Purified Saponins in Momordica charantia Treated with High Hydrostatic Pressure and Ionic Liquid-Based Aqueous Biphasic Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Total Saponins
2.2.1. Single Factor Experiments for Extraction
2.2.2. Box–Behnken Design for Extraction Process
2.3. Purification of Total Saponins
2.3.1. Single Factor Experiments for Purification
2.3.2. Box–Behnken Design Optimization for Purification Process
2.4. Determination of the Total Saponins Content
2.5. Purification of Total Saponins from M. charantia
2.6. Calculation of Extraction Efficiency and Purity of Total Saponins
2.7. LC/MS Conditions and Parameters
2.8. Statistical Analysis
3. Results and Discussion
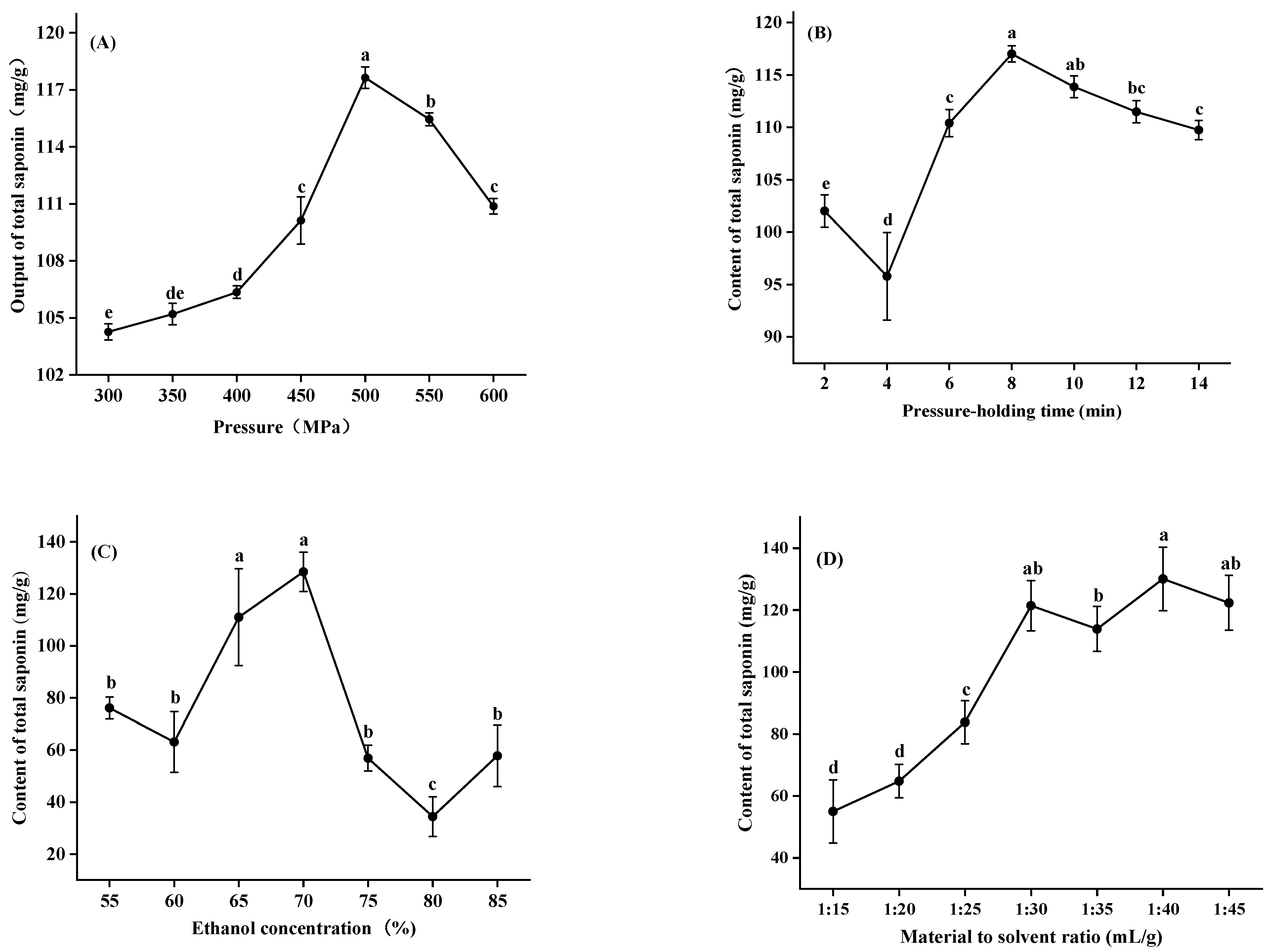
3.1. Single Factor Experiments of Extraction Conditions
3.1.1. Pressure
3.1.2. Pressure-Holding Time
3.1.3. Ethanol Concentration
3.1.4. Material to Solvent Ratio
3.1.5. Optimization of Extraction Conditions by Box–Behnken Design
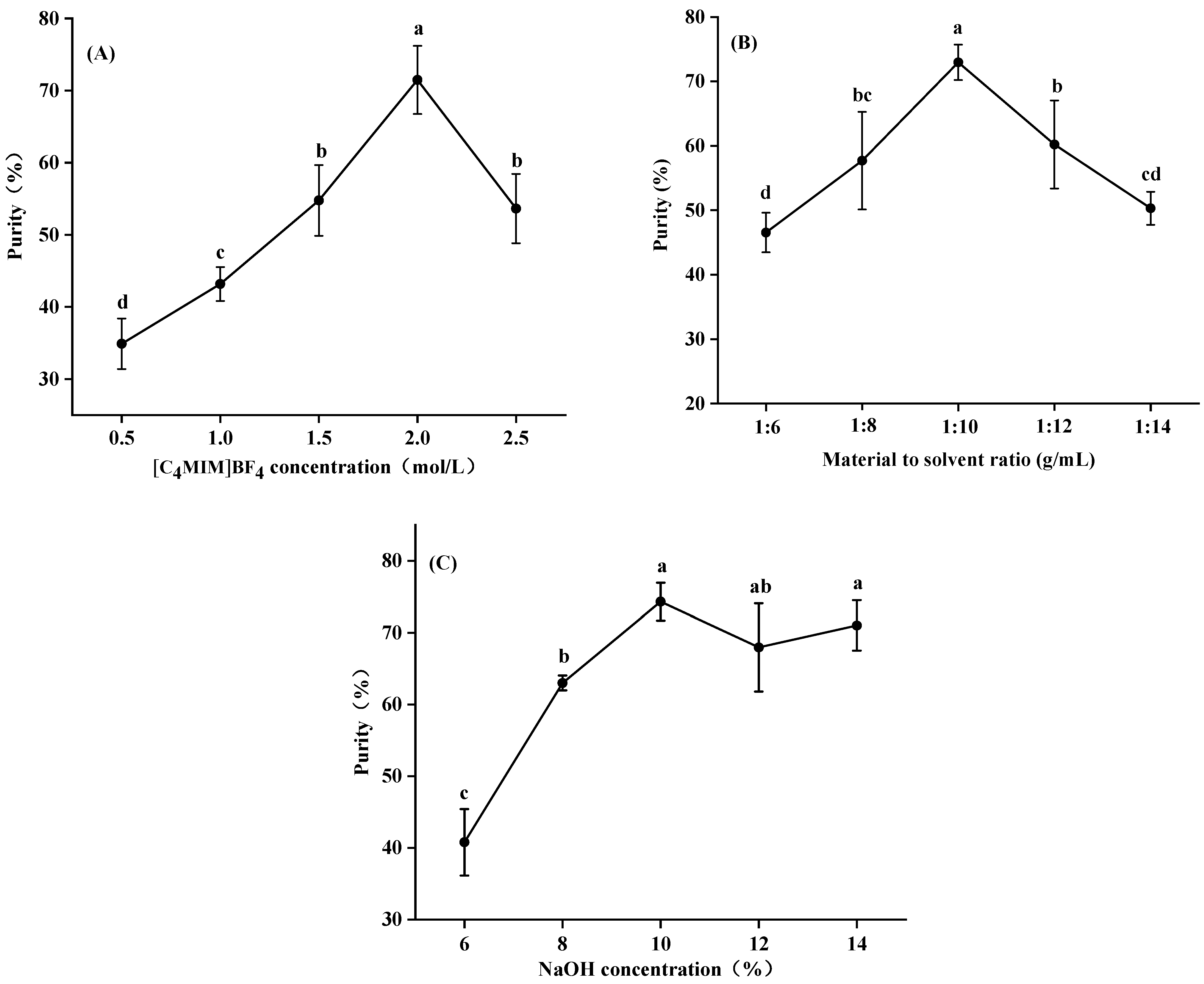
3.2. Purification of Total Saponins under the Optimized Extraction Conditions
3.2.1. Effect of [C4MIM]BF4 Concentration, Sodium Hydroxide Concentration and Material to Solvent Ratio on Purification Efficiency of Saponins by IL-Based ABS
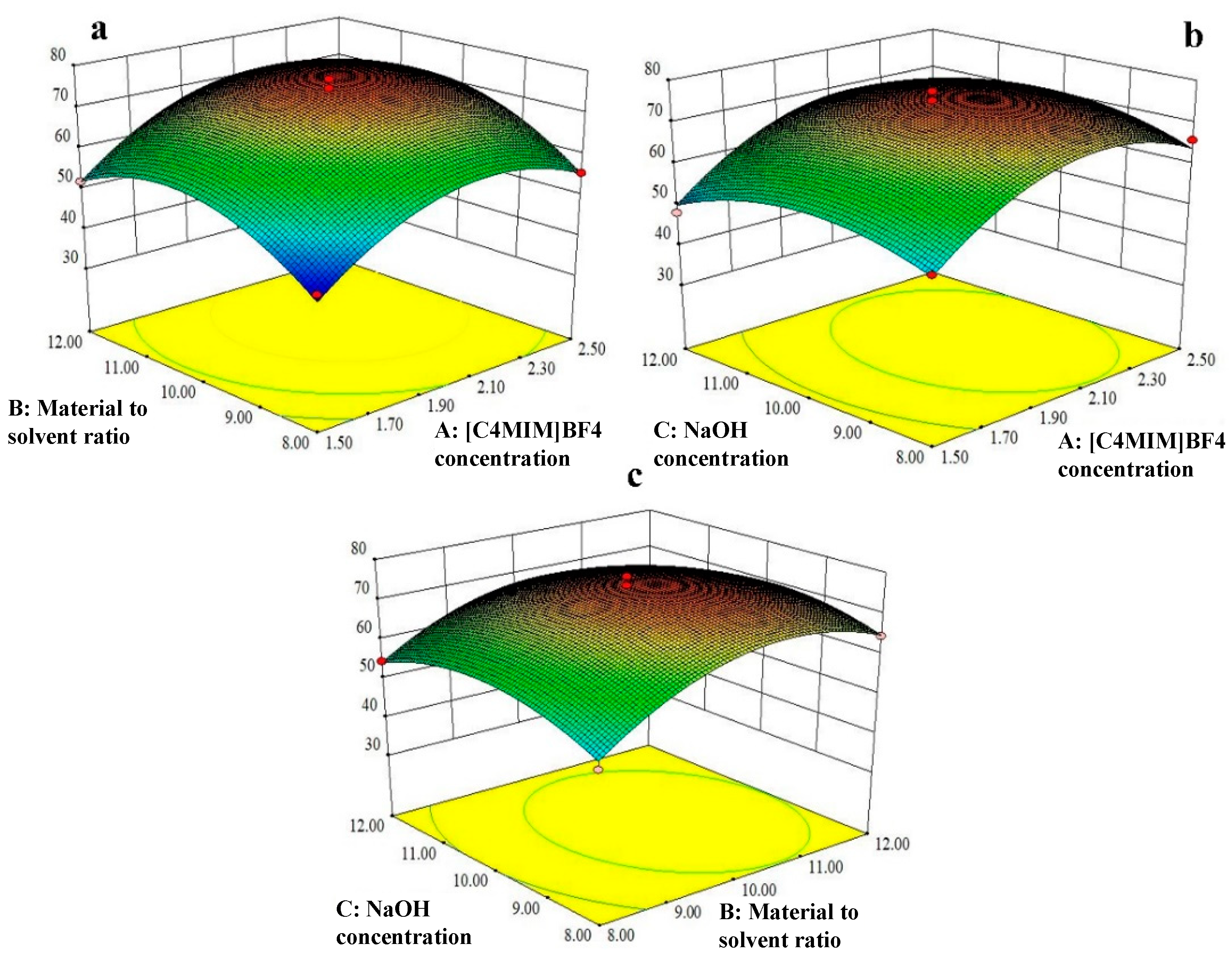
3.2.2. Optimization of Purification Conditions by BBD
3.3. Mass Spectral Identification of Saponins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grover, J.K.; Yadav, S.P. Pharmacological actions and potential uses of Momordica charantia: A review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Xie, J.H.; Zhu, J.H.; Peng, Y. Ethanol modified supercritical carbon dioxide extraction of flavonoids from Momordica charantia L. and its antioxidant activity. Food Bioprod. Process. 2012, 90, 579–587. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.; Tsai, T.H.; Li, Y.Y.; Wu, W.H.; Huang, C.J.; Tsai, P.J. Wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) extract and its bioactive components suppress Propionibacterium acnes -induced inflammation. Food Chem. 2012, 135, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.X.; Meng, Z.F.; Kuerban, K.; Qi, F.L.; Liu, J.Y.; Wei, Y.X.; Wang, Q.; Jiang, S.S.L.; Feng, M.Q.; Ye, L. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018, 9, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, A.C.; Ma, J.; Kavalier, A.; He, K.; Brillantes, A.B.; Kennelly, E.J. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine 2011, 19, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Clouatre, D.L.; Rao, S.N.; Preuss, H.G. Bitter Melon Extracts in Diabetic and Normal Rats Favorably Influence Blood Glucose and Blood Pressure Regulation. J. Med. Food 2011, 14, 1496–1504. [Google Scholar] [CrossRef]
- Perez, J.L.; Jayaprakasha, G.K.; Patil, B.S. Metabolite profiling and in vitro biological activities of two commercial Momordica charantia (M. charantia Linn.) cultivars. Food Chem. 2019, 288, 178–186. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Tan, S.P.; Vuong, Q.V.; Stathopoulos, C.E.; Parks, S.E.; Roach, P.D. Optimized aqueous extraction of saponins from bitter melon for production of a saponin-enriched bitter melon powder. J. Food Sci. 2014, 79, 1372–1381. [Google Scholar] [CrossRef]
- Lin, H.M.; Zhang, Y.G.; Han, M.; Yang, L.M. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason. Sonochem. 2013, 20, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wang, Y.; Shao, X.; Zhu, C.; Lin, Y.; Gao, S.; Tang, D. Extraction and purification of triterpenoid saponins from licorice by ionic liquid based extraction combined with in situ alkaline aqueous biphasic systems. Sep. Purif. Technol. 2020, 247, 116953. [Google Scholar] [CrossRef]
- Chen, R.Z.; Meng, F.L.; Zhang, S.Q.; Liu, Z.Q. Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside from ginseng. Sep. Purif. Technol. 2018, 66, 340–346. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Chen, R.Z.; Wang, C.Z. Experiment study on ultrahigh pressure extraction of ginsenosides. J. Food Eng. 2017, 79, 1–5. [Google Scholar] [CrossRef]
- Ma, J.; Tong, P.Y.; Chen, Y.J.; Wang, Y.; Ren, H.; Gao, Z.P.; Yue, T.L.; Long, F.Y. The inhibition of pectin oligosaccharides on degranulation of RBL-2H3 cells from apple pectin with high hydrostatic pressure assisted enzyme treatment. Food Chem. 2022, 371, 131097. [Google Scholar] [CrossRef]
- Wu, W.; Xiang, F.; He, F. Polyphenols from Artemisia argyi leaves: Environmentally friendly extraction under high hydrostatic pressure and biological activities. Ind. Crops Prod. 2021, 171, 113951. [Google Scholar] [CrossRef]
- Li, W.H.; Zhang, F.S.; Liu, P.L.; Bai, Y.F.; Gao, L.; Shen, Q. Effect of high hydrostatic pressure on physicochemical, thermal and morphological properties of mung bean (Vigna radiata L.) starch. J. Food Eng. 2011, 103, 388–393. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Wang, Z.L.; Dai, J.G.; Chen, L.; Huang, Y.F. Preparation and quality assessment of high-purity ginseng total saponins by ion exchange resin combined with macroporous adsorption resin separation. Chin. J. Nat. Med. 2014, 12, 382. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Cognigni, A.; de Faria, E.L.P.; Silvestre, A.J.D.; Zirbs, R.; Freire, M.G.; Bica, K. Valorization of olive tree leaves: Extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids. Sep. Purif. Technol. 2018, 204, 30–37. [Google Scholar] [CrossRef]
- Yu, T.H.; Olsson, E.; Lian, G.P.; Liu, L.; Huo, F.; Zhang, X.P.; Cai, Q. Prediction of the Liquid–Liquid Extraction Properties of Imidazolium-Based Ionic Liquids for the Extraction of Aromatics from Aliphatics. J. Chem. Inf. Modeling 2021, 61, 3376–3385. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Romero, J. Solvent extraction of rare-earth elements with ionic liquids: Toward a selective and sustainable extraction of these valuable elements. Curr. Opin. Green Sustain. Chem. 2021, 27, 100428. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, A.R.; Lu, B.L.; Li, Z.Y.; Wang, J.J. Dissolution of cellulose in 1-allyl-3-methylimizodalium carboxylates at room temperature: A structure–property relationship study. Carbohydr. Polym. 2015, 117, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, W.L.; Feng, Y.C.; Fang, X.; Li, J.Y.; Gao, X.H.; Li, H.L. Simultaneous recovery of glycyrrhizic acid and liquiritin from Chinese licorice root (Glycyrrhiza uralensis Fisch) by aqueous two-phase system and evaluation biological activities of extracts. Sep. Sci. Technol. 2018, 53, 1342–1350. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef]
- Santos, J.H.P.M.; Trigo, J.P.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Ventura, S.P.M. Fractionation of Isochrysis galbana proteins, arabinans, and glucans using ionic-liquid-based aqueous biphasic systems. ACS Sustain. Chem. Eng. 2018, 6, 14042–14053. [Google Scholar] [CrossRef]
- Popovich, D.; Li, L.; Zhang, W. Bitter melon (Momordica charantia) triterpenoid extract reduces preadipocyte viability, lipid accumulation and adiponectin expression in 3T3-L1 cells. Food Chem. Toxicol. 2010, 48, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, X.; Liu, Y.; Wu, Q.; Jin, Y. An optimized microwave-assisted extraction method for increasing yields of rare ginsenosides from Panax quinquefolius L. J. Ginseng. Res. 2016, 40, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Xie, C.; Wei, Y.; Li, J.; Yang, X. Purification and characterisation of an antifungal protein, MCha-Pr, from the intercellular fluid of bitter gourd (Momordica charantia) leaves. Protein Expr. Purif. 2015, 107, 43–49. [Google Scholar] [CrossRef]
- Quental, M.V.; Pereira, M.M.; Ferreira, A.M.; Pedro, S.N.; Shahriari, S.; Mohamadou, A.; Coutinho, J.A.P.; Freire, M.G. Enhanced separation performance of aqueous biphasic systems formed by carbohydrates and tetralkylphosphonium- or tetra-lkylammonium-based ionic liquids. Green Chem. 2018, 20, 2978–2983. [Google Scholar] [CrossRef]
- Tan, Z.; Yi, Y.; Wang, H.; Zhou, W.; Wang, C. Extraction, Preconcentration and Isolation of Flavonoids from Apocynum venetum L. Leaves Using Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with an Aqueous Biphasic System. Molecules 2016, 21, 262. [Google Scholar] [CrossRef] [Green Version]
- Tuan, P.A.; Kim, J.K.; Park, N.I.; Lee, S.Y.; Park, S.U. Carotenoid content and expression of phytoene synthase and phytoene desaturase genes in bitter melon (Momordica charantia). Food Chem. 2011, 126, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Cumming, E.; Manoharan, G.; Kalasz, H.; Adeghate, E. Medicinal chemistry of the anti-diabetic effects of Momordica charantia: Active constituents and modes of actions. Open Med. Chem. J. 2011, 5, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| No. | Pressure (MPa) | Pressure-Holding Time (min) | Ethanol Concentration (%) | Ratio of Material to Solvent (g/mL) | Output (mg/g) |
|---|---|---|---|---|---|
| 1 | 500 | 6 | 75 | 1:35 | 99.701 |
| 2 | 450 | 8 | 75 | 1:35 | 97.916 |
| 3 | 500 | 8 | 70 | 1:35 | 123.638 |
| 4 | 500 | 8 | 70 | 1:35 | 128.730 |
| 5 | 450 | 8 | 65 | 1:35 | 108.257 |
| 6 | 500 | 8 | 75 | 1:30 | 102.640 |
| 7 | 500 | 8 | 70 | 1:35 | 123.690 |
| 8 | 500 | 8 | 75 | 1:40 | 101.223 |
| 9 | 500 | 8 | 70 | 1:35 | 120.856 |
| 10 | 500 | 6 | 70 | 1:40 | 103.690 |
| 11 | 500 | 10 | 75 | 1:35 | 86.315 |
| 12 | 500 | 10 | 70 | 1:30 | 118.021 |
| 13 | 500 | 10 | 70 | 1:40 | 111.722 |
| 14 | 500 | 6 | 65 | 1:35 | 107.260 |
| 15 | 500 | 10 | 65 | 1:35 | 118.598 |
| 16 | 550 | 8 | 70 | 1:30 | 117.076 |
| 17 | 550 | 10 | 70 | 1:35 | 109.622 |
| 18 | 450 | 8 | 70 | 1:30 | 111.512 |
| 19 | 500 | 6 | 70 | 1:30 | 112.457 |
| 20 | 550 | 8 | 70 | 1:40 | 111.932 |
| 21 | 450 | 8 | 70 | 1:40 | 108.047 |
| 22 | 450 | 10 | 70 | 1:35 | 113.087 |
| 23 | 450 | 6 | 70 | 1:35 | 103.165 |
| 24 | 500 | 8 | 65 | 1:40 | 110.094 |
| 25 | 550 | 6 | 70 | 1:35 | 121.906 |
| 26 | 550 | 8 | 75 | 1:35 | 84.478 |
| 27 | 500 | 8 | 70 | 1:35 | 126.052 |
| 28 | 500 | 8 | 65 | 1:30 | 114.504 |
| 29 | 550 | 8 | 65 | 1:35 | 115.711 |
| No. | A [C4MIM]BF4 Concentration (mol/L) | B Material to Solvent Ratio | C Sodium Hydroxide Concentration (%) | Purity (%) |
|---|---|---|---|---|
| 1 | 1.5 | 1:8 | 10.0 | 45.28 |
| 2 | 1.5 | 1:10 | 8.0 | 52.61 |
| 3 | 1.5 | 1:12 | 10.0 | 51.95 |
| 4 | 2.0 | 1:10 | 10.0 | 77.33 |
| 5 | 2.0 | 1:10 | 10.0 | 74.98 |
| 6 | 2.5 | 1:10 | 12.0 | 63.69 |
| 7 | 2.0 | 1:10 | 10.0 | 75.15 |
| 8 | 2.0 | 1:10 | 10.0 | 74.31 |
| 9 | 2.5 | 1:12 | 10.0 | 64.21 |
| 10 | 2.0 | 1:10 | 10.0 | 72.93 |
| 11 | 2.0 | 1:12 | 12.0 | 63.20 |
| 12 | 1.5 | 1:10 | 12.0 | 48.16 |
| 13 | 2.5 | 1:8 | 10.0 | 55.55 |
| 14 | 2.0 | 1:8 | 12.0 | 54.62 |
| 15 | 2.0 | 1:8 | 8.0 | 50.71 |
| 16 | 2.5 | 1:10 | 8.0 | 65.87 |
| 17 | 2.0 | 1:12 | 8.0 | 64.56 |
| Compound | Formula | Theoretical (m/z) | Experimental (m/z) | Adduct | Δppm |
|---|---|---|---|---|---|
| Kuguaglycoside A | C37H62O8 | 657.43 | 657.41 | M + Na | 30.42 |
| Momordicoside L | C36H58O9 | 658.44 | 658.45 | M + Na | −15.19 |
| Kuguacin B | C30H48O3 | 457.34 | 457.36 | M + H | −43.73 |
| Kuguacin J | C30H46O3 | 455.35 | 455.34 | M + H | 21.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Yang, H.; Chen, Y.; Feng, X.; Wu, C.; Long, F. Purified Saponins in Momordica charantia Treated with High Hydrostatic Pressure and Ionic Liquid-Based Aqueous Biphasic Systems. Foods 2022, 11, 1930. https://doi.org/10.3390/foods11131930
Ma J, Yang H, Chen Y, Feng X, Wu C, Long F. Purified Saponins in Momordica charantia Treated with High Hydrostatic Pressure and Ionic Liquid-Based Aqueous Biphasic Systems. Foods. 2022; 11(13):1930. https://doi.org/10.3390/foods11131930
Chicago/Turabian StyleMa, Jing, Hongkai Yang, Yajing Chen, Xiaoping Feng, Chunyu Wu, and Fangyu Long. 2022. "Purified Saponins in Momordica charantia Treated with High Hydrostatic Pressure and Ionic Liquid-Based Aqueous Biphasic Systems" Foods 11, no. 13: 1930. https://doi.org/10.3390/foods11131930
APA StyleMa, J., Yang, H., Chen, Y., Feng, X., Wu, C., & Long, F. (2022). Purified Saponins in Momordica charantia Treated with High Hydrostatic Pressure and Ionic Liquid-Based Aqueous Biphasic Systems. Foods, 11(13), 1930. https://doi.org/10.3390/foods11131930





