Peanut Butter Food Safety Concerns—Prevalence, Mitigation and Control of Salmonella spp., and Aflatoxins in Peanut Butter
Abstract
1. Introduction
1.1. Status of the Peanut Butter Market
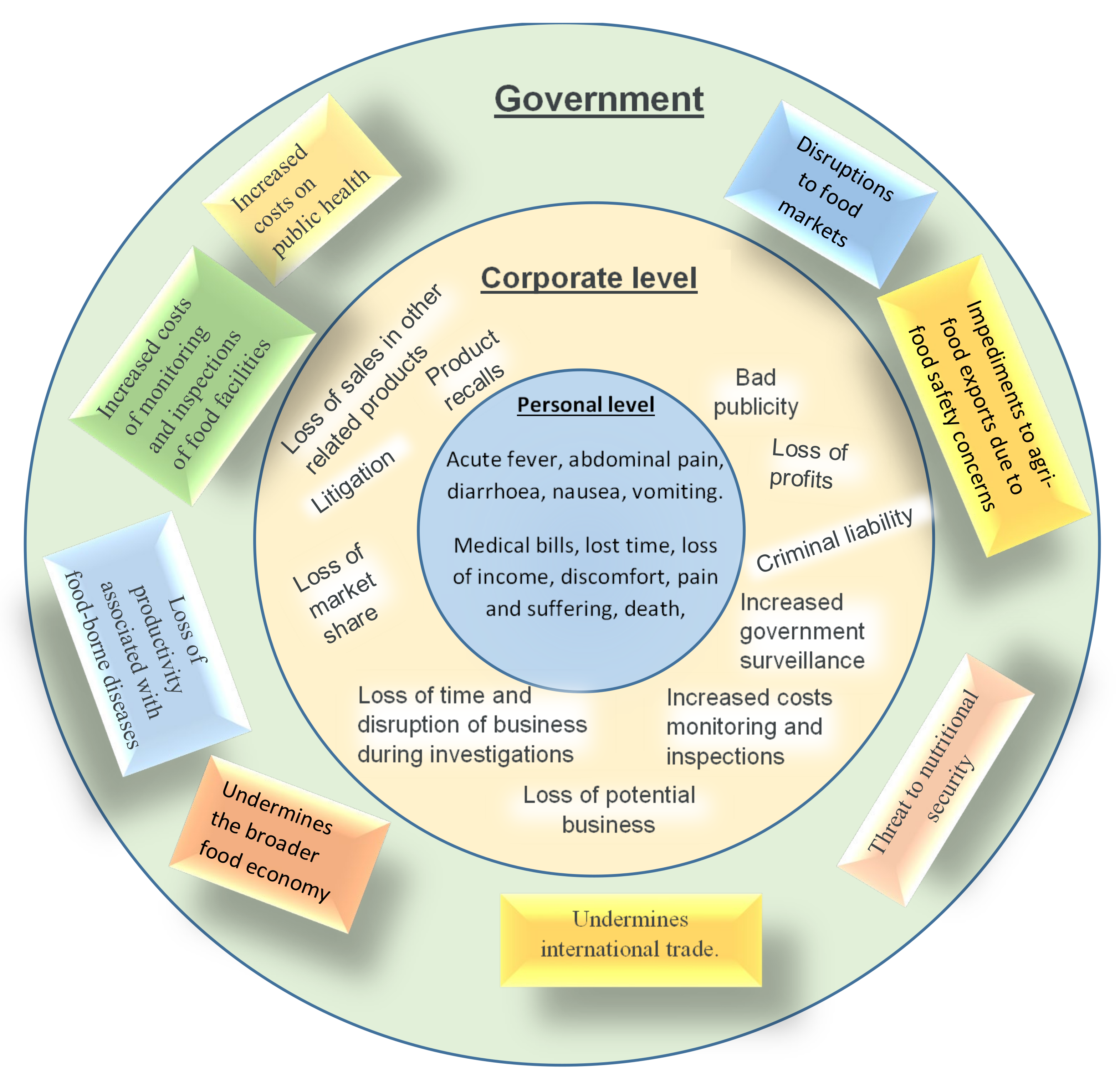
1.2. The Food Safety Risk Associated with Peanut Butter
2. The Food Safety Risks of Butter Contamination by Salmonella spp.
2.1. Technical Challenges of Inactivating Salmonella spp. in Peanut Butter
2.2. Nature of Salmonella spp.
2.3. Adaptation of Salmonella spp. in Stress Environments
2.4. Nature of Peanut Butter
2.5. Influence of Product Formulations on the Efficacy of Inactivation of Salmonella spp. in Contaminated Peanut Butter
2.6. Thermal Inactivation of Salmonella spp. in Contaminated Peanut Butter
2.7. Effects of Storage Temperature on Inactivation of Salmonella spp. in Peanut Butter
2.8. Effects of Water Activity on Thermal Inactivation of Salmonella spp. in Contaminated Peanut Butter
2.9. Variations in Thermal Inactivation of Various Salmonella spp. Serotypes in Peanut Butter
2.10. Microwave and Radiofrequency Heating Inactivation of Salmonella spp. in Contaminated Peanut Butter
2.11. Irradiation Inactivation of Salmonella spp. in Contaminated Peanut Butter
2.12. High Hydrostatic Pressure Inactivation of Salmonella spp. in Contaminated Peanut Butter
2.13. Use of Competitive and Antagonistic Bacterial to Inactivate Salmonella spp. in Contaminated Peanut Butter
2.14. Cleaning and Decontamination of Peanut Butter Plant Contaminated with Salmonella spp.
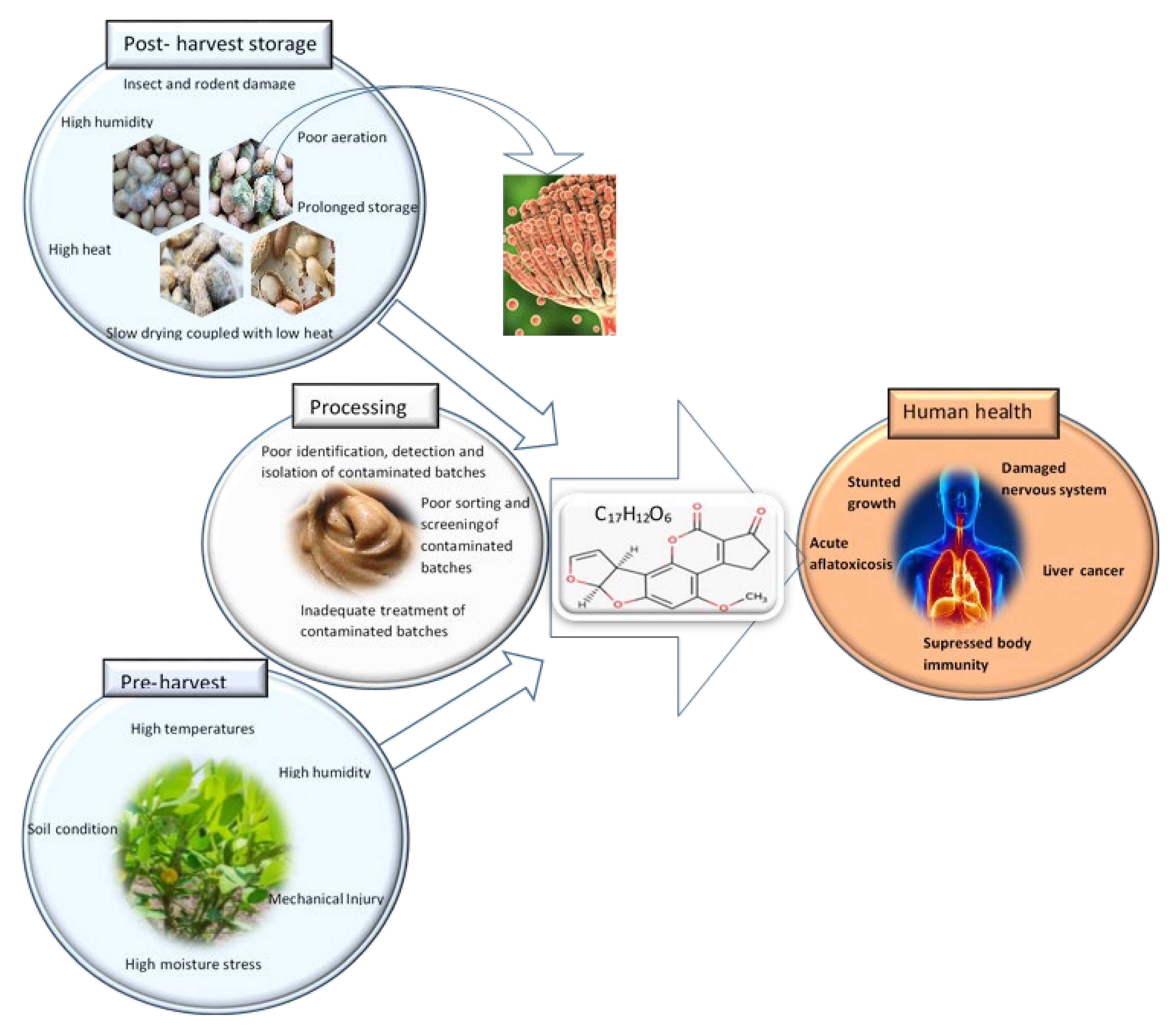
3. Food Safety Risk of Contamination of Peanut Butter by Aflatoxins
Control and Monitoring of Aflatoxin Contamination in Peanut Butter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Expert Market Research. Global Peanut Butter Market: By Spread Type: Creamy, Crunchy/Chunky, Old Fashioned/Natural, Reduced Fat, Honey Roasted; by End-Use: Cooking, Health Product; Regional Analysis; Historical Market and Forecast (2017–2027); Market Dynamics: Swot Analysis; Competitive Landscape; Industry Events and Developments. 2021. Available online: https://www.expertmarketresearch.com/reports/peanut-butter-market (accessed on 5 January 2022).
- Market Data Forecast. Global Peanut Butter Market by Product Type (Plain, Regular, Low Sodium and Low Sugar), by Distribution Channel (Hypermarkets, Supermarkets, Retailers and Other Distribution Channels) and by Regional Analysis (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa) and Global Industry Analysis, Size, Share, Growth, Trends, and Forecast (2022–2027). 2022. Available online: https://www.marketdataforecast.com/market-reports/peanut-butter-market (accessed on 13 January 2022).
- IMARC Group. Peanut Butter Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2022–2027. 2022. Available online: https://www.imarcgroup.com/peanut-butter-manufacturing-plant (accessed on 15 January 2022).
- 360 Research Reports. Global Peanut Butter Market Insights and Forecast 2028. 2022. Available online: https://www.360researchreports.com/global-peanut-butter-market-20312008 (accessed on 14 December 2021).
- Food Processing Technology. Post Holdings Acquires Peter Pan Peanut Butter Brand. 2021. Available online: https://www.foodprocessing-technology.com/news/post-holdings-peter-pan/ (accessed on 8 February 2022).
- Kemp, B. Gov. Kemp: American Peanut Growers Group to Open New Processing Facility, Expand Shelling Plant. State of Georgia. 2021. Available online: https://gov.georgia.gov/press-releases/2021-11-09/gov-kemp-american-peanut-growers-group-open-new-processing-facility (accessed on 14 December 2021).
- National Peanut Board. Why America’s Favorite Spread Is Now a “Favourite” Across the Pond. 2020. Available online: https://www.nationalpeanutboard.org/news/why-americas-favorite-spread-is-now-favourite-across-pond-conversation-between-american-peanut-council-and-national-peanut-board.htm (accessed on 14 December 2021).
- Facts and Factors. Peanut Butter Market by Type (Smooth Peanut Butter, Crunchy Peanut Butter, and Others) and by Distribution Channel (Supermarkets and Hypermarkets, Convenience Stores, Online Stores, and Others): Global Industry Outlook, Market Size, Business Intelligence, Consumer Preferences, Statistical Surveys, Comprehensive Analysis, Historical Developments, Current Trends, and Forecast 2020–2026. 2020. Available online: https://www.fnfresearch.com/peanut-butter-market-by-type-smooth-peanut-butter-1295 (accessed on 11 April 2022).
- Statista. Peanut Butter Industry—Statistics & Facts; Statista Research Department: New York, NY, USA, 2021; Available online: https://www.statista.com/topics/2287/peanut-butter-industry/#topicHeader__wrapper (accessed on 15 December 2021).
- CBI Ministry of Foreign Affairs of the Netherlands (CBIN). The European Market Potential for Groundnuts. CBI Ministry of Foreign Affairs of The Netherlands. 2020. Available online: https://www.cbi.eu/market-information/processed-fruit-vegetables-edible-nuts/groundnuts/market-potential (accessed on 14 December 2021).
- IndexBox. EU–Peanut Butter and Prepared or Preserved Groundnuts–Market Analysis, Forecast, Size, Trends and Insights. 2020. Available online: https://www.indexbox.io/blog/peanut-butter-market-in-the-eu-key-insights-2020/ (accessed on 16 January 2022).
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.S.; Sreedharan, A.; Schneider, K.R. Peanut and peanut products: A food safety perspective. Food Control 2013, 32, 296–303. [Google Scholar] [CrossRef]
- Njoroge, S.M.C.; Matumba, L.; Kanenga, K.; Siambi, M.; Waliyar, F.; Maruwo, J.; Monyo, E.S. A Case for Regular Aflatoxin Monitoring in Peanut Butter in Sub-Saharan Africa: Lessons from a 3-Year Survey in Zambia. J. Food Prot. 2016, 79, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Mupunga, I.; Mnggawa, P.; Katerere, D. Peanuts, aflatoxins and undernutrition in children in Sub-Saharan Africa. Nutrients 2017, 9, 1287. [Google Scholar] [CrossRef]
- Rahmat, S.; Cheong, B.; Hamid, M. Challenges of developing countries in complying quality and enhancing standards in food industries. Procedia Soc. Behav. Sci. 2016, 224, 445–451. [Google Scholar] [CrossRef]
- Humphrey, J. Food Safety, Trade, Standards and the Integration of Smallholders into Value Chains—A Review of the Literature. 2017. Available online: https://digitallibrary.un.org/record/1665029?ln=en (accessed on 13 January 2022).
- Grocery Manufacturers Association (GMA). Control of Salmonella in Low-Moisture Foods. 2009. Available online: http://graphics8.nytimes.com/packages/pdf/business/20090515_moss_ingredients/SalmonellaControlGuidance.pdf (accessed on 13 January 2022).
- Anderson, D.G.; Lucore, L.A. Validating the Reduction of Salmonella and Other Pathogens in Heat Processed Low-Moisture Foods. Alliance for Innovation & Operational Excellence. Alexandria. 2012. Available online: https://ucfoodsafety.ucdavis.edu/sites/g/files/dgvnsk7366/files/inline-files/224455.pdf (accessed on 15 December 2021).
- Oguntoyinbo, F.A. Safety Challenges Associated with Traditional Foods of West Africa. Food Rev. Int. 2014, 30, 338–358. [Google Scholar] [CrossRef]
- Ncube, F.; Kanda, A.; Chijokwe, M.; Mabaya, G.; Nyamugure, T. Food safety knowledge, attitudes and practices of restaurant food handlers in a lower-middle-income country. Food Sci. Nutr. 2020, 8, 1677–1687. [Google Scholar] [CrossRef]
- Wittenberger, K.; Dohlman, E. Peanut Outlook Impacts of the 2008-09 Foodborne Illness Outbreak Linked to Salmonella in Peanuts. 2010. Available online: https://www.ers.usda.gov/publications/pub-details/?pubid=37836 (accessed on 5 December 2021).
- Bakhtavoryan, R.; Capps, O.; Salin, V. Impact of Food Contamination on Brands: A Demand Systems Estimation of Peanut Butter. Agric. Resour. Econ. Rev. 2012, 41, 327–339. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Im, J.; Lee, J.-S.; Jeon, H.J.; Mogeni, O.D.; Kim, J.H.; Rakotozandrindrainy, R.; Baker, S.; Marks, F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum. Vaccines Immunother. 2018, 15, 1421–1426. [Google Scholar] [CrossRef]
- World Bank. The Safe Food Imperative: Accelerating Progress in Low- and Middle-Income Countries. 2018. Available online: https://www.worldbank.org/en/topic/agriculture/publication/the-safe-food-imperative-accelerating-progress-in-low-and-middle-income-countries (accessed on 14 December 2021).
- Britton, B.C.; Sarr, I.; Oliver, H.F. Enterobacteriaceae, coliform, yeast, and mold contamination patterns in peanuts compared to production, storage, use practices, and knowledge of food safety among growers in Senegal. Int. J. Food Microbiol. 2021, 360, 109437. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease “Burden of Illness” Studies. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Finn, S.; Condell, O.; McClure, P.; Amézquita, A.; Fanning, S. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef]
- Grasso, E.M.; Grove, S.F.; Halik, L.A.; Arritt, F.; Keller, S.E. Cleaning and sanitation of Salmonella-contaminated peanut butter processing equipment. Food Microbiol. 2015, 46, 100–106. [Google Scholar] [CrossRef]
- Mermelstein, N. Validating the Safety of Low-Moisture Foods. Institute of Food Technologist. 2018. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2018/august/columns/food-safety-and-quality-validating-safety-low-moisture-foods (accessed on 10 December 2021).
- Snyder, T.; Boktor, S.; M’Ikanatha, N.M. Salmonellosis Outbreaks by Food Vehicle, Serotype, Season, and Geographical Location, United States, 1998 to 2015. J. Food Prot. 2019, 82, 1191–1199. [Google Scholar] [CrossRef]
- Sher, A.A.; Mustafa, B.E.; Grady, S.C.; Gardiner, J.C.; Saeed, A.M. Outbreaks of foodborne Salmonella enteritidis in the United States between 1990 and 2015: An analysis of epidemiological and spatial-temporal trends. Int. J. Infect. Dis. 2021, 105, 54–61. [Google Scholar] [CrossRef]
- Wason, S.; Verma, T.; Subbiah, J. Validation of process technologies for enhancing the safety of low-moisture foods: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4950–4992. [Google Scholar] [CrossRef]
- He, Y.; Guo, D.; Yang, J.; Tortorello, M.L.; Zhang, W. Survival and Heat Resistance of Salmonella enterica and Escherichia coli O157:H7 in Peanut Butter. Appl. Environ. Microbiol. 2011, 77, 8434–8438. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Salazar, J.K.; Yang, J.; Tortorello, M.L.; Zhang, W. Increased Water Activity Reduces the Thermal Resistance of Salmonella enterica in Peanut Butter. Appl. Environ. Microbiol. 2013, 79, 4763–4767. [Google Scholar] [CrossRef]
- Jin, Y.; Pickens, S.R.; Hildebrandt, I.M.; Burbick, S.J.; Grasso-Kelley, E.M.; Keller, S.E.; Anderson, N.M. Thermal Inactivation of Salmonella Agona in Low–Water Activity Foods: Predictive Models for the Combined Effect of Temperature, Water Activity, and Food Component. J. Food Prot. 2018, 81, 1411–1417. [Google Scholar] [CrossRef]
- Song, W.-J.; Kang, D.-H. Inactivation of Salmonella Senftenberg, Salmonella Typhimurium and Salmonella Tennessee in peanut butter by 915 MHz microwave heating. Food Microbiol. 2016, 53, 48–52. [Google Scholar] [CrossRef]
- Ziegler, V.; Ferreira, C.; Rockembach, C.; Pereira de Pereira, M.; Maurício de Oliveira, M.; Elias, M. Sensory and chemical properties of peanut grains (Arachis hypogaea L) roasted in microwave or oven. Semin. Ciências Agrárias 2017, 38, 197–208. [Google Scholar] [CrossRef][Green Version]
- Scheil, W.; Cameron, S.; Dalton, C.; Murray, C.; Wilson, D. A South Australian Salmonella Mbandaka outbreak investigation using a database to select controls. Aust. N. Z. J. Public Health 1998, 22, 536–539. [Google Scholar] [CrossRef]
- Centre for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Typhimurium Infections Linked to Peanut Butter, 2008–2009 (Final Update). 2009. Available online: https://www.cdc.gov/Salmonella/2009/peanut-butter-2008-2009.html (accessed on 20 December 2021).
- Sheth, A.N.; Hoekstra, M.; Patel, N.; Ewald, G.; Lord, C.; Clarke, C.; Villamil, E.; Niksich, K.; Bopp, C.; Nguyen, T.-A.; et al. A National Outbreak of Salmonella Serotype Tennessee Infections From Contaminated Peanut Butter: A New Food Vehicle for Salmonellosis in the United States. Clin. Infect. Dis. 2011, 53, 356–362. [Google Scholar] [CrossRef]
- Centre for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Bredeney Infections Linked to Peanut Butter Manufactured by Sunland, Inc. (Final Update). 2012. Available online: https://www.cdc.gov/Salmonella/Bredeney-09-12/index.html (accessed on 20 December 2021).
- Centre for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Braenderup Infections Linked to Nut Butter Manufactured by nSpired Natural Foods, Inc. (Final Update). 2014. Available online: https://www.cdc.gov/Salmonella/Braenderup-08-14/index.html (accessed on 20 December 2021).
- Weise, E. Salmonella Outbreaks Lead to Food-Safety Changes. Marle Clark. 2009. Available online: https://about-Salmonella.com/Salmonella_outbreaks/news/Salmonella-outbreaks-lead-to-food-safety-changes (accessed on 2 December 2021).
- Tavernise, S.U.S. Makes Final an Array of Rules on Food Safety. The New York Times. 2015. Available online: https://www.nytimes.com/2015/09/11/science/food-industry-gets-new-safety-rules-to-prevent-illness.html (accessed on 22 December 2021).
- The United States Department of Justice. Conagra Subsidiary Sentenced in Connection with Outbreak of Salmonella Poisoning Related to Peanut Butter. 2016. Available online: https://www.justice.gov/opa/pr/conagra-subsidiary-sentenced-connection-outbreak-Salmonella-poisoning-related-peanut-butter (accessed on 2 December 2021).
- Liu, S.; Tang, J.; Tadapaneni, R.K.; Yang, R.; Zhu, M.-J. Exponentially increased thermal resistance of Salmonella spp. and Enterococcus faecium at reduced water activity. Appl. Environ. Microbiol. 2018, 84, e02742-17. [Google Scholar] [CrossRef]
- Burnett, S.; Gehm, E.; Weissinger, W.; Beuchat, L. Survival ofSalmonellain peanut butter and peanut butter spread. J. Appl. Microbiol. 2000, 89, 472–477. [Google Scholar] [CrossRef]
- Kilonzo-Nthenge, A.; Rotich, E.; Godwin, S.; Huang, T. Consumer storage period and temperature for peanut butter and their effects on survival of Salmonella and Escherichia coli O157:H7. Food Prot. Trends 2009, 29, 787–792. [Google Scholar]
- World Health Organisation (WHO). Salmonella (Non-Typhoidal). 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/Salmonella-(non-typhoidal) (accessed on 2 December 2021).
- Percival, S.; Williams, D. Salmonella. In Microbial of Waterborne Diseases; Percival, S., Williams, D., Gray, N., Yates, M., Chalmers, R., Eds.; Academic Press: Cambridge, UK, 2014; pp. 209–222. [Google Scholar] [CrossRef]
- White, A.P.; Surette, M.G. Comparative Genetics of the rdar Morphotype in Salmonella. J. Bacteriol. 2006, 188, 8395–8406. [Google Scholar] [CrossRef]
- White, A.P.; Gibson, D.L.; Kim, W.; Kay, W.W.; Surette, M.G. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J. Bacteriol. 2006, 188, 3219–3227. [Google Scholar] [CrossRef]
- US Food and Drug Administration (US FDA). How FDA Has Used Whole-Genome Sequencing of Foodborne Pathogens for Regulatory Purposes. 2017. Available online: https://www.fda.gov/food/whole-genome-sequencing-wgs-program/examples-how-fda-has-used-whole-genome-sequencing-foodborne-pathogens-regulatory-purposes (accessed on 2 December 2021).
- Xie, Y.; Xu, J.; Yang, R.; Alshammari, J.; Zhu, M.-J.; Sablani, S.; Tang, J. Moisture Content of Bacterial Cells Determines Thermal Resistance of Salmonella enterica Serotype Enteritidis PT 30. Appl. Environ. Microbiol. 2021, 87, e02194-20. [Google Scholar] [CrossRef]
- Zhang, Y.; Keller, S.E.; Grasso-Kelley, E.M. Fate of Salmonella throughout Production and Refrigerated Storage of Tahini. J. Food Prot. 2017, 80, 940–946. [Google Scholar] [CrossRef]
- Römling, U. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 2005, 62, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Milanov, D.; Prunic, B.; Velhner, M.; Pajic, M.; Cabarkapa, I.S. RDAR morphotype: A resting stage of some Enteroba cteriaceae. Food Feed Res. 2015, 42, 43–50. [Google Scholar] [CrossRef]
- Lee, K.; Shoda, M.; Kawai, K.; Koseki, S. Relationship between glass transition temperature, and desiccation and heat tolerance in Salmonella enterica. PLoS ONE 2020, 15, e0233638. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; Yousef, A.E. Collateral adaptive responses induced by desiccation stress in Salmonella enterica. LWT 2020, 133, 110089. [Google Scholar] [CrossRef]
- Hernández, S.B.; Cota, I.; Ducret, A.; Aussel, L.; Casadesús, J. Adaptation and Preadaptation of Salmonella enterica to Bile. PLoS Genet. 2012, 8, e1002459. [Google Scholar] [CrossRef]
- Pradhan, D.; Negi, V.D. Stress-induced adaptations in Salmonella: A ground for shaping its pathogenesis. Microbiol. Res. 2019, 229, 126311. [Google Scholar] [CrossRef]
- Baert, L.; Gimonet, J.; Barretto, C.; Fournier, C.; Jagadeesan, B. Genetic changes are introduced by repeated exposure of Salmonella spiked in low water activity and high fat matrix to heat. Sci. Rep. 2021, 11, 8144. [Google Scholar] [CrossRef]
- Urdaneta, V.; Hernández, S.B.; Casadesús, J. Mutational and non mutational adaptation of Salmonella enterica to the gall bladder. Sci. Rep. 2019, 9, 5203. [Google Scholar] [CrossRef]
- Wright, D.G.; Minarsich, J.; Daeschel, M.A.; Waite-Cusic, J. Thermal inactivation of Salmonella spp. in commercial tree nut and peanut butters in finished packaging. J. Food Saf. 2017, 38, e12371. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Ali, T. Textural Quality of Peanut Butter as Influenced by Peanut Seed and Oil Contents. Peanut Sci. 1986, 13, 18–20. [Google Scholar] [CrossRef]
- Perlman, D. Method and Composition for Preventing Oil Separation in Vegetable Kernel Butters by Combining with Microparticulate Silicon Dioxide. United States Patent No. US08/941,569, 27 January 1997. [Google Scholar]
- EAS 60: 2000; Peanut Butter–Specification. East African Community: 2000. Available online: https://law.resource.org/pub/eac/ibr/eas.60.2006.html (accessed on 24 January 2022).
- US Food and Drug Admiration (US FDA). Requirements for Specific Standardized Tree Nut and Peanut Products. 21CFR164.150, Silver Spring, United States. 2000. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=164&showFR=1&subpartNode=21:2.0.1.1.40.2 (accessed on 5 December 2021).
- SPX Corporation. Peanut Butter. Delavan, Wisconsin, US. 2008. Available online: https://www.spxflow.com/assets/pdf/apv-homogenizer-peanut-butter-processing-us.pdf (accessed on 16 January 2022).
- How Products Are Made. (n.d). Peanut Butter. Available online: http://www.madehow.com/Volume-1/Peanut-Butter.html (accessed on 4 September 2021).
- Dhamsaniya, N.K.; Patel, N.C.; Dabhi, M.N. Selection of groundnut variety for making a good quality peanut butter. J. Food Sci. Technol. 2011, 49, 115–118. [Google Scholar] [CrossRef]
- Shibli, S.; Siddique, F.; Raza, S.; Ahsan, Z.; Raza, I. Chemical Composition and Sensory Analysis of Peanut Butter from Indigenous Peanut Cultivars of Pakistan. Pak. J. Agric. Res. 2019, 32, 159–169. [Google Scholar] [CrossRef]
- Li, C.; Huang, L.; Chen, J. Comparative study of thermal inactivation kinetics of Salmonella spp. in peanut butter and peanut butter spread. Food Control 2014, 45, 143–149. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, G.; Gerner-Smidt, P.; Mantripragada, V.; Ezeoke, I.; Doyle, M.P. Thermal Inactivation of Salmonella in Peanut Butter. J. Food Prot. 2009, 72, 1596–1601. [Google Scholar] [CrossRef]
- D’Souza, T.; Karwe, M.; Schaffner, D.W. Effect of High Hydrostatic Pressure on Salmonella Inoculated into Creamy Peanut Butter with Modified Composition. J. Food Prot. 2014, 77, 1664–1668. [Google Scholar] [CrossRef]
- Pelaez, M.A.B.; Anapi, G.R.; Bautista, D.V.; Dallo, M.D.P.; Libunao, J.C.M.; Gabriel, A.A. Thermal inactivation of Salmonella enterica in Philippine flowing-type peanut butter. LWT 2020, 129, 109507. [Google Scholar] [CrossRef]
- Ha, J.-W.; Kim, S.-Y.; Ryu, S.-R.; Kang, D.-H. Inactivation of Salmonella enterica serovar Typhimurium and Escherichia coli O157:H7 in peanut butter cracker sandwiches by radio-frequency heating. Food Microbiol. 2013, 34, 145–150. [Google Scholar] [CrossRef]
- Shachar, D.; Yaron, S. Heat Tolerance of Salmonella enterica Serovars Agona, Enteritidis, and Typhimurium in Peanut Butter. J. Food Prot. 2006, 69, 2687–2691. [Google Scholar] [CrossRef]
- Keller, S.E.; Grasso, E.M.; Halik, L.A.; Fleischman, G.J.; Chirtel, S.J.; Grove, S.F. Effect of Growth on the Thermal Resistance and Survival of Salmonella Tennessee and Oranienburg in Peanut Butter, Measured by a New Thin-Layer Thermal Death Time Device. J. Food Prot. 2012, 75, 1125–1130. [Google Scholar] [CrossRef]
- Park, E.-J.; Oh, S.-W.; Kang, D.-H. Fate of Salmonella Tennessee in Peanut Butter at 4 and 22 °C. J. Food Sci. 2008, 73, M82–M86. [Google Scholar] [CrossRef]
- Mattick, K.L.; Jørgensen, F.; Wang, P.; Pound, J.; Vandeven, M.H.; Ward, L.R.; Legan, J.D.; Lappin-Scott, H.M.; Humphrey, T.J. Effect of Challenge Temperature and Solute Type on Heat Tolerance of Salmonella Serovars at Low Water Activity. Appl. Environ. Microbiol. 2001, 67, 4128–4136. [Google Scholar] [CrossRef]
- Garces-Vega, F.J.; Ryser, E.T.; Marks, B.P. Relationships of Water Activity and moisture content to the thermal inactivation kinetics of Salmonella in low-moisture foods. J. Food Prot. 2019, 82, 963–970. [Google Scholar] [CrossRef]
- Institute of Food Technologists. Evaluation and Definition of Potentially Hazardous Foods—A Report of the Institute of Food Technologists for the Food and Drug Administration of the United States Department of Health and Human Service. 2021. Available online: https://www.fda.gov/files/food/published/Evaluation-and-Definition-of-Potentially-Hazardous-Foods.pdf (accessed on 19 December 2021).
- US Food and Drug Administration. Guidance for Industry: Measures to Address the Risk for Contamination by Salmonella Species in Food Containing a Peanut-Derived Product as an Ingredient. 2009. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-measures-address-risk-contamination-Salmonella-species-food-containing-peanut (accessed on 28 November 2021).
- Kottapalli, B.; Nguyen, O.; Perez, T.; Cunningham, A. Thermal Inactivation of Salmonella and Listeria monocytogenes in peanut Butter–filled pretzels and whole wheat pita chips. J. Food Prot. 2019, 82, 238–246. [Google Scholar] [CrossRef]
- Algorri, R. Foodborne Illness Part 3: How does Salmonella Make Us Sick? American Society for Microbiology. 2019. Available online: https://asm.org/Articles/2019/April/Foodborne-Illness-Part-3-How-does-Salmonella-make (accessed on 14 January 2022).
- Marra, F.; Zhang, L.; Lyng, J. Radio frequency treatment of foods: Review of recent advances. J. Food Eng. 2009, 91, 497–508. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, R.; Zhang, B.; Erdogdu, F.; Wang, S. A comprehensive review on recent developments of radio frequency treatment for pasteurizing agricultural products. Crit. Rev. Food Sci. Nutr. 2020, 61, 380–394. [Google Scholar] [CrossRef]
- Ling, B.; Cheng, T.; Wang, S. Recent developments in applications of radio frequency heating for improving safety and quality of food grains and their products: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 2622–2642. [Google Scholar] [CrossRef]
- Hvizdzak, A.L.; Beamer, S.; Jaczynski, J.; Matak, K.E. Use of Electron Beam Radiation for the Reduction of Salmonella enterica Serovars Typhimurium and Tennessee in Peanut Butter. J. Food Prot. 2010, 73, 353–357. [Google Scholar] [CrossRef]
- Ban, G.-H.; Kang, D.-H. Effects of gamma irradiation for inactivating Salmonella Typhimurium in peanut butter product during storage. Int. J. Food Microbiol. 2014, 171, 48–53. [Google Scholar] [CrossRef]
- Matak, K.E.; Hvizdzak, A.L.; Beamer, S.; Jaczynski, J. Recovery of Salmonella enterica Serovars Typhimurium and Tennessee in Peanut Butter after Electron Beam Exposure. J. Food Sci. 2010, 75, M462–M467. [Google Scholar] [CrossRef]
- El-Rawas, A.; Hvizdzak, A.; Davenport, M.; Beamer, S.; Jaczynski, J.; Matak, K. Effect of electron beam irradiation on quality indicators of peanut butter over a storage period. Food Chem. 2012, 133, 212–219. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Yusop, S.M.; Rahman, I.A.; Dauqan, E.; Abdullah, A. Efficacy of Gamma Irradiation in Improving the Microbial and Physical Quality Properties of Dried Chillies (Capsicum annuum L.): A Review . Foods 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A.K. Wholesomeness and safety aspects of irradiated foods. Food Chem. 2019, 285, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Desrosier, N.; Singh, R. Food Irradiation. Encyclopaedia Britannica. 2022. Available online: https://www.britannica.com/topic/food-preservation/Food-irradiation (accessed on 10 March 2022).
- Szczawińska, M.E. Application of Ionizing Radiation for Control of Salmonella in Food. In Current Topics in Salmonella and Salmonellosis; Mares, M., Ed.; InTech: Rijeka, Croatia, 2017; pp. 253–274. [Google Scholar] [CrossRef]
- FAO/IAEA/WHO Study Group. High-Dose Irradiation: Wholesomeness of Food Irradiated with Doses Above 10 kGy: Report of a Joint FAO/IAEA/WHO Study Group; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 1999; Volume 890, p. i-197. Available online: https://apps.who.int/iris/handle/10665/42203 (accessed on 15 December 2021).
- Castell-Perez, M.E.; Moreira, R.G. Irradiation and Consumers Acceptance. Innov. Food Process. Technol. 2021, 2, 122–135. [Google Scholar] [CrossRef]
- Román, S.; Sanchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Siegrist, M.; Hartmann, C. Consumer acceptance of novel food technologies. Nat. Food 2020, 1, 343–350. [Google Scholar] [CrossRef]
- Grasso, E.M.; Somerville, J.A.; Balasubramaniam, V.; Lee, K. Minimal Effects of High-Pressure Treatment on Salmonella enterica Serovar Typhimurium Inoculated into Peanut Butter and Peanut Products. J. Food Sci. 2010, 75, E522–E526. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, G.S. Effects of High-Pressure Processing (HPP) on Bacterial Spores: An Overview. Food Rev. Int. 2008, 24, 330–351. [Google Scholar] [CrossRef]
- Podolak, R.; Whitman, D.; Black, D.G. Factors Affecting Microbial Inactivation during High Pressure Processing in Juices and Beverages: A Review. J. Food Prot. 2020, 83, 1561–1575. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial inactivation by high pressure processing: Principle, mechanism and factors responsible. Food Sci. Biotechnol. 2020, 30, 19–35. [Google Scholar] [CrossRef]
- Tamber, S. Population-Wide Survey of Salmonella enterica Response to High-Pressure Processing Reveals a Diversity of Responses and Tolerance Mechanisms. Appl. Environ. Microbiol. 2018, 84, e01673-17. [Google Scholar] [CrossRef]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-pressure processing—Effects on microbial food safety and food quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Zhang, W.; Zhang, J.; Pérez-Santaescolástica, C.; Lorenzo, J.M. High-Pressure Pro-cessing in inactivation of Salmonella spp. in food products. Trends Food Sci. Technol. 2021, 107, 31–37. [Google Scholar] [CrossRef]
- Stiles, T.K. The Effects of High Prects of High Pressure Processing on Processing on Peanut Sauce Inoculated with Salmonella. Master’s Thesis, University of Nebraska, Lincoln, OR, USA, 2010. Available online: https://core.ac.uk/download/pdf/17210701.pdf (accessed on 2 December 2021).
- Fayol-Messaoudi, D.; Coconnier-Polter, M.-H.; Moal, V.-L.; Atassi, F.; Berger, C.; Servin, A. The Lactobacillus plantarum strain ACA-DC287 isolated from a Greek cheese demonstrates antagonistic activity in vitro and in vivo against Salmonella enterica serovar Typhimurium. J. Appl. Microbiol. 2007, 103, 657–665. [Google Scholar] [CrossRef]
- Klu, Y.A.K.; Phillips, R.D.; Chen, J. Survival of four commercial probiotic mixtures in full fat and reduced fat peanut butter. Food Microbiol. 2014, 44, 34–40. [Google Scholar] [CrossRef]
- Klu, Y.A.K.; Chen, J. Influence of probiotics, included in peanut butter, on the fate of selected Salmonella and Listeria strains under simulated gastrointestinal conditions. J. Appl. Microbiol. 2016, 120, 1052–1060. [Google Scholar] [CrossRef]
- Filbert, M.E.; Brown, D.L. Aflatoxin Contamination in Haitian and Kenyan Peanut Butter and Two Solutions for Reducing Such Contamination. J. Hunger Environ. Nutr. 2012, 7, 321–332. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Aflatoxins. 2018. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/aflatoxins (accessed on 14 January 2022).
- Chen, Y.-C.; Liao, C.-D.; Lin, H.-Y.; Chiueh, L.-C.; Shih, D.Y.-C. Survey of aflatoxin contamination in peanut products in Taiwan from 1997 to 2011. J. Food Drug Anal. 2013, 21, 247–252. [Google Scholar] [CrossRef]
- Njoroge, S.M.C. A Critical Review of Aflatoxin Contamination of Peanuts in Malawi and Zambia: The Past, Present, and Future. Plant Dis. 2018, 102, 2394–2406. [Google Scholar] [CrossRef]
- Lien, K.-W.; Wang, X.; Pan, M.-H.; Ling, M.-P. Assessing Aflatoxin Exposure Risk from Peanuts and Peanut Products Imported to Taiwan. Toxins 2019, 11, 80. [Google Scholar] [CrossRef]
- Jallow, E.A.; Jarju, O.M.; Oyelakin, O.; Jallow, D.B.; Mendy, B. Evaluation of aflatoxin B1 contamination of peanut butter in Gambia. Afr. J. Food Sci. 2020, 15, 360–366. [Google Scholar]
- FAO/IAEA. Training and Reference Centre for Food and Pesticide Control. Manual on the Application of the HACCP System in Mycotoxin Prevention and Control. Rome: FAO/IAEA Training and Reference Centre for Food and Pesticide Control. Food & Agriculture Org., No. 73. 2001. Available online: https://www.fooddiagnostics.dk/seekings/uploads/FAO-Manual_of_Application_of_HACCP_on_Mycotoxin_Prevention_and_Control.pdf (accessed on 10 December 2021).
- World Health Organisation (WHO). Aflatoxins. (Ref No: WHO/NHM/FOS/RAM/18.1). 2018. Available online: https://www.who.int/foodsafety/FSDigest_Aflatoxins_EN.pdf (accessed on 2 December 2021).
- Gong, Y.; Routledge, M.; Bombana, A.; Kimanya, M.; Nelson, F.; Sonoiya, S.; Manyong, V. Aflatoxin and Human Health Knowledge Platform 2015 Situational Analysis for East Africa Region. 2015. Available online: https://aflasafe.com/wp-content/uploads/pdf/TPP-1-Aflatoxin-and-Human-Health.pdf (accessed on 15 December 2021).
- East African Community. Harmful Effects of Aflatoxin and Its Impact on Human Health. Report Brief (No. 1). 2018. Available online: https://aflasafe.com/wp-content/uploads/pdf/Policy-Brief-1-Harmful-Effects-of-Aflatoxin.pdf (accessed on 14 December 2021).
- Mutegi, C.K.; Cotty, P.J.; Bandyopadhyay, R. Prevalence and mitigation of aflatoxins in Kenya (1960-to date). World Mycotoxin J. 2018, 11, 341–357. [Google Scholar] [CrossRef]
- Sundsmo, A.; Gwinner, V.; Nelson, F.; Othieno, O.R.; Manyong, V. Five-Year Communication Strategy for an Aflatoxin Safe East African Community Knowledge Platform 2015 Situational Analysis. 2015. Available online: https://www.aflatoxinpartnership.org/wp-content/uploads/2021/05/G5-Five-Year-Communication-Strategy-for-an-Aflatoxin-Safe-East-African-Community.pdf (accessed on 14 December 2021).
- Dorner, J.W. Management and prevention of mycotoxins in peanuts. Food Addit. Contam. Part A 2008, 25, 203–208. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of ozone for degradation of mycotoxins in food: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar] [CrossRef]
- Chiou, R.Y.-Y.; Lin, C.M.; Shyu, S.-L. Property Characterization of Peanut Kernels Subjected to Gamma Irradiation and Its Effect on the Outgrowth and Aflatoxin Production by Aspergillus parasiticus. J. Food Sci. 1990, 55, 210–213. [Google Scholar] [CrossRef]
- de Camargo, A.C.; de Souza Vieira, T.F.; Regitano-d’Arce, M.; de Alencar, S.M.; Calori-Domingues, M.; Spoto, M.; Canniatti-Brazaca, S. Gamma irradiation of in-shell and blanched peanuts protects against mycotoxin fungi and retains their nutraceutical components during long-term storage. Int. J. Mol. Sci. 2012, 13, 10935–10958. [Google Scholar] [CrossRef]
- Gapasin, D.; Cherry, J.; Gilbert, J.; Gibbons, R.; Hildebrand, G.; Nelson, D.; Valentine, H.; Williamson, H. The Peanut Collaborative Research Support Program (CRSP)—2005 External Evaluation Report; USAID: Washington, DC, USA, 2005. Available online: https://pdf.usaid.gov/pdf_docs/PDACF623.pdf (accessed on 14 December 2021).
- European Commission (EC). Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Myco-Toxins in Foodstuffs. Reg-ulation (EC) No 401/2006 of 23 February 2006. 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2006R0401:20100313:EN:PDF (accessed on 10 December 2021).
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for Detection of Aflatoxins in Agricultural Food Crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- Nilüfer, D.; Boyacıoǧlu, D. Comparative Study of Three Different Methods for the Determination of Aflatoxins in Tahini. J. Agric. Food Chem. 2002, 50, 3375–3379. [Google Scholar] [CrossRef]
- Ono, E.Y.S.; Da Silva, M.; Ribeiro, R.M.R.; Ono, M.A.; Hayashi, L.; Garcia, G.T.; Hirooka, E.Y. Comparison of thin-layer chromatography, spectrofluorimetry and bright greenish-yellow fluorescence test for aflatoxin detection in corn. Braz. Arch. Biol. Technol. 2010, 53, 687–692. [Google Scholar] [CrossRef]
| Specification | Country/Region | ||
|---|---|---|---|
| USA | Malaysia | East African Community | |
| % peanuts (minimum) | 90 | 85 | 90 |
| % lipids (maximum) | 55 | 55 | 55 |
| % Salt (maximum) | 1.6 | 2 | 2 |
| % Moisture | - | 3 | 2 |
| Permitted additives | |||
| % Stabiliser (maximum) | 4 | 5 | 3 |
| % Dextrose (maximum) | 6 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sithole, T.R.; Ma, Y.-X.; Qin, Z.; Wang, X.-D.; Liu, H.-M. Peanut Butter Food Safety Concerns—Prevalence, Mitigation and Control of Salmonella spp., and Aflatoxins in Peanut Butter. Foods 2022, 11, 1874. https://doi.org/10.3390/foods11131874
Sithole TR, Ma Y-X, Qin Z, Wang X-D, Liu H-M. Peanut Butter Food Safety Concerns—Prevalence, Mitigation and Control of Salmonella spp., and Aflatoxins in Peanut Butter. Foods. 2022; 11(13):1874. https://doi.org/10.3390/foods11131874
Chicago/Turabian StyleSithole, Tapiwa Reward, Yu-Xiang Ma, Zhao Qin, Xue-De Wang, and Hua-Min Liu. 2022. "Peanut Butter Food Safety Concerns—Prevalence, Mitigation and Control of Salmonella spp., and Aflatoxins in Peanut Butter" Foods 11, no. 13: 1874. https://doi.org/10.3390/foods11131874
APA StyleSithole, T. R., Ma, Y.-X., Qin, Z., Wang, X.-D., & Liu, H.-M. (2022). Peanut Butter Food Safety Concerns—Prevalence, Mitigation and Control of Salmonella spp., and Aflatoxins in Peanut Butter. Foods, 11(13), 1874. https://doi.org/10.3390/foods11131874





