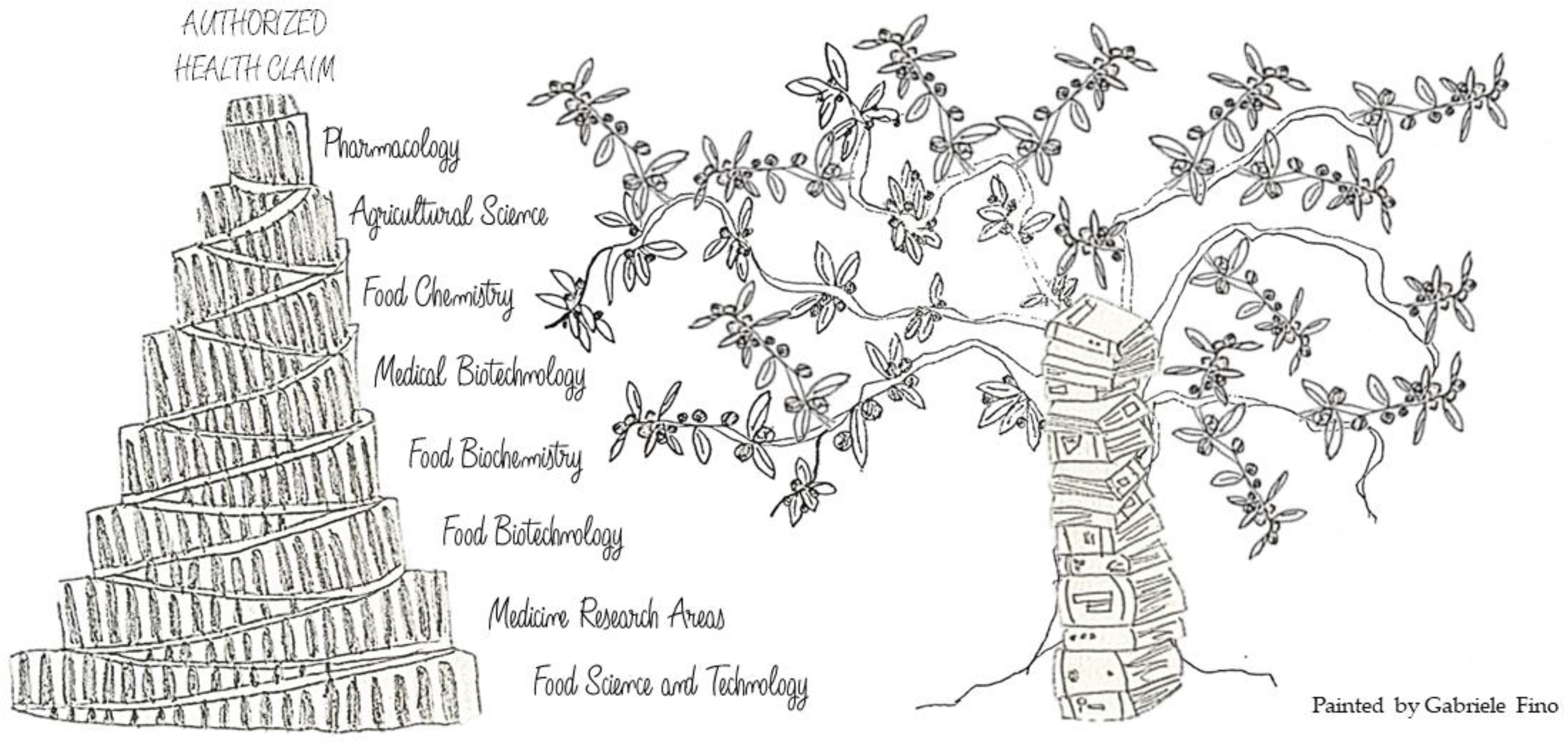
The Tower of Babel of Pharma-Food Study on Extra Virgin Olive Oil Polyphenols
Abstract
:1. Introduction
The Tower of Babel Model: A Challenge for Pharma-Food Science Communication on EVOO Polyphenols
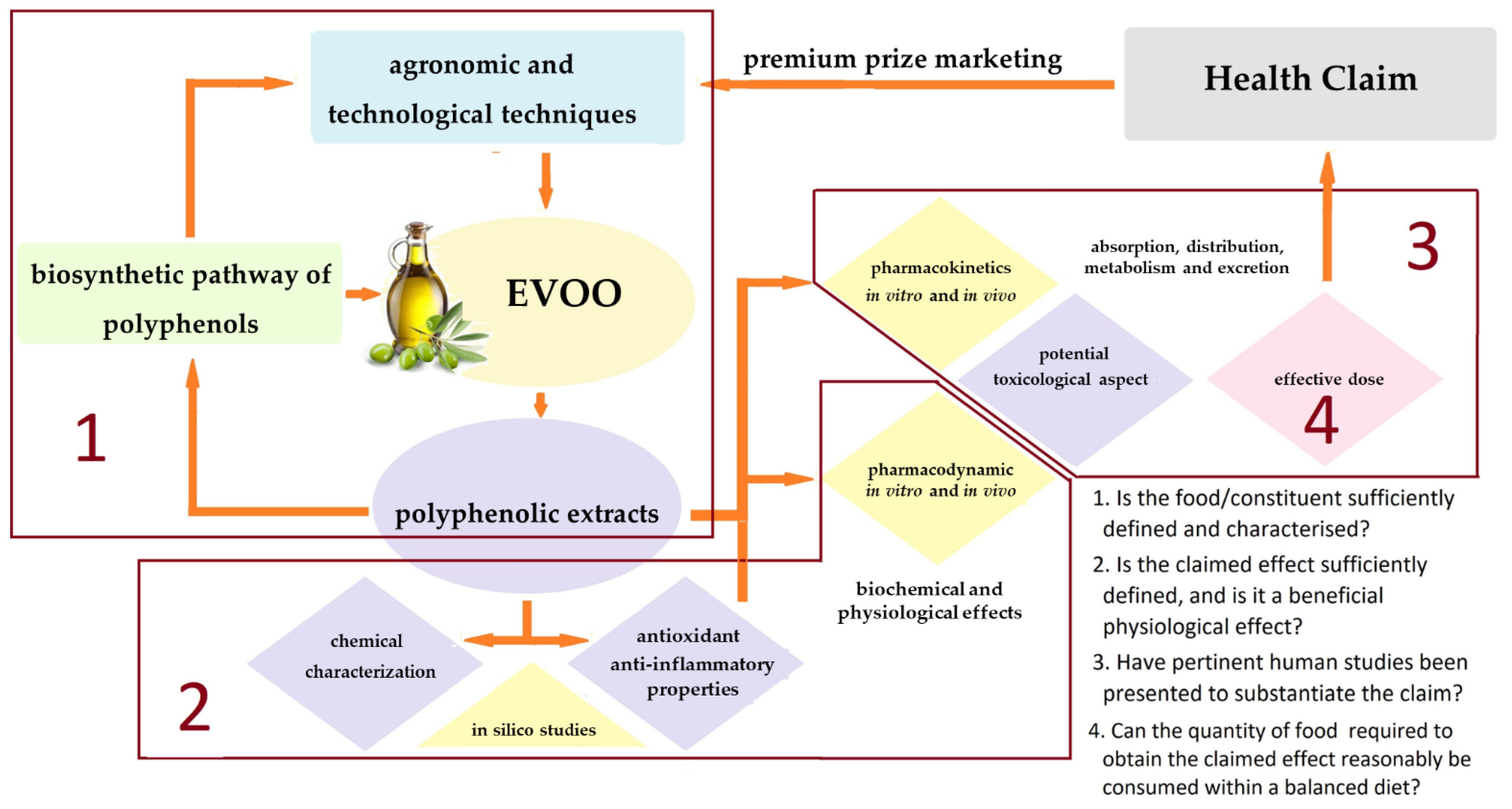
2. EVOO Polyphenols
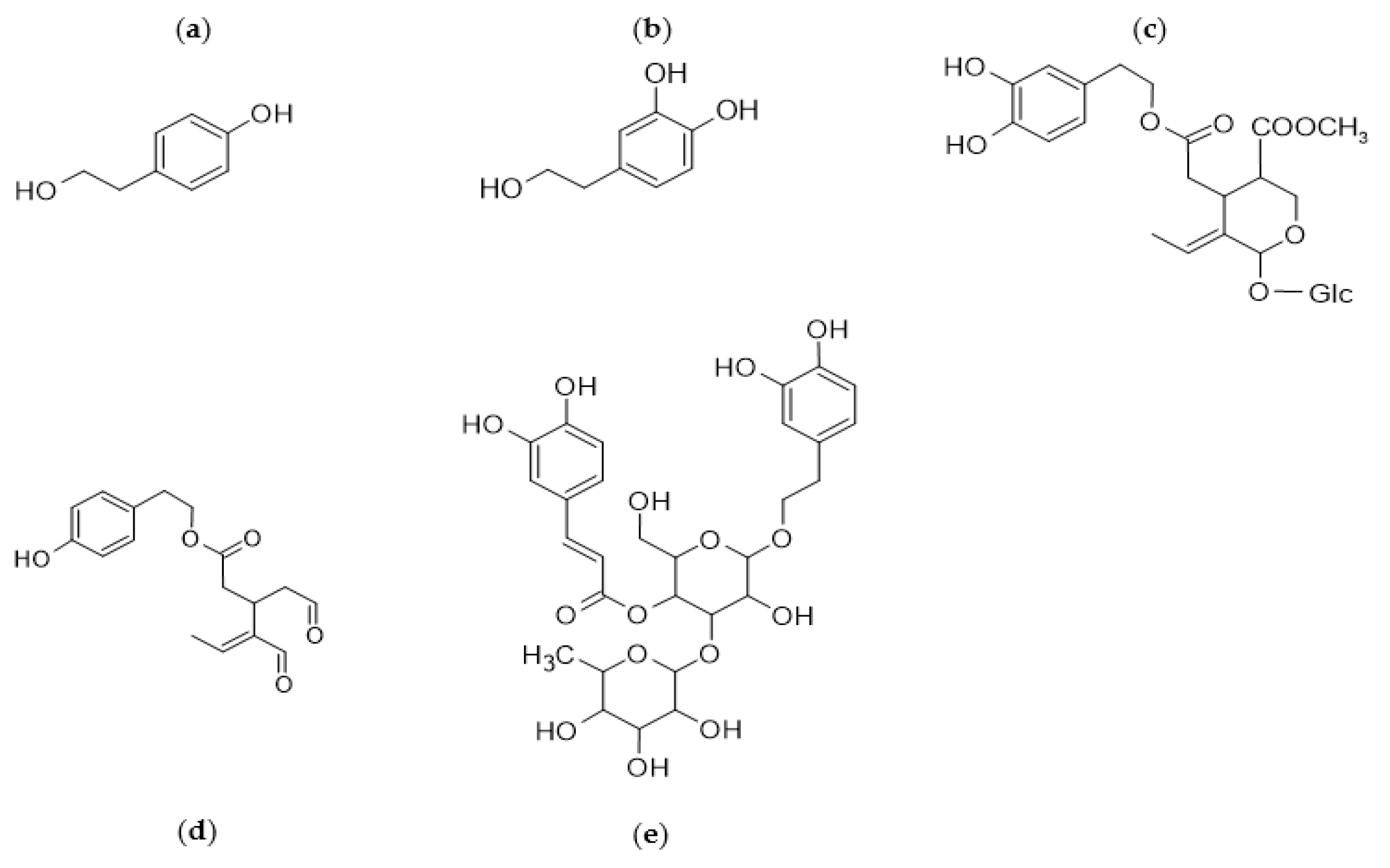
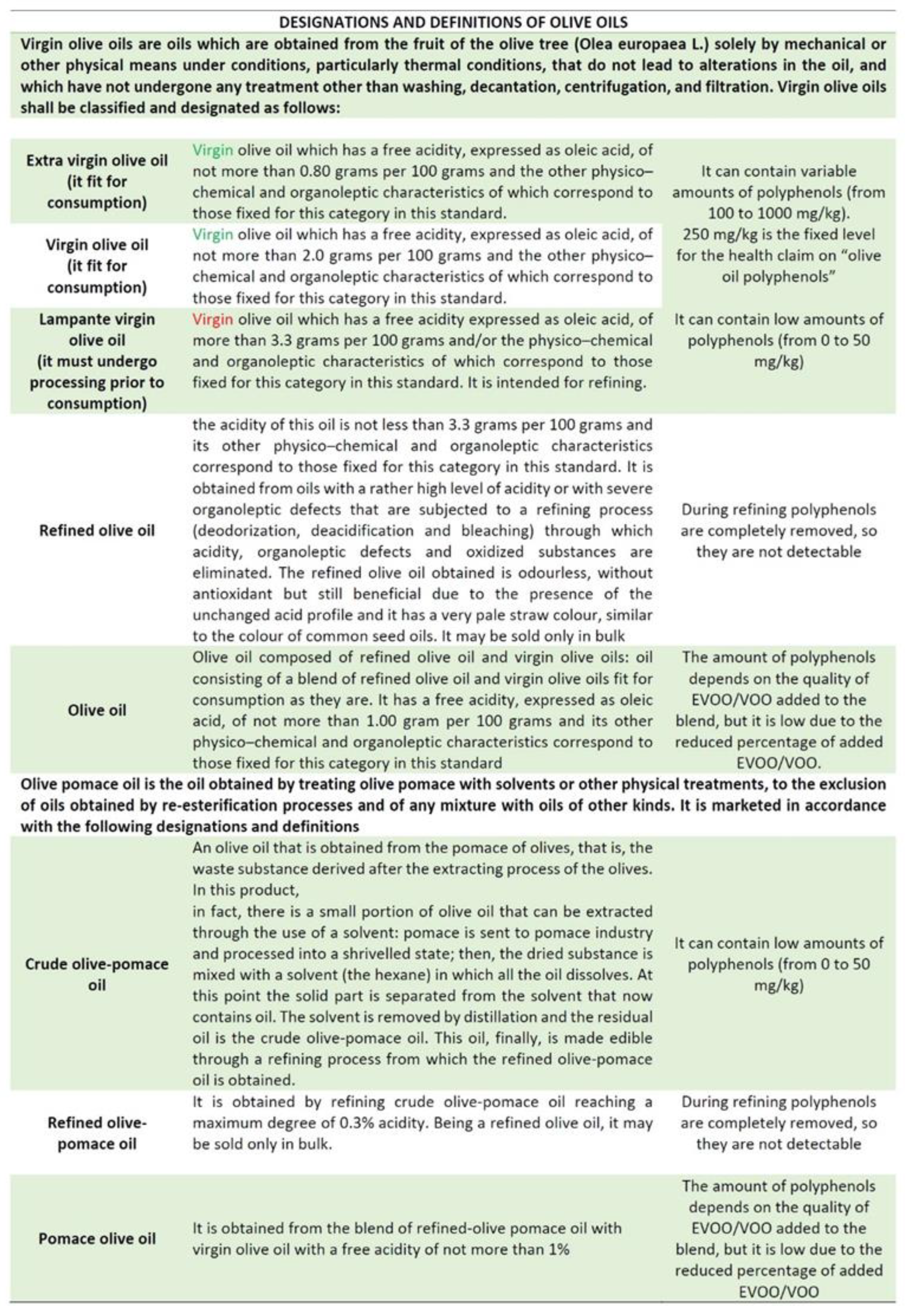
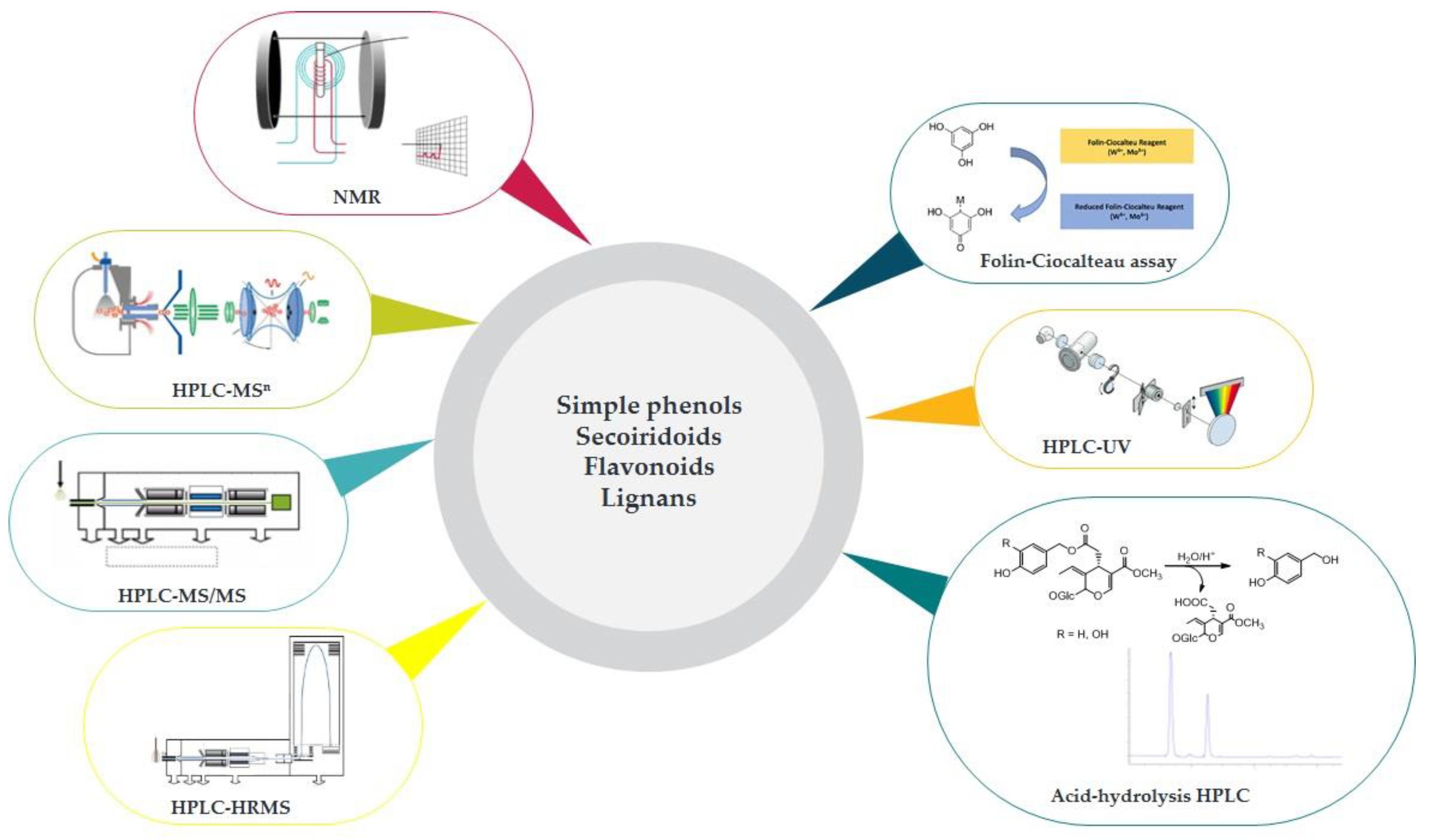
2.1. Quali- and Quantitative EVOO Polyphenols Characterization: A Dual Communication?
2.2. Characterization of EVOO Polyphenols: The Lack of an Official Methodology
3. EVOO Polyphenols Antioxidant and Anti-Inflammatory Properties
3.1. Chemical Antioxidant EVOO Polyphenols Assays: A Preliminary Investigation
- The type of antioxidant measured (e.g., lipophilic, hydrophilic, enzymatic, and non-enzymatic);
- The character of assay medium (e.g., aqueous, and organic solvent, direct or indirect, in situ and ex situ);
- The type of assay reagent (e.g., radical and non-radical initiated reactions);
- The mechanism of action.
- Single electron transfer (SET);
- Hydrogen atom transfer (HAT).
- Folin–Ciocalteu reagent (FCR);
- Trolox equivalence antioxidant capacity (TEAC);
- 2,2-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay (ABTS+);
- Ferric ion reducing antioxidant power (FRAP);
- CUPRAC: “total antioxidant potential” assay using a Cu(II) complex as an oxidant;
- 2,2-Diphenyl-1-picrylhydrazyl radical scavenging capacity assay (DPPH˙);
- Fast Blue BB diazonium salt (FBBB).
- Inhibition of induced low-density lipoprotein autoxidation;
- Oxygen radical absorbance capacity (ORAC);
- Total radical trapping antioxidant parameter (TRAP);
- Crocin bleaching assays [55].
3.2. In Vitro Antioxidant and Anti-Inflammatory EVOO Polyphenols Screening
3.2.1. Inhibition of Protein Denaturation Assay
3.2.2. Membrane Stabilization Method
- a.
- Membrane hemolysis induced by hypotonic solution
- b.
- Heat-induced hemolysis
3.2.3. Assay of Cyclooxygenase and 5-Lipooxygenase Inhibition
4. In Vitro EVOO Polyphenols Biological Screening: A Point about the Potential Mechanism of Action
5. In Silico Studies on EVOO Polyphenols
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Caputo, P.; Oliviero Rossi, C.; Crupi, P.; Muraglia, M.; Rago, V.; Malivindi, R.; Clodoveo, M.L.; Restuccia, D.; Aiello, F. A Tara Gum/Olive Mill Wastewaters Phytochemicals Conjugate as a New Ingredient for the Formulation of an Antioxidant-Enriched Pudding. Foods 2022, 11, 158. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. History and characteristics of the olive tree. In Olive Oil Chemistry and Technology; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 1996. [Google Scholar]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; de Cássia Freitas, K.; Santana, L.F.; Pott, A.; Donadon, J.R.; de Cássia Avellaneda Guimarães, R. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, S.; Liso, M.; Verna, G.; Curci, F.; Milani, G.; Faienza, M.F.; Franchini, C.; Moschetta, A.; Chieppa, M.; Clodoveo, M.L.; et al. Extra Virgin Olive Oil Extracts Modulate the Inflammatory Ability of Murine Dendritic Cells Based on Their Polyphenols Pattern: Correlation between Chemical Composition and Biological Function. Antioxidants 2021, 10, 1016. [Google Scholar] [CrossRef]
- Sarapis, K.; Thomas, C.J.; Hoskin, J.; George, E.S.; Marx, W.; Mayr, H.L.; Kennedy, G.; Pipingas, A.; Willcox, J.C.; Prendergast, L.A.; et al. The Effect of High Polyphenol Extra Virgin Olive Oil on Blood Pressure and Arterial Stiffness in Healthy Australian Adults: A Randomized, Controlled, Cross-Over Study. Nutrients 2020, 12, 2272. [Google Scholar] [CrossRef]
- Marrano, N.; Spagnuolo, R.; Biondi, G.; Cignarelli, A.; Perrini, S.; Vincenti, L.; Laviola, L.; Giorgino, F.; Natalicchio, A. Effects of Extra Virgin Olive Oil Polyphenols on Beta-Cell Function and Survival. Plants 2021, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Khandouzi, N.; Zahedmehr, A.; Nasrollahzadeh, J. Effect of polyphenol-rich extra-virgin olive oil on lipid profile and inflammatory biomarkers in patients undergoing coronary angiography: A randomised, controlled, clinical trial. Int. J. Food Sci. Nutr. 2021, 72, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Franco, M.; Toledo, E.; Luchsinger, J.; Willett, W.C.; Hu, F.B.; Martinez-Gonzalez, M.A. Olive oil and prevention of chronic diseases: Summary of an International conference. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 649–656. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on the substantiation of a health claim related to polyphenols in olive and maintenance of normal blood HDL cholesterol concentrations (ID 1639, further assessment) under Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2848. [Google Scholar] [CrossRef] [Green Version]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA Health Claim on Olive Oil Polyphenols: Acid Hydrolysis Validation and Total Hydroxytyrosol and Tyrosol Determination in Italian Virgin Olive Oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Romero-Segura, C.; Sanz, C.; Pérez, A.G. Modulating oxidoreductase activity modifies the phenolic content of virgin olive oil. Food Chem. 2015, 171, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hbaieb, R.H.; Kotti, F.; García-Rodríguez, R.; Gargouri, M.; Sanz, C.; Pérez, A.G. Monitoring endogenous enzymes during olive fruit ripening and storage: Correlation with virgin olive oil phenolic profiles. Food Chem. 2015, 174, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hbaieb, R.H.; Kotti, F.; Gargouri, M.; Msallem, M.; Vichi, S. Ripening and storage conditions of Chétoui and Arbequina olives: Part I. Effect on olive oils volatiles profile. Food Chem. 2016, 203, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Hbaieb, R.H.; Kotti, F.; Cortes-Francisco, N.; Caixach, J.; Gargouri, M.; Vichi, S. Ripening and storage conditions of Chétoui and Arbequina olives: Part II. Effect on olive endogenous enzymes and virgin olive oil secoiridoid profile determined by high resolution mass spectrometry. Food Chem. 2016, 210, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Servili, M. Characterization of phenolic and volatile composition of extra virgin olive oil extracted from six Italian cultivars using a cooling treatment of olive paste. LWT 2018, 87, 523–528. [Google Scholar] [CrossRef]
- Almeida, B.; Valli, E.; Bendini, A.; Gallina Toschi, T. Semi-industrial ultrasound-assisted virgin olive oil extraction: Impact on quality. Eur. J. Lipid Sci. Technol. 2017, 119, 1600230. [Google Scholar] [CrossRef]
- Aymen Bejaoui, M.; Sánchez-Ortiz, A.; Paz Aguilera, M.; Ruiz-Moreno, M.J.; Sánchez, S.; Jiménez, A.; Beltrán, G. High power ultrasound frequency for olive paste conditioning: Effect on the virgin olive oil bioactive compounds and sensorial characteristics. Innov. Food Sci. Emerg. Technol. 2018, 47, 136–145. [Google Scholar] [CrossRef]
- Miho, H.; Moral, J.; López-González, M.A.; Díez, C.M.; Priego-Capote, F. The phenolic profile of virgin olive oil is influenced by malaxation conditions and determines the oxidative stability. Food Chem. 2020, 314, 126183. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Minnocci, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Sebastiani, L.; Servili, M. High vacuum-assisted extraction affects virgin olive oil quality: Impact on phenolic and volatile compounds. Food Chem. 2021, 342, 128369. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; Durante, V.; Pesce, V.; Maiellaro, I.; Lovece, A.; Mercurio, A.; Laghezza, A.; Corbo, F.; et al. Comparison between Different Flavored Olive Oil Production Techniques: Healthy Value and Process Efficiency. Plant Foods Hum. Nutr. 2016, 71, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Moramarco, V.; Paduano, A.; Sacchi, R.; Di Palmo, T.; Crupi, P.; Corbo, F.; Pesce, V.; Distaso, E.; Tamburrano, P.; et al. Engineering design and prototype development of a full scale ultrasound system for virgin olive oil by means of numerical and experimental analysis. Ultrason. Sonochem. 2017, 37, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, R.; Losito, I.; Castellaneta, A.; De Ceglie, C.; Calvano, C.D.; Cataldi, T.R.I. Insight into the Storage-Related Oxidative/Hydrolytic Degradation of Olive Oil Secoiridoids by Liquid Chromatography and High-Resolution Fourier Transform Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 12310. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Regulation (EU) No 1308/2013 of the European Parliament and of the Council Establishing a Common Organization of the Markets in Agricultural Products and Repealing Council Official. J. Eur. Union Bruss. Belg. 2013, L 347/671, 1. Available online: https://data.europa.eu/eli/reg/2013/1308/oj (accessed on 22 May 2022).
- Buttriss, J.L.; Benelam, B. Nutrition and health claims: The role of food composition data. Eur. J. Clin. Nutr. 2010, 64 (Suppl. S3), S8–S13. [Google Scholar] [CrossRef]
- International Olive Council. Determination of the Content of Waxes, Fatty Acid Methyl Esters and Fatty Acid Ethyl Esters by Capillary Gas Chromatography. 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/Method-COI-T.20-Doc.-No-28-Rev.2-2017.pdf (accessed on 22 May 2022).
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct Measurement of Oleocanthal and Oleacein Levels in Olive Oil by Quantitative 1H NMR. Establishment of a New Index for the Characterization of Extra Virgin Olive Oils. J. Agric. Food Chem. 2012, 60, 11696. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Boskou, D. The health claim on “olive oil polyphenols” and the need for meaningful terminology and effective analytical protocols. Eur. J. Lipid Sci. Technol. 2015, 117, 1091–1094. [Google Scholar] [CrossRef]
- Dini, I.; Graziani, G.; Gaspari, A.; Fedele, F.L.; Sicari, A.; Vinale, F.; Cavallo, P.; Lorito, M.; Ritieni, A. New Strategies in the Cultivation of Olive Trees and Repercussions on the Nutritional Value of the Extra Virgin Olive Oil. Molecules 2020, 25, 2345. [Google Scholar] [CrossRef]
- Cerretani, L.; Gallina Toschi, T.; Bendini, A. Phenolic fraction of virgin olive oil: An overview on identified compounds and analytical methods for their determination. Funct. Plant Sci. Biotechnol 2009, 3, 69. [Google Scholar]
- Reboredo-Rodríguez, P.; Valli, E.; Bendini, A.; Di Lecce, G.; Simal-Gándara, J.; Gallina Toschi, T. A widely used spectrophotometric assay to quantify olive oil biophenols according to the health claim (EU Reg. 432/2012). Eur. J. Lipid Sci. Technol. 2016, 118, 1593–1599. [Google Scholar] [CrossRef]
- International Olive Council. Determination of Biophenols in Olive Oils by HPLC. 2009 COI/T.20/Doc.No 29/1-8. Available online: https://www.oelea.de/downloads/COI-T20-DOC-29-2009-DETERMINATION-OF-BIOPHENOLS-IN-OLIVE-OILSBY-HPLC.pdf (accessed on 22 May 2022).
- Romani, A.; Pinelli, P.; Mulinacci, N.; Galardi, C.; Vincieri, F.F.; Liberatore, L.; Cichelli, A. HPLC and HRGC analyses of polyphenols and secoiridoid in olive oil. Chromatographia 2001, 53, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Mastralexi, A.; Nenadis, N.; Tsimidou, M.Z. Addressing analytical requirements to support health claims on “olive oil polyphenols” (EC Regulation 432/2012). J. Agric. Food Chem. 2014, 62, 2459–2461. [Google Scholar] [CrossRef] [PubMed]
- Purcaro, G.; Codony, R.; Pizzale, L.; Mariani, C.; Conte, L. Evaluation of total hydroxytyrosol and tyrosol in extra virgin olive oils. Eur. J. Lipid Sci. Technol. 2014, 116, 805–811. [Google Scholar] [CrossRef]
- European Commission. Regulation EC No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Off. J. Eur. Union 2012, L136, 1. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32012R0432 (accessed on 22 May 2022).
- Mulinacci, N.; Giaccherini, C.; Ieri, F.; Innocenti, M.; Romani, A.; Vincieri, F.F. Evaluation of lignans and free and linked hydroxy-tyrosol and tyrosol in extra virgin olive oil after hydrolysis processes. J. Sci. Food Agric. 2006, 86, 757–764. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2010, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Losito, I.; Abbattista, R.; De Ceglie, C.; Castellaneta, A.; Calvano, C.D.; Cataldi, T. Bioactive Secoiridoids in Italian Extra-Virgin Olive Oils: Impact of Olive Plant Cultivars, Cultivation Regions and Processing. Molecules 2021, 26, 743. [Google Scholar] [CrossRef]
- Innocenti, M.; La Marca, G.; Malvagia, S.; Giaccherini, C.; Vincieri, F.F.; Mulinacci, N. Electrospray ionisation tandem mass spectrometric investigation of phenylpropanoids and secoiridoids from solid olive residue. Rapid Commun. Mass Spectrom. 2006, 20, 2013–2022. [Google Scholar] [CrossRef]
- Di Donna, L.; Mazzotti, F.; Napoli, A.; Salerno, R.; Sajjad, A.; Sindona, G. Secondary metabolism of olive secoiridoids. New microcomponents detected in drupes by electrospray ionization and high-resolution tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 273–278. [Google Scholar] [CrossRef]
- Christophoridou, S.; Dais, P. Novel approach to the detection and quantification of phenolic compounds in olive oil based on 31P nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2006, 54, 656–664. [Google Scholar] [CrossRef]
- Starec, M.; Calabretti, A.; Berti, F.; Forzato, C. Oleocanthal Quantification Using 1H NMR Spectroscopy and Polyphenols HPLC Analysis of Olive Oil from the Bianchera/Belica Cultivar. Molecules 2021, 26, 242. [Google Scholar] [CrossRef] [PubMed]
- Christophoridou, S.; Dais, P.; Tseng, L.H.; Spraul, M. Separation and identification of phenolic compounds in olive oil by coupling high-performance liquid chromatography with postcolumn solid-phase extraction to nuclear magnetic resonance spectroscopy (LC-SPE-NMR). J. Agric. Food Chem. 2005, 53, 4667–4679. [Google Scholar] [CrossRef]
- Pérez-Trujillo, M.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Parella, T. Separation and identification of phenolic compounds of extra virgin olive oil from Olea europaea L. by HPLC-DAD-SPE-NMR/MS. Identification of a new diastereoisomer of the aldehydic form of oleuropein aglycone. J. Agric. Food Chem. 2010, 58, 9129–9136. [Google Scholar] [CrossRef]
- Klikarová, J.; Rotondo, A.; Cacciola, F.; Ceslova, L.; Dugo, P.; Mondello, L.; Rignano, F. The Phenolic Fraction of Italian Extra Virgin Olive Oils: Elucidation through Combined Liquid Chromatography and NMR Approaches. Food Anal. Methods 2019, 12, 1759–1770. [Google Scholar] [CrossRef]
- Sánchez de Medina, V.; Calderón-Santiago, M.; El Riachy, M.; Priego-Capote, F.; Luque de Castro, M.D. High-resolution mass spectrometry to evaluate the influence of cross-breeding segregating populations on the phenolic profile of virgin olive oils. J. Sci. Food Agric. 2014, 94, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- Gosetti, F.; Bolfi, B.; Manfredi, M.; Calabrese, G.; Marengo, E. Determination of eight polyphenols and pantothenic acid in extra-virgin olive oil samples by a simple, fast, high-throughput and sensitive ultra high performance liquid chromatography with tandem mass spectrometry method. J. Sep. Sci. 2015, 38, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Aprile, A.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; Sabella, E.; De Bellis, L. Phenolic Profile and Antioxidant Activity of Italian Monovarietal Extra Virgin Olive Oils. Antioxidants 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasi, F.; Rocchetti, G.; Montesano, D.; Lucini, L.; Chiodelli, G.; Ghisoni, S.; Baccolo, G.; Simonetti, M.S.; Cossignani, L. Changes in extra-virgin olive oil added with Lycium barbarum L. carotenoids during frying: Chemical analyses and metabolomic approach. Food Res. Int. 2018, 105, 507–516. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Rui, H.L. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134 (Suppl. S12), 3479S–3485S. [Google Scholar] [CrossRef] [Green Version]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety-Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Augusto Ballus, C.; Dillenburg Meinhart, A.; de Souza Campos, F.A., Jr.; Teixeira Godoy, H. Total Phenolics of Virgin Olive Oils Highly Correlate with the Hydrogen Atom Transfer Mechanism of Antioxidant Capacity. J. Am. Oil Chem. Soc. 2015, 92, 843–851. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Celik, S. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Ninfali, P.; Aluigi, G.; Bacchiocca, M.; Magnani, M. Antioxidant capacity of extra-virgin olive oils. J. Am. Oil Chem. Soc. 2001, 78, 243–247. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Opitz, S.E.; Smrke, S.; Goodman, B.A.; Keller, M.; Schenker, S.; Yeretzian, C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary. Assays. Foods 2014, 3, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Putnik, P.; Kovačević, D.B.; Santos, J.S.; Calado, V.; Rocha, R.S.; Cruz, A.G.D.; Jarvis, B.; Rodionova, O.Y.; Pomerantsev, A. Trends in Chemometrics: Food Authentication, Microbiology, and Effects of Processing. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y.; Lee, J.; Lee, H.B.; Son, Y.Y.; Park, C.S.; Shetty, K.; Kim, Y.C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Paunkov, A.; Chartoumpekis, D.V.; Ziros, P.G.; Sykiotis, G.P. A Bibliometric Review of the Keap1/Nrf2 Pathway and its Related Antioxidant Compounds. Antioxidants 2019, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Zhongyi, J.; Haitao, Z.; Pusen, W.; Weitao, Q.; Lin, Z.; Xiao-Kang, L.; Futian, D. Different subpopulations of regulatory T cells in human autoimmune disease, transplantation, and tumor immunity. MedComm 2022, 3, e137. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.; Mercadante, A.Z. Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar. Drugs 2012, 10, 1784–1798. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Barrow, C.J. Lipase-Produced Hydroxytyrosyl Eicosapentaenoate is an Excellent Antioxidant for the Stabilization of Omega-3 Bulk Oils, Emulsions and Microcapsules. Molecules 2018, 23, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condelli, N.; Caruso, M.C.; Galgano, F.; Russo, D.; Milella, L.; Favati, F. Prediction of the antioxidant activity of extra virgin olive oils produced in the Mediterranean area. Food Chem. 2015, 177, 233–239. [Google Scholar] [CrossRef]
- Della Pelle, F.; Compagnone, D. Nanomaterial-Based Sensing and Biosensing of Phenolic Compounds and Related Antioxidant Capacity in Food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambini, J.; Stromsnes, K. Oxidative Stress and Inflammation: From Mechanisms to Therapeutic Approaches. Biomedicines 2022, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Candales, A.; Hernández Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar]
- Rojas-Morales, P.; León-Contreras, J.C.; Sánchez-Tapia, M.; Silva-Palacios, A.; Cano-Martínez, A.; González-Reyes, S.; Jiménez-Osorio, A.S.; Hernández-Pando, R.; Osorio-Alonso, H.; Sánchez-Lozada, L.G.; et al. A ketogenic diet attenuates acute and chronic ischemic kidney injury and reduces markers of oxidative stress and inflammation. Life Sci. 2022, 289, 120227. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Yeo, B.T.T.; Spreng, R.N. Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topogr. 2019, 32, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Aswad, M.; Rayan, M.; Abu-Lafi, S.; Falah, M.; Raiyn, J.; Abdallah, Z.; Rayan, A. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Inflamm. Res. 2018, 67, 67–75. [Google Scholar] [CrossRef]
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients 2022, 14, 534. [Google Scholar] [CrossRef]
- De Santis, S.; Clodoveo, M.L.; Corbo, F. Correlation between Chemical Characterization and Biological Activity: An Urgent Need for Human Studies Using Extra Virgin Olive Oil. Antioxidants 2022, 11, 258. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; De Sancti, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord.-Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J. Pharm. Sci. 2012, 20, 76. [Google Scholar] [CrossRef] [Green Version]
- Labu, Z.K.; Laboni, F.R.; Tarafdar, M.; Howlader, M.S.I.; Rashid, M.H. Membrane stabilization as a mechanism of anti-inflammatory and thrombolytic activities of ethanolic extract of arial parts of Spondiasis pinanata (Family: Anacardiaceae). Health Environ. Res. Online 2015, 2, 44. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/8324349 (accessed on 22 May 2022).
- Giménez-Bastida, J.A.; González-Sarrías, A.; Laparra-Llopis, J.M.; Schneider, C.; Espín, J.C. Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7937. [Google Scholar] [CrossRef]
- Mogana, R.; Teng-Jin, K.; Wiart, C. Anti-Inflammatory, Anticholinesterase, and Antioxidant Potential of Scopoletin Isolated from Canarium patentinervium Miq. (Burseraceae Kunth). Evid.-Based Complement. Altern. Med. 2013, 2013, 734824. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218. [Google Scholar] [CrossRef] [Green Version]
- Silenzi, A.; Giovannini, C.; Scazzocchio, B.; Varì, R.; D’Archivio, M.; Santangelo, C.; Masella, R. Chapter 22—Extra virgin olive oil polyphenols: Biological properties and antioxidant activity. In Oxidative Stress and Dietary Antioxidants; Victor, R., Preedy, P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 225–233. ISBN 9780128159729. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerković, I.; Maldini, M.; Serreli, G. Phenolic Compounds, Antioxidant Activity, and Other Characteristics of Extra Virgin Olive Oils from Italian Autochthonous Varieties Tonda di Villacidro, Tonda di Cagliari, Semidana, and Bosana. J. Chem. 2016, 2016, 8462741. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, C.G.; Dutta, A.; Averill, C.L.; McKie, S.; Akiki, T.J.; Averill, L.A.; Deakin, J. Ketamine, but Not the NMDAR Antagonist Lanicemine, Increases Prefrontal Global Connectivity in Depressed Patients. Chronic Stress 2018, 2, 2470547018796102. [Google Scholar] [CrossRef]
- Gambino, C.M.; Accardi, G.; Aiello, A.; Candore, G.; Dara-Guccione, G.; Mirisola, M.; Procopio, A.; Taormina, G.; Caruso, C. Effect of Extra Virgin Olive Oil and Table Olives on the ImmuneInflammatory Responses: Potential Clinical Applications. Endocr. Metab. Immune Disord.-Drug Targets 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; De Feo, V.; Cruz, A.G.; d’Acierno, A. Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms 2019, 7, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, F.; Md Aslam, A.; Shaoying, G.; Qi, S.; Xue, B.; Sifan, L.; Ling, G. Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control 2018, 94, 289–294. [Google Scholar] [CrossRef]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.B.; Gautam, S.; Sharma, A. Free Phenolics and Polyphenol Oxidase (PPO): The Factors Affecting Post-Cut Browning in Eggplant (Solanum melongena). Food Chem. 2013, 139, 105–114. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Bubonja-Šonje, M.; Knežević, S.; Abram, M. Challenges to antimicrobial susceptibility testing of plant-derived polyphenolic compounds. Arch. Ind. Hyg. Toxicol. 2020, 71, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2020, 19, 3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradiso, V.M.; Flamminii, F.; Pittia, P.; Caponio, F.; Mattia, C.D. Radical Scavenging Activity of Olive Oil Phenolic Antioxidants in Oil or Water Phase during the Oxidation of O/W Emulsions: An Oxidomics Approach. Antioxidants 2020, 9, 996. [Google Scholar] [CrossRef]
- Scoditti, E.; Capurso, C.; Capurso, A.; Massaro, M. Vascular effects of the Mediterranean diet-part II: Role of omega-3 fatty acids and olive oil polyphenols. Vascul Pharmacol. 2014, 63, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Rice-Evans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cerretani, L.; Lerma-García, M.J.; Herrero-Martínez, J.M.; Gallina-Toschi, T.; Simó-Alfonso, E.F. Determination of Tocopherols and Tocotrienols in Vegetable Oils by Nanoliquid Chromatography with Ultraviolet−Visible Detection Using a Silica Monolithic Column. J. Agric. Food Chem. 2010, 58, 757–761. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clodoveo, M.L.; Muraglia, M.; Crupi, P.; Hbaieb, R.H.; De Santis, S.; Desantis, A.; Corbo, F. The Tower of Babel of Pharma-Food Study on Extra Virgin Olive Oil Polyphenols. Foods 2022, 11, 1915. https://doi.org/10.3390/foods11131915
Clodoveo ML, Muraglia M, Crupi P, Hbaieb RH, De Santis S, Desantis A, Corbo F. The Tower of Babel of Pharma-Food Study on Extra Virgin Olive Oil Polyphenols. Foods. 2022; 11(13):1915. https://doi.org/10.3390/foods11131915
Chicago/Turabian StyleClodoveo, Maria Lisa, Marilena Muraglia, Pasquale Crupi, Rim Hachicha Hbaieb, Stefania De Santis, Addolorata Desantis, and Filomena Corbo. 2022. "The Tower of Babel of Pharma-Food Study on Extra Virgin Olive Oil Polyphenols" Foods 11, no. 13: 1915. https://doi.org/10.3390/foods11131915
APA StyleClodoveo, M. L., Muraglia, M., Crupi, P., Hbaieb, R. H., De Santis, S., Desantis, A., & Corbo, F. (2022). The Tower of Babel of Pharma-Food Study on Extra Virgin Olive Oil Polyphenols. Foods, 11(13), 1915. https://doi.org/10.3390/foods11131915









