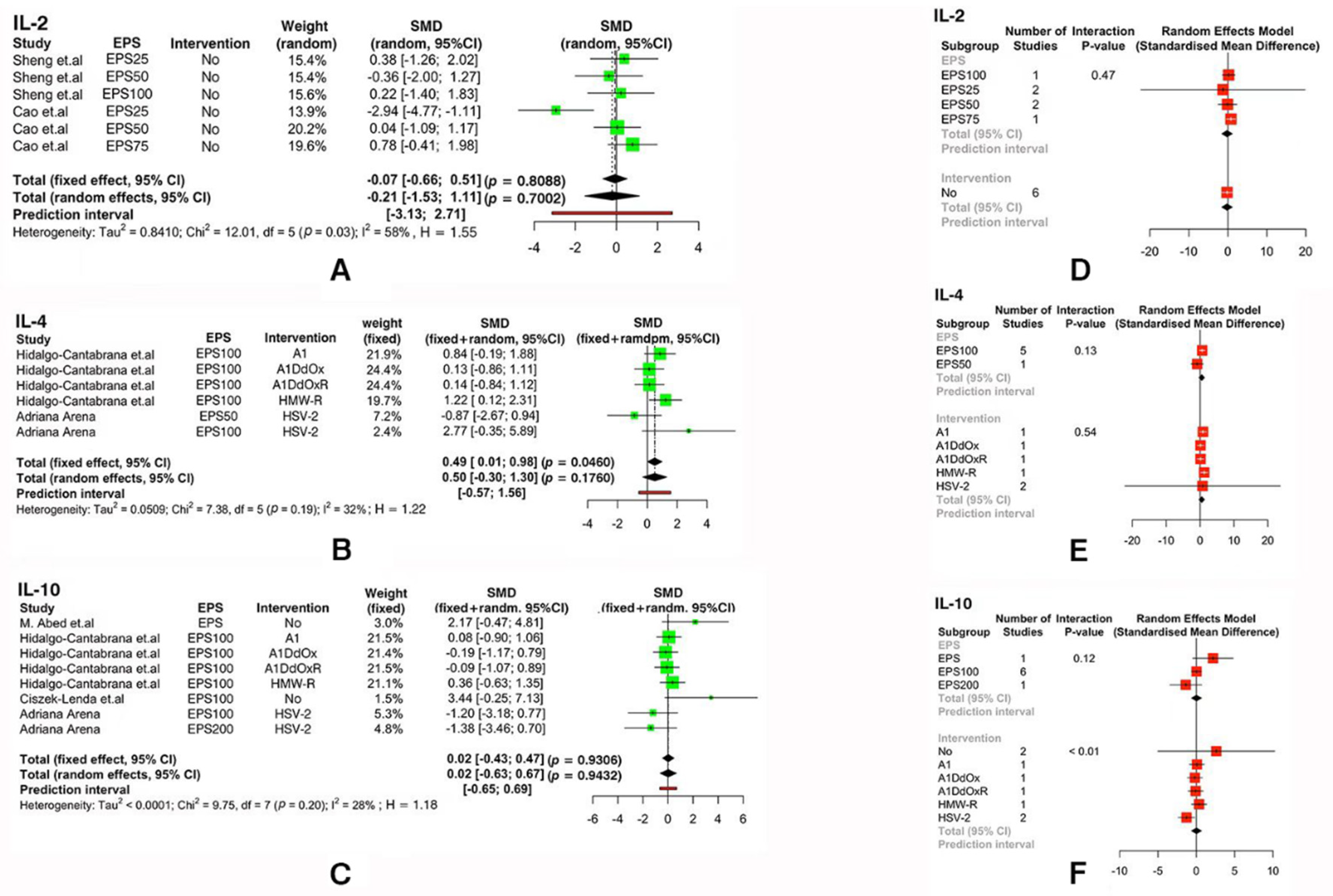
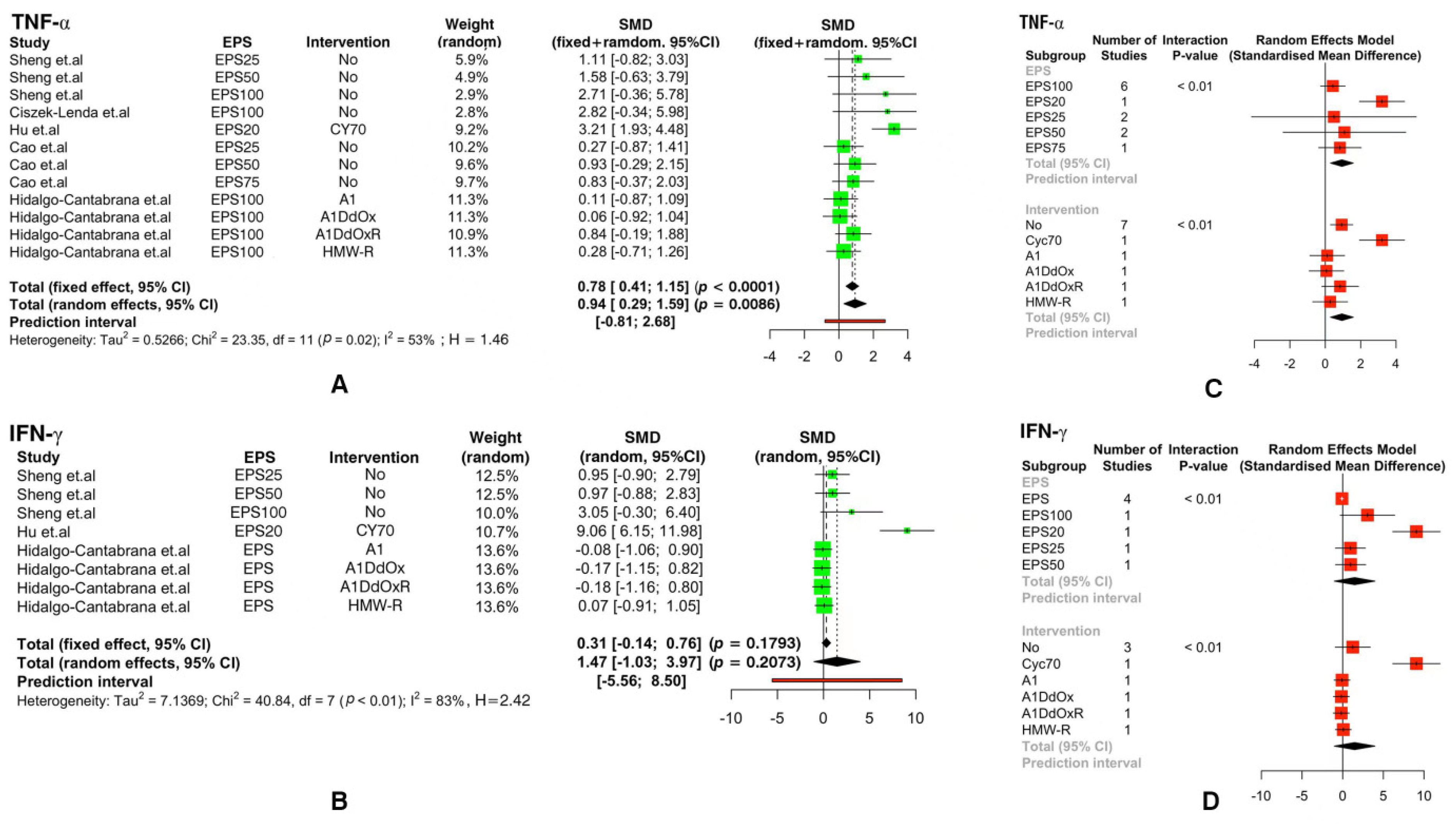
| Parameter | Study |
|---|---|
| Population | Mice, people |
| Intervention | EPSs from Fungi, bacteria, mold; any time, dosage, or form |
| Comparator | Placebo (with any co-intervention) |
| Outcomes | IFN-γ, TNF-α, IL-2, IL-4, IL-10, IgG, IgA, Thymus index, Splenomeric index |
| Study | Randomized controlled trials, published in English |
| Data from | Research Objects | Number | Treatment Duration | Medicament | Research Indicators |
|---|---|---|---|---|---|
| Reprinted/adapted with permission from Ref. [26]. 2016, Jianfeng Cao | Mouse serum, spleen, and thymus from S180 tumor-bearing mice | ① n = 7 | ① 17 d | ①, ② 25, 50, 75 mg/kg EPS from Rhizopus nigricans Ehrenb; S180 tumor-bearing mice | Spleen and thymus index, IL-2, TNF-a |
| ② n = 6 | ② 10 d | ||||
| Reprinted/adapted with permission from Ref. [27]. 2016, Ting Hu | Mouse serum, Spleen, and thymus of mice | n = 12 | 8 d | EPSs of a Cordyceps sinensis fungus (20 mg/kg); cyclophosphamide-induced-mice (70 mg/kg) | Spleen and thymus index, IL-10, TNF-α, INF-γ |
| Reprinted/adapted with permission from Ref. [28]. 2011, Marta Ciszek-Lenda | Mouse macrophage | 5 × 105 cell/mL | 1 d | LPS (1 μg/mL); Lactobacillus rhamnosus KL37, Escherichia coli 0111:B4, whole heat-killed bacteria, EPSs from Lactobacillus rhamnosus KL37(100 μg/mL) | TNF-α, IL-6, IL-10, IL-12p40 |
| Reprinted/adapted with permission from Ref. [29]. 2014, Claudio Hidalgo-Cantabrana | Immune cells isolated from mouse GALT and PBMC | n = 8 2 × 106 cell/mL | 5.25 d | EPSs of sub-species Bifidobacterium (100 μg/mL) PHA (2.5 μg/mL) | IFNγ, IL-10, IL-1a, IL-4, TNF-α, IL-17, TGF-β |
| Reprinted/adapted with permission from Ref. [30]. 2011, Lu Sheng | Splenocytes, thymocytes | ①, ②: n = 6 1 × 106 cell/mL | ① 1 d, 2 d ② 20 h | ① Add 10 μg of the mitogen concanavalin A and different doses of EPSs (the ultimate concentrations were 12.5, 25, 50, 100 μg/mL, respectively) ② Treat with 25, 50, 100 μg/mL of Cordyceps sinensis | Splenocytes, thymocytes, TNF-α, IFN-γ, IL-2 |
| Reprinted/adapted with permission from Ref. [31]. 2010, Jun Liu | Spleen and thymus of mice | n = 6 | 42 d | EPS from endophytic bacterium Paenibacillus polymyxa EJS-3 (100, 200, 400 mg/kg body weight per day); d-Gal-induced mice (100 mg/kg) | Spleen and thymus index |
| Reprinted/adapted with permission from Ref. [32]. 2013, Yuxing Guo | Spleen and thymus of mice | n = 10 | 2 d | Selenium EPS produced by Lactococcus lactis subsp. Lactis (50, 100, 150 mg/kg); EPSs produced by Lactococcus lactis subsp. Lactis (50, 100, 150 mg/kg); CY treatment of mice (50 mg/kg) | Spleen and thymus index |
| Reprinted/adapted with permission from Ref. [33]. 2016, Lei Zhu | Thymus and spleen of mice | n = 7 | 14 d | 5-Fu, (20 mg/kg); EPS from Rhizopus nigricans, at the doses of 50, 100, 200 mg/kg combined with 5-Fu at a dose of 20 mg/kg | Index and weight for thymus and spleen |
| Reprinted/adapted with permission from Ref. [34]. 2020, Saif M. Abed | Mouse serum | Triple dilution | 14 d | EPSs produced from Vancomycin Resistant Enterococcus faecalis (0.5, 1 mg/mL) | IL-10 |
| Reprinted/adapted with permission from Ref. [35]. 2009, Adriana Arena | Human PMBC | 2 × 106 cell/mL | 1 d | PBMC was added with EPSs (0, 50, 100, 200, and 300 μg/mL); | IFN-α, TNF-α, IL-4, IL-10, IL-12, IL-18 |
| Reprinted/adapted with permission from Ref. [36]. 2015, Chiaki Matsuzaki | Rat plasma | n = 4 | 42 d | Add ad labium the NTM048 EPS-containing experimental water at a concentration of 0%, 0.05%, 0.1%, 0.5%, or 1% w/v | IgA, IgG |
| Reprinted/adapted with permission from Ref. [37]. 2020, Bukola Adebayo-Tayo | Mouse serum | n = 4 Diluted 10 times normal saline | 15 d | Cigarettes; 20 mg/kg/day 5-fluorouracil; with 500 nM aqueous solution of EPSWLD or EPSMLD through intra-peritoneal (IP) administration | IgG, IgA, IgM |
| Study | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Reprinted/adapted with permission from Ref. [26]. 2016, Jianfeng Cao | ✓ | - | ✓ | - | - | - | - | ✓ | ✓ | 4 |
| Reprinted/adapted with permission from Ref. [27]. 2016, Ting Hu | ✓ | - | ✓ | - | - | ✓ | - | ✓ | ✓ | 5 |
| Reprinted/adapted with permission from Ref. [28]. 2011, Marta Ciszek-Lenda | ✓ | - | - | - | - | - | - | ✓ | ✓ | 3 |
| Reprinted/adapted with permission from Ref. [29]. 2014, Claudio Hidalgo-Cantabrana | ✓ | - | - | - | - | ✓ | - | ✓ | ✓ | 4 |
| Reprinted/adapted with permission from Ref. [30]. 2011, Lu Sheng | ✓ | - | - | - | - | - | - | ✓ | ✓ | 3 |
| Reprinted/adapted with permission from Ref. [31]. 2010, Jun Liu | ✓ | - | ✓ | - | - | ✓ | - | ✓ | ✓ | 5 |
| Reprinted/adapted with permission from Ref. [32]. 2013, Yuxing Guo | ✓ | - | ✓ | - | - | ✓ | - | ✓ | ✓ | 5 |
| Reprinted/adapted with permission from Ref. [33]. 2016, Lei Zhu | ✓ | - | - | - | - | ✓ | - | ✓ | ✓ | 4 |
| Reprinted/adapted with permission from Ref. [34]. 2020, Saif M. Abed | ✓ | - | ✓ | - | - | ✓ | - | ✓ | ✓ | 5 |
| Reprint-ed/adapted with permission from Ref. [35]. 2009, Adriana Arena | ✓ | ✓ | ✓ | ✓ | 4 | |||||
| Reprinted/adapted with permission from Ref. [36]. 2015, Chiaki Matsuzaki | ✓ | - | - | - | - | ✓ | - | ✓ | ✓ | 4 |
| Reprinted/adapted with permission from Ref. [37]. 2020, Bukola Adebayo-Tayo | - | - | - | - | - | - | - | - | ✓ | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, Y.; Zhang, J.; Wang, Y.; Liu, Y. The Regulation of Micro-Organisms’ Extra-Cellular Polysaccharides on Immunity: A Meta-Analysis. Foods 2022, 11, 1949. https://doi.org/10.3390/foods11131949
Zhang J, Chen Y, Zhang J, Wang Y, Liu Y. The Regulation of Micro-Organisms’ Extra-Cellular Polysaccharides on Immunity: A Meta-Analysis. Foods. 2022; 11(13):1949. https://doi.org/10.3390/foods11131949
Chicago/Turabian StyleZhang, Jin, Yirui Chen, Jiaqi Zhang, Yitong Wang, and Yanan Liu. 2022. "The Regulation of Micro-Organisms’ Extra-Cellular Polysaccharides on Immunity: A Meta-Analysis" Foods 11, no. 13: 1949. https://doi.org/10.3390/foods11131949
APA StyleZhang, J., Chen, Y., Zhang, J., Wang, Y., & Liu, Y. (2022). The Regulation of Micro-Organisms’ Extra-Cellular Polysaccharides on Immunity: A Meta-Analysis. Foods, 11(13), 1949. https://doi.org/10.3390/foods11131949






