Abstract
With the dramatic increase in mortality of cardiovascular diseases (CVDs) caused by thrombus, this has sparked an interest in seeking more effective thrombolytic drugs or dietary nutriments. The dietary consumption of natto, a traditional Bacillus-fermented food (BFF), can reduce the risk of CVDs. Nattokinase (NK), a natural, safe, efficient and cost-effective thrombolytic enzyme, is the most bioactive ingredient in natto. NK has progressively been considered to have potentially beneficial cardiovascular effects. Microbial synthesis is a cost-effective method of producing NK. Bacillus spp. are the main production strains. While microbial synthesis of NK has been thoroughly explored, NK yield, activity and stability are the critical restrictions. Multiple optimization strategies are an attempt to tackle the current problems to meet commercial demands. We focus on the recent advances in NK, including fermented soybean foods, production strains, optimization strategies, extraction and purification, activity maintenance, biological functions, and safety assessment of NK. In addition, this review systematically discussed the challenges and prospects of NK in actual application. Due to the continuous exploration and rapid progress of NK, NK is expected to be a natural future alternative to CVDs.
1. Introduction
At present, cardiovascular diseases (CVDs) have become the leading cause of human death among various diseases, and are characterized by high morbidity, disability and mortality [1]. Even with the application of the most advanced therapies, most patients are still too late for treatment [2,3]. With the accumulation of experience in the treatment of CVDs and the increase in public awareness of nutrition and health, people gradually realize that dietary is an effective way to prevent and treat CVDs [4].
The consumption of fermented soybean products has shown a significant negative correlation with the risk of CVDs [5,6]. Natto is a traditional soybean food fermented by Bacillus subtilis (Bacillus-fermented food, BFF) and rich in nattokinase (NK), which is an alkaline serine protease and exhibits strong thrombolytic activity and substrate specificity [7]. NK has a multipotent thrombolytic mechanism (Figure 1) [8,9,10]. For example, NK not only directly degrades fibrin and dissolve thrombi, but also indirectly drives the conversion of endogenous prourokinase to urokinase and plasminogen to plasmin by increasing the level of t-PA (tissue plasminogen activator) and inhibits the level of PAI-1 (plasminogen activator inhibitor) [11,12]. In addition, NK exhibits advantages of high thrombolytic activity, rapid and long duration of action, non-toxic side effects, convenient administration and low production cost, when compared with clinical drugs or other fibrinolytic components, such as urokinase, streptokinase, lumbrokinase, snake venoms and centipede venoms [7,13]. Therefore, NK is expected to be a promising dietary supplement or nutraceutical for the preventive and therapeutic treatment of CVDs [8,14].
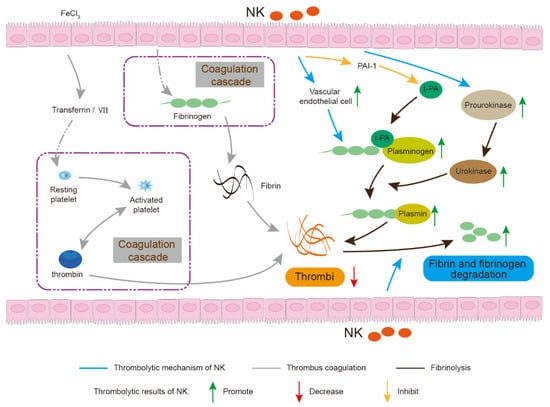
Figure 1.
Thrombolytic mechanism of NK. NK not only degrades fibrin or dissolve thrombi directly, but also activates the body’s own thrombolytic system and inhibits its thrombi coagulation.
This review systematically discusses the recent advances in NK, including fermented soybean foods, production strain sources, optimization strategies, extraction and purification procession, activity maintenance, functional evaluation, and toxicity assessment of NK. Meanwhile, it reveals the serious challenges for NK production by microorganism, as well as the prospects for NK application.
2. Fermented Soybean Foods Rich in NK
The consumption of soybean as a food has been recorded for centuries [15]. There are a variety of soybean products, including soybean milk, tofu, bean curd shin, and fermented soybean products. Among the fermented soybean products, such as natto, thua nao, tempeh, knema, douche, gochujang, moromi, chungkookjang, ganjang, doenjang, sieng and pepok are favored by consumers due to their rich flavor, taste and high nutritional value (Table 1) [16,17,18]. Natto is the main representative of leguminous BFFs. There are three types of natto foods, including itohiki-natto, yukiwari-natto and hama-natto, which were extensively consumed in Japan [19]. Fermentation of Bacillus spp. results in the extensive degradation of soybean components and the massive production of novel bioactive compounds, such as NK, riboflavin, polyglutamic acid, etc. [15,20]. NK is an important functional factor with thrombolytic activity. In 1906, Sawamura reported the protease activity from natto for the first time [21]. In addition, its fibrinolytic activity was first described by Oshima in 1925 [22]. Miyake et al. succeeded in isolating the protein crystals and determining its amino acid composition [23]. In 1987, Sumi et al. first used the name “NK” in the extraction and isolation of fibrinolytic protein from natto [24]. The protein sequence of NK is highly homologous to the subtilisin NAT (EC 3.4.21.62) produced by Bacillus; however, only NK exhibits strong thrombolytic activity and substrate specificity [7].

Table 1.
Traditional fermented soybean foods.
3. Production Strains of NK
Production strains of NK have been isolated from various sources, such as fermented legumes foods, soils, marine water, plants, dairy products, rust, cow dung and others (Table 2), of which BFFs have been proved to be good resources of NK for a long time, such as natto (Figure 2) [39]. Bacillus spp. are potential microbial sources of NK, which contains B. subtilis, B. amyloliquefaciens, B. licheniformis, B. megaterium, B. pumilus, B.cereus and others [16,40,41].
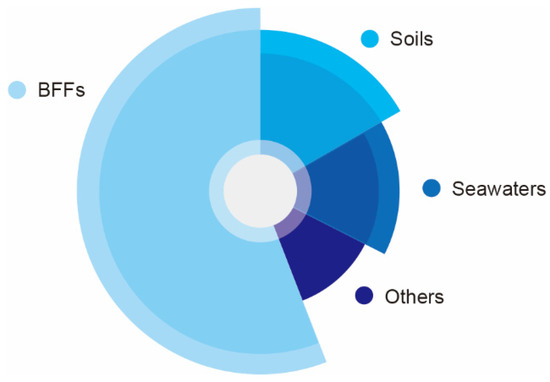
Figure 2.
Sources of NK producing strains.
B. subtilis, as a group of Gram-positive, endospore-forming and rod-shaped bacteria, is generally regarded as a safe (GRAS) microbial producer in fermented legumes foods [16,42]. B. subtilis has a short growth cycle, easy fermentation process, high genetic engineering operability, high genetic stability, clear metabolic networks, and no obvious codon preference. Most importantly, it has good protein secretion and expression capacity [42,43]. B. subtilis natto, mainly isolated from the fermented vegetable cheese natto, exhibited a multiple-effect fibrinolytic activity of approximately 40 IU/g (wet weight) [24]. B. subtilis REVS 12, a production strain of NK, was isolated from a natto food collected in Chennai [44]. Similarly, B. subtilis MX-6 from Chinese douche showed the presence of aprN encoding NK, which was verified with the polymerase chain reaction (PCR) method [45]. In addition, B. amyloliquefaciens, B. licheniformis, B. megaterium, B. pumilus, B.cereus and others have also been exploited for NK production [16,40,41]. In addition, other microorganisms are also used as alternatives, such as endophytic Fusarium spp., Zygosaccharomyces rouxii, Tetragenococcus halophilus, and Pseudomonas spp. et al. [17,46].

Table 2.
Sources of NK production strains.
Table 2.
Sources of NK production strains.
| Sources | Strains | Ref. | ||
|---|---|---|---|---|
| Fermented soybean foods | Natto | B. subtilis natto | [24] | |
| B. subtilis natto | [47] | |||
| B. subtilis REVS12 | [44] | |||
| B. subtilis natto B-12 | [48] | |||
| Da jang | B. subtilis LSSE-62 | [49] | ||
| Moromi | B. subtilis K2 | [17] | ||
| Douchi | B. subtilis DC33 | [50] | ||
| B. subtilis YF38 | [51] | |||
| B. subtilis MX-6 | [45] | |||
| B. amyloliquefaciens DC-4 | [52] | |||
| Thua nao | B. subtilis | [37] | ||
| Chungkukjang | Bacillus sp. CK 11-4 | [53] | ||
| Doenjang | B. subtilis WRL101 | [54] | ||
| Bacillus sp. DJ-4 | [55] | |||
| Gembus | B. pumilus | [56] | ||
| Soils | B. subtilis natto WTC016 | [57] | ||
| B. sublitis RJAS19 | [58] | |||
| B. subtilis IMR-NK1 | [59] | |||
| B. subtilis TKU007 | [60] | |||
| B. subtilis | [61] | |||
| Pseudomonas sp. TKU015 | [62] | |||
| Marine | Marine water | B. subtilis | [63] | |
| Marine cultures | B. subtilis ICTF-1 | [64] | ||
| Plants | Red alga Porphyra Dentata | B. subtilis N1 | [65] | |
| The root tissue of Stemona japonica (Blume) Miq | Endophytic Strain EJS-3 | [66] | ||
| Dairy products | Bovine milk | Pseudomonas aeruginosa CMSS | [67] | |
| Fermented milk | B. subtilis VITMS 2 | [68] | ||
| Rust | B. cereus VITSDVM3 | [69] | ||
| Cow dung | Bacillus spp. IND7 | [70] | ||
4. Optimization Strategies for NK
Although the microbial production of NK has been intensively studied, yield, activity and stability of NK continue to be the critical restraints on the industrial application of NK. Screening and breeding of NK production strains, culture medium and condition optimization, recombinant expression, and molecular modification are normally used as effective strategies to overcome the above restraints [71,72].
4.1. Screening and Breeding of NK Production Strains
Strain screening and breeding are proved to be an important way to improve the yield, activity and stability of NK. Production strains of NK are usually screened from traditional BFFs, which were identified to have fibrinolytic capacity [44]. The expression of NK in the wide-type strains is generally low. Therefore, researchers have used various breeding methods to enhance NK expression. Mutation breeding, including physical mutagenesis and chemical mutagenesis, are the most used methods, which can improve the expression yield, activity and stability of NK produced by the production strains. 60Co-γ irradiation mutagenesis increased the fibrin hydrolytic activity of the NK production strain by 29.62% and the thermal stability by 82.31% [73]. After the radiation to the protoplasts of B. subtills natto by ultraviolet (UV), the viability of NK production strains improved by 16.6%, and the thermal stability increased by 20% after 15 min treatment at 65 °C [74]. NK expression was also enhanced by 2-fold in P. aeruginosa CMSS obtained by UV mutagenesis [67]. In addition, the combination of hydroxylamine hydrochloride and UV mutagenesis increased the NK yield of the strain by 68% [75]. In the process of strain screening and breeding, the key to obtain excellent NK production strains is established rapid screening techniques, combined with high throughput screening methods of fibrinolytic activity assays [76].
4.2. Culture Medium and Culture Condition Optimization
Culture media composition, culture conditions, and fermentation methods are the main factors influencing NK yield, which are commonly used strategies to improve NK yield.
Optimized medium composition can efficiently facilitate the strains’ growth, which results in the large expression of NK (Table 3). Carbon sources, nitrogen sources, and inorganic salts have a large effect on NK yield of production strains. Carbon sources act as the main substrates to provide energy for the microorganisms to produce NK. In addition, the type and concentration of carbon source can both affect the NK yield. Several studies have shown that glucose was superior to maltose and sucrose as carbon sources for the productivity of NK and increased by 1.23- and 1.25-fold after the replacements, respectively [77]. The NK activity of the medium with yeast extract as the carbon source was 171.1 ± 0.27 U/mL, which was 10 U/mL higher than that of beef extract [68]. The adjustment of the concentration of yeast extract from 3% to 6% in the medium resulted in a 1.12-fold increase in NK activity [78]. Optimal glycerol concentration can also enhance the NK activity. When the concentration of glycerol in the medium was increased by 1%, the NK activity improved by 38.40 U/mL [79]. In addition, rice husk, cassava starch, and other agricultural and sideline products can also be used as carbon sources. When the fermentation medium contained 13.3% rice husk, the NK yield reached 2503.4 IU/g, which was 7-fold of that of the medium without rice husk [80]. In another study, similar NK activity about 1754 U/mL in fermentation media containing tapioca starch and soluble starch was found [81]. In addition, the fibrinolytic activity reached 3682 ± 43 IU/g and 6479 IU/g by using Ginkgo seeds and chestnuts as carbon sources in solid media, respectively [82,83].
Nitrogen-containing compounds also can affect NK yield and activity. In addition to the usual nitrogen sources, substrates rich in legume proteins are the main nitrogen sources for NK production, such as soybean milk, soybean protein hydrolysate, soybean peptone, and soybean meal. When soybean milk in the medium increased from 120 to 180 g/L, the NK yield increased by 1.20-fold to 28.3 g/L [77]. Similarly, when the optimized medium consisted of 20 g/L soybean meal, the NK activity increased up to 2174 U/mL, which was 1.24-fold of that of the 10 g/L soybean meal [81]. In addition, the composite nitrogen sources consisting of soybean hydrolysate and soybean meal achieved a higher level of NK activity about 4876 IU/mL, which was 6.5-fold higher than the only medium containing soybean hydrolysate [77]. Notably, some nutrient-rich by-products in food processing are also suitable alternatives of nitrogen sources, such as tofu processing wastewater, cheese whey, and glutamate waste liquid [84,85]. For culture medium with tofu processing wastewater, the NK activity reached 7209.15 ± 195.46 IU/mL and increased by 19.25% compared to soy peptone [84]. Furthermore, the final NK activity was 789.93 U/mL in the low-cost substrate of cheese whey, while the medium cost reduced by 55–60% [85].
Inorganic salts can also promote NK yield and enhance its activity [61,68]. Metal ions usually serve as cofactors in NK production. Ca2+ can stimulate NK yield; the maximum fibrinolytic activity reached 3787 U/mL at 1.6g/L CaCl2 [81]. As the concentration of MgSO4 increased from 0 to 0.72%, the NK activity increased by 71% [79]. In addition, when the optimum ratio of Na2HPO4 and NaH2PO4 was 4:1, the optimal NK activity was 871.56 IU/mL [86].
It has been demonstrated that suitable culture conditions, such as inoculum size, pH, temperature, and dissolved oxygen, can effectively improve NK activity and yield [87]. Most Bacillus species are mesophilic and aerobic strains, tend to thrive well in optimum conditions, and effect NK yield and activity. The optimized NK activity was 3284 ± 58 IU/mL under the culture condition of 30 °C pH 7.0, inoculum amount 2%, and 60 mL of loading volume in a 250 mL conical flask [57]. The NK activity improved by 381 U/mL when adjusting the aeration rate from 1.3 to 10 vvm [81]. Similarly, the NK activity reached 587 U/mL in the fermenter, which was 1.23-fold higher than B shake flask fermentation [78]. In another scale-up experiment in a 100 L fermenter, NK activity reached the maximum of 10,661.97 ± 72.47 IU/mL [84].
The optimization and integration of conditions is the focus of the current research, with higher efficiency, lower production costs, higher NK activity, and more consistent productivity and yields. Various strategies, such as fed-batch and continuous culture, were tested to optimize the NK yield. A supplement with glycerol maintained at a concentration of 20 mL/L resulted in a final NK activity of 7778 17.28 U/mL, 2.6-fold higher than that of the batch [88]. In addition, the highest NK activity reached 654.84 U/mL by fed-batch 3% glycerol and improved by 12% compared to that of the batch culture [78].

Table 3.
Culture medium and condition optimization of NK.
Table 3.
Culture medium and condition optimization of NK.
| Strains | Culture Medium | Culture Methods | Results | Ref. |
|---|---|---|---|---|
| B. subtilis natto WTC016 | Luria–Bertani (LB) liquid medium: peptone (10 g/L), yeast extract (5 g/L), NaCl (10 g/L), agar (15 g/L), | Fermented at 30 °C, pH 7.0, and 60 mL of loading volume in 250 mL conical flask for 24 h | 3284 ± 58 IU/mL | [57] |
| B. subtilis VITMS2 | Sucrose (1%), soybean meal (2%), malt extract (2%), and 10 mM of CaCl2, MgSO4, Na2HPO4 and K2HPO4 | Inoculated with 4.0% inoculum, pH 7.0, 30 °C for 48 h | 171.1 ± 0.27 U/mL | [68] |
| B. subtilis NDF | Soybean milk (180 g/L) and glucose (105 g/L) | Inoculated with the 5% (V/V) of inoculant 3.7 × 108 colony forming unit/mL (CFU/mL) and fed-batch fermented at 30 °C, initial pH 7.0, 600 rpm, 1.0 vvm in 6-L fermenter with 4 L of loading volume for 25 h | 10,220 IU/mL | [77] |
| B. subtilis natto | Yeast extract (6%), soy peptone (1.2%), and glycerol (6%) | Inoculated with the 2% volume/volume (V/V) spore solution (5.2 ± 0.5 × 1010 spores/mL) and fed-batch fermented at 40 °C for 10 h in fermenter | 587 U/mL | [78] |
| B. subtilis natto | Soybean flour (16.7%) and rice husk (13.3%) with 70% water content | Solid-state fermentation incubated at 37 °C for 24 h | 2503.4 IU/g dry substrate | [80] |
| B. subtilis D21-8 | Cassava starch (20 g/L), soybean meal (10 g/L), K2HPO4 (3 g/L), KH2PO4 (1 g/L), MgSO4·7H2O (3.5 g/L), and CaCl2 (0.2 g/L) | 2% (V/V) inoculated and fermented at 34 °C and 180 rpm for 72 h in 250 mL Erlenmeyer flask with loading 50 mL medium liquid | 1754 U/mL | [81] |
| B. subtilis natto 1A752 | Ginkgo seeds | Relative humidity 80%, initial water content 73%, at 38 °C, inoculation volume 18% for 38 h | 3682 ± 43 IU/g dry substrate | [82] |
| B. subtilis natto | Chestnut | 5% (V/V) inoculum concentration at 38 °C for 56 h | 6479 IU/g dry substrate | [83] |
| B. subtilis MTCC 2616 | Tryptone (10 g/L), yeast extract (10 g/L), K2HPO4·3H2O (1 g/L), MgSO4·7H2O (0.5 g/L), and CaCl2·2H2O (0.5 g/L). | Fermented at 30 °C and 150 rpm in an orbital shaker for 51 h | 789.93 U/mL | [84] |
| B. subtilis GXA-28 | The cane molasses contained 35.2% (w/w) sucrose, 9.0% (w/w) glucose, 15.8% (w/w) fructose; the monosodium glutamate waste liquor consisted of 2.0% (w/w) glutamate, 0.3% (w/w) (NH4)2SO4 | 2% (V/V) inoculum in solid medium at pH 8.0 and 45 °C for 24 h in shallow tray | 986 U/g-substrate | [85] |
| B. subtilis natto | Glucose (6.10 g/L), soybean peptone (5.00 g/L), K2HPO4 (3.00 g/L), MgSO4.7H2O (0.25 g/L), NaCl (4.27 g/L), CaCl2 (0.05 g/L) | Inoculated 5 billion colony-forming units/mL of medium in 37 °C and pH 7.5 for 20 h | 55.82 U/mL | [87] |
| B. subtilis 13,932 | Glucose (30.868 g/L), tofu processing wastewater (93.669%), MgSO4·7H2O (1.129 g/L), CaCl2 (0.791 g/L) | Fermented at 37 °C, pH 7.0, 70 mL liquid medium, and 200 rpm in 100 L bioreactors | 7209.15 ± 195.46 IU/mL | [89] |
| B. subtilis 14,715 | 25 g of pigeon pea | 2% (V/V) inoculum (107 CFU/mL) fermented at 35 °C for 32 h | 53.03 U/g | [90] |
| B. subtilis natto | LB medium, glycerol (20 mL/L) | Fermented at 37 °C, pH 7.0, in fermenter | 7778 ± 17.28 U/mL | [91] |
| P. aeruginosa CMSS | Shrimp shell (1%), KH2PO4 (0.1%), MgSO4 (0.05%) | 1% (V/V) inoculum at 37 °C, 120 rpm, and pH 7 for 24 h in a shaker | 2581 U/ mL | [67] |
4.3. Recombinant Expression
At present, genetic manipulation is commonly used to improve NK yield. Undoubtedly, recombinant technology offers a promising approach for enhancing NK yield and extensive application. The aprN gene encoding NK has been cloned and recombinantly expressed in various suitable expression systems, including microbes, insects, and plants [9,92,93]. Microbes such as Bacillus spp., Escherichia coli (E. coli), lactic acid bacteria (LAB), and eucaryotes are always feasible host strains for NK production (Table 4) [9]. B. subtilis, as the main source strain of NK, is a food-grade expression and secretory system. Therefore, it became the first choice for NK recombinant expression. However, in addition to NK, it also expresses various natural extracellular proteases, which may substantially degrade the recombinant proteins expressed by the systems [9]. The genetically improved strain of B. subtilis, which lacks extracellular proteases, can solve this problem [94]. The NK yield of the recombinant mutant B. subtilis LM2531 with multiple deficient lytic genes was significantly increased about 2.6-fold compared with that of prototypical B. subtilis 168 [95]. The NK activity and product activity of mutant B. licheniformis BL10 with eight deficient proteases increased by 39%, compared to wild-type WX-02 [96]. E. coli has a shorter growth cycle, simpler cultural medium, and easier downstream purification processes, which make it a potential way of producing NK. Both the pro-NK and NK were recombinantly expressed in E. coli, but the fibrinolytic activity of the recombinant NK was considerably lower than that of wild-type NK [97]. Recombinant E. coli DH5α constructed in B. licheniformis DW2 as a host for NK resulted in a 6.54-fold increase in aprE transcript levels and a 50.53% increase in NK activity [98]. Although NK was expressed largely in E. coli, the recombinant protein aggregated to form insoluble and inactive inclusion bodies, which finally reduced NK yield and made it difficult to recover [51,99]. A food-grade LAB expression system has enabled the development of oral NK formulations [100]. Recombinant LAB can enhance the stability of NK in intestinal fluid, which retained approximately 32% of the fibrinolytic activity after 3 h [100]. Moreover, eukaryotic systems such as Pichia pastoris, can also be used for expressing NK [97,101]. However, these hosts exposed the disadvantage of post-translational modification, and long expression times simultaneously.

Table 4.
Recombinant expression of NK in different hosts.
In addition, other recombinant expression systems, such as animal and plant, have also been used to express NK. Insects such as Spodoptera frugiperda are the normally used animal recombinant expression system. The modified baculovirus expression system successfully expressed NK with 60 U/mL fibrinolytic activity in S. frugiperda [92]. Plant systems, named plant molecular farming (PMF), represent hopeful alternative methods for producing recombinant proteins [104,105]. A synthetic gene (sNK) was constructed and transiently expressed in melon fruit, with the maximum fibrinolytic activity of 79.30 U/mL [102]. Although PMF-expressed NK had the ability to dissolve fibrin and blood clots in vitro and is not immunogenic by oral intake, PMF application in NK is hampered by some clear restrictions, such as the high cost of downstream extraction processing and low feasibility [106,107].
4.4. Molecular Modification
Optimization of the promoter element of aprN gene is an effective way to increase NK yields. The expression of aprN gene in recombinant B. subtilis can be increased to 643 mg/L by replacing the promoter (from TACAAT to TATAAT) [108]. The exogenous expression of NK by cloning the promoter PnisZ and signal peptide SPUsp in L. lactis increased by about 94% [109]. High yields of recombinant NK were also achieved using the tandem promoter PHpaII-PHpaII-PP43 in B. subtilis WB800, with a final maximum amplified yield of 816.7 ± 30.0 FU/mL of NK [110]. Besides the optimization of the promoter elements, gene modification is used to improve the fibrinolytic activity of NK. A mutant library of NK has been generated to obtain mutants with higher catalytic efficiency; the S101L, S101F and S101W mutants possessed a 1.45-fold, 1.80-fold and 1.91-fold higher catalytic efficiency, respectively, compared with wild-type NK [111]. In addition, based on several conserved amino acid residues in the determined stereo structure of NK, site-directed mutagenesis was also used to improve the enzymatic properties, such as catalytic activity, stability and antioxidative activity [112]. For a single mutant (I31L) constructed by site-directed mutagenesis, the catalytic efficiency and fibrinolytic activity of this mutant increased about 2- and 1.92-fold relative to the wild-type NK, respectively [113]. The mutant Q59E constructed by site-directed mutagenesis increased specific activity to 10.28 ± 0.5 U/mg and the mutant SY exhibited better stability than other mutants and the wild type under acidic conditions at pH 4, with the activity maintained at 93.3% [114]. Furthermore, 3D model-validated mutants S18D, Q19I, T242Y and Q245W were more stable and less immunogenic than native NK, but without causing any changes in the catalytic site [115]. In another study, for the site-directed mutant M222A optimized amino acid residues Thr220 and Met222 close to the catalytic residue Ser221, the oxidative stability of NK was improved by 10-fold compared to natural NK [112].
5. Enrichment Extraction of NK
Enrichment extraction remains an important way to improve NK purity and broaden its application areas. NK is produced by Bacillus spp. and secreted extracellularly, which makes it relatively simple to extract. Some traditional protein extraction processes are also applicable to the purification and enrichment of NK, such as protein salting-out, organic solvent distillation, chromatographic chromatography, and dialysis, etc. [9,72]. However, they suffer from operational complexity, inferior efficiency, and low recovery rates [8,39]. Therefore, combined methods are often used in practical production. Microfiltration and ultrafiltration combine to purify NK from fermentation broth with a recovery of 96%, and purity above 92% [72]. Alternatively, NK can be purified by salting-out and then column chromatography [39,116]. NK purified by using a gel filtration (Sephadex G-75) column and hydrophobic interaction chromatography obtained a 56.1-fold activity of the initial activity and the recovery of 43.2% [48]. The purified NK was obtained by ammonium sulfate precipitation and nickel column affinity chromatography with a total NK recovery of 65.2% [91]. The purified NK activity by membrane filtration and Q-Sepharose ion exchange chromatography was 5457 U/mg, with recoveries of 82.45% and 66.91%, respectively [82].
In addition, researchers have developed cheaper, more effective extraction methods and purification techniques, such as reverse micellar extraction, magnetic bead, and three-phase partitioning (TPP) technique [117,118]. The recovery of the AOT (sodium di [2-ethylhexyl] sulfosuccinate)/isooctane reverse micelles system extraction efficiency was 80% with the purification factor of 3.9 [119]. The efficient magnetic poly methyl methacrylate beads immobilized with p-aminobenzamidine have a strong capacity for NK purification with the recovery of 85% and purification factor of 8.7 [120]. NK purified by the TPP technique combined with t-butanol and ammonium sulphate not only showed improved pH and temperature stability, but also achieved 129.5% recovery of NK activity [118]. After graded enrichment, the purified NK was obtained by spray drying or lyophilization, etc.
6. Maintenance of NK Activity
The in vivo experiment results confirmed that NK was absorbed through the intestinal tract, and thus took on a functional role [121]. However, free NK is less resistant to the highly acidic pH and was completely inactivated after 1 h in the gastric environment at pH 1.2 [76]. Maintenance of NK activity is the main obstacle to its oral administration effect. Several enzyme immobilization and delivery systems can solve this problem, thus improving the stability and bioavailability of NK.
Enzyme immobilization is commonly used to improve the fibrinolytic activity and stability of NK [122]. The traditional methods are adsorption, embedment, covalent binding, and cross-linking. Immobilized NK can maintain its activity in storage at 4 °C for 25 days using poly hydroxybutyrate physisorption of NK [123]. Embedment usually utilizes biomolecular immobilization. Proteins, polysaccharides, liposomes and biopolymers are all available materials for NK microcapsules. NK activity modified with folic acid-modified chitosan was at least 36% higher than unmodified NK [124]. Phytosterol liposomes (lecithin and phytosterol) encapsulated in NK by a thin film reported an optimal encapsulation rate of 65.25% and NK activity of 2500 U/mL [125].
Although the traditional method is simple and inexpensive, there are still certain defects, such as low efficiency and poor stability. Therefore, researchers have developed even more superior enzyme-targeted drug delivery systems [126,127]. A novel chitosan/dialdehyde starch derivative micelle-hydrogel loaded with NK has an NK release rate of 89.4% [128]. Another targeted delivery vehicle using a mesoporous silica/polyglutamic acid peptide dendrimer and NK for electrostatic interaction maximum NK activity of 75% when the ratio was 4/1 [129]. Immobilized NK onto fine magnetic Fe3O4 nanoparticles with a coupling agent resulted in a higher thrombolytic activity of 91.89%, clearly superior to that of pure NK by 9.03% [130]. In addition to enzyme immobilization, other forms of NK protection and delivery systems are also available matrices, such as tablets and nano-emulsion. NK tablets made from enteric pH-responsive material Eudragit L100-55 and hydroxypropyl cellulose prevented NK inactivation in gastric juice and enabled the controlled release of NK in vitro [131]. High self-double-emulsifying nano emulsions with NK showed a slow-release effect; the encapsulation rate of NK was 86.8 ± 8.2%. The cumulative release after 8h was about 30% [132]. Conjugated poly (lactic-co-glycolic acid) encapsulated NK also works as an effective drug for AD, with a cumulative release rate of 80.23% ± 3.675% over 24 h [133].
7. Biological Functions and Pharmaceutical Value of NK
NK can inhibit coagulation, dissolve blood clots, reduce hypertension and hyperlipidemia, promote apoptosis, and regulate autophagy [134,135]. Therefore, NK is highly promising as a natural, safe, effective, and low-cost clinical agent or food supplement for the treatment or prevention of CVDs (Figure 3) [10,15].
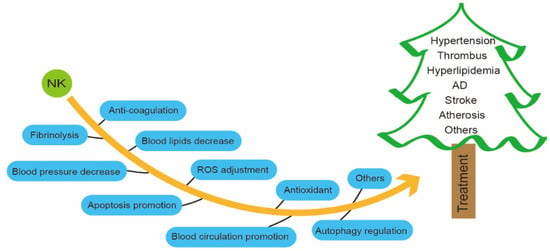
Figure 3.
Biological functions of NK.
7.1. Antithrombotic and Fibrinolytic Efficacy of NK
Thrombus is formed by the massive coagulation of fibrin and platelets within blood vessels. The coagulation can be reversed through fibrinolysis. In various vitro and vivo tests, NK has exhibited extremely potent thrombolytic activity with a relatively low risk of hemorrhage, including shortening plasma clot lysis time and reducing the features associated with an increased risk of CVDs [136]. NK delayed thrombosis by over 90% at 75 mg/kg in the model of blood clots, and the safe dose of NK was 15-fold of that of t-PA [137]. Both oral doses and injected NK resulted in a significant reduction by 67.3 to 83.6% in the induced rat models [82]. In a human trial, two NK capsules (2000 FU/capsule) administered orally and daily reduced the features associated with an increased risk of CVDs, plasma fibrinogen levels, factor VII and factor VIII by 9%, 14% and 17%, respectively, for patients with CVDs [12]. In another study, factor VIII activity decreased by an average of 7.4% and 7.6% after 4 and 6 hours of oral 2000 FU NK, respectively, compared to the initial time [138].
7.2. Anti-Atherosclerotic Effect of NK
The underlying pathological change common to various CVDs is atherosclerosis (AS), which results in heart disease and stroke. AS is a group of lesions based on disorders of lipid metabolism with some symptoms such as thickening and hardening of the arterial blood vessel walls. NK suppresses intimal thickening through its synergistic antioxidant and anti-apoptotic effects. In addition, NK prevents AS by reducing lipid peroxidation, improving lipid metabolism, and inhibiting low-density lipo-protein (LDL) oxidation [139]. NK treatment resulted in a significant reduction in intima-media thickness and carotid plaque size by 36% and 10.62%, respectively, for patients. Therefore, NK is promised to be a viable alternative therapy for atherosclerotic plaque-induced CVDs and stroke [140].
7.3. Hyperlipidemia Reducing Effects of NK
Hyperlipidemia (HLP) is a condition in which blood lipid levels are too high, directly causing several serious diseases such as atherosclerosis. NK prevented HLP by reducing lipid peroxidation and improving lipid metabolism. In a clinical study, total serum cholesterol (TC) levels decreased by 6.8% after 8 weeks in patients with primary hypercholesterolemia treated with 4000FU NK, compared to the placebo group [141]. In addition, the combination of NK with red yeast rice extract had a hypolipidemic effect for patients with hyperlipidemia, which decreased triglycerides (TG), TC and low-density lipoprotein cholesterol (LDL-C) by 15%, 25%, 41%, respectively, and increased HDL-C by 7.5% [142]. In male white rabbit models, the combination of NK and red ginseng reduced the serum cholesteryl ester transfer protein activity by an average of 22%, compared with the high cholesterol groups [143].
7.4. Antihypertensive Effects of NK
Hypertension is characterized by an increase in arterial blood pressure (BP) in the body circulation, which is the most common chronic disease and the most important risk factor for CVDs. NK supplementation reduces systolic and diastolic blood pressure through the cleavage of fibrinogen in plasma, suggesting its role in the prevention and treatment of hypertension [144]. In addition, NK was considered a relatively strong angiotensin-converting enzyme inhibitor (ACEI), which may be attributed to the reduction in BP [14]. In a vitro coagulation lysis assay, the ACEI activity of NK reached a maximum of 87.45% at the NK concentration of 4.8 mg/mL [68]. In addition, an 8-week intake of NK decreased the average baseline diastolic BP from 86 to 81 ± 2.5 mmHg in North American hypertensives [145].
7.5. Neuroprotective Effects of NK
Degenerative diseases of the central nervous system are a group of diseases caused by chronic degenerative tissue degeneration. The main pathological feature is the extracellular deposition of β-amyloid. Memory dysfunction, cognitive function deficit, language function decline, etc., are common clinical symptoms. In vivo and vitro studies demonstrated that the neuroprotective effect of NK was associated with inhibiting β-amyloid deposition, promoting proteolysis, anti-inflammatory and antiapoptotic effects [146]. NK significantly reduced the cerebral infarct volume by approximately 61% in photothrombotic stroke patients [147]. NK also played an important role in the treatment of amyloid-related diseases, such as Alzheimer’s disease (AD) [148]. In rat models, NK can exhibit a positive effect on regulating specific factors in the AD pathway, including enhancing impaired learning, memory capability, and effective suppressing of β-amyloid.
7.6. Other Diseases
NK is also an effective treatment for other diseases, due to its protease properties. NK can be an alternative treatment strategy for retinal neovascularization by the anti-angiogenic effect [149]. NK can treat patients with proliferative vitreoretinal disease by hydrolyzing collagen fiber [150]. NK also treats chronic rhinosinusitis and asthma by hydrolyzing fibrin in nasal polyps of patients with chronic sinusitis [151]. In addition, NK improved survival and inhibited tumor growth in mice with liver cancer [152].
8. Toxicity Assessment of NK
Given the growing public interest in the prospective health benefits of NK, it is important to assess the potential toxicity and overall safety of NK. NK as a bioactive ingredient of fermented food has been consumed for over a thousand years. NK is virtually safe when administered at the commended dose (200 mg per day) and does not show any signs of toxicity in the histopathological examination of organs and tissues. In a 14-day acute toxicity study, no significant signs of adverse effects or mortality were observed in rats gavaged with 2000 mg/kg of NK and those that continued gavaging up to 90 days also showed no abnormalities [153]. Similarly, there was no mortality, adverse clinical signs, any tissue damage, or gross pathological findings in rat models exposed to single gavage doses of NK at 1000 and 2000 mg/kg during the acute 28-day and 90-day repeated dose toxicity study period. Moreover, in human trials, healthy volunteers taking daily oral NK (10 mg/kg) for 28 days also did not exhibit any adverse effects [154]. In animal and human trials, the same physiological effects indicate the bioequivalence of NK. The safety of NK still needs to be thought about and studied, including the effects of doses, administration time, toxicity and genotoxicity [144,154].
9. Conclusions and Prospects
Nattokinase (NK) is a natural, safe, efficient and cost-effective thrombolytic enzyme, which exhibits potentially beneficial cardiovascular effects. This review focused on NK-enriched fermented soybean foods, and more systematically, elaborated on microbial synthesis, extraction and purification, activity maintenance, thrombolytic activity, and safety assessment of NK. Undoubtedly, microbial synthesis by Bacillus spp. is a commonly used method of producing NK. The yield, fibrinolytic activity and bioavailability of NK directly determined its industrial applications. At present, with the gradual increase in practical demand, the public are becoming more interested in the health-related potential of NK and fermented soybean foods enriched with NK. Therefore, although microbial synthesis is an extremely cost-effective way to produce NK, its yield, activity and stability are the primary constraints of industrial applications. The improvement of NK yield, fibrinolytic activity and stability, including potential high throughput microbial producer screening, optimization of fermentation medium and conditions, recombinant expression, and molecular modification, can increase the expression of NK. Moreover, the novel enrichment and purification techniques could enhance the activity and stability of NK. In addition, the development of various stable deliveries and formulations of NK could also meet commercial demands. The biological value and functional activity of NK still need to be validated to enhance the bioavailability. Further studies will certainly focuse on multiple theoretical studies and advanced technologies of NK microbial synthesis and contributions to CVDs.
Author Contributions
Conceptualization, S.L. and F.W.; writing—original draft preparation, D.L.; writing—review and editing, supervision, D.L., M.H. and Y.G.; investigation, L.H. and Z.T.; validation, M.H. and S.L.; funding acquisition, S.L. and F.W.; project administration, F.W., B.F. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System (CARS-04) and Central Public-interest Scientific Institution Basal Research Fund (S2021JBKY-02).
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the funds and thank the Knowledge Innovation Program Funding of Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Richard, A.; Margaritis, A. World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, The Netherlands, Licence: CC BY-NC-SA 3.0 IGO; 2022; Available online: https://www.who.int/data/gho/publications/world-health-statistics (accessed on 19 May 2022).
- Geraghty, L.; Figtree, G.A.; Schutte, A.E.; Patel, S.; Arnott, C. Cardiovascular disease in women: From pathophysiology to novel and emerging risk factors. Heart Lung Circ. 2020, 30, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Falkner, B.; Gidding, S. Life-course implications of pediatric risk factors for cardiovascular disease. Can. J. Cardiol. 2021, 37, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Nozue, M.; Shimazu, T.; Charvat, H.; Mori, N.; Tsugane, S. Fermented soy products intake and risk of cardiovascular disease and total cancer incidence: The Japan Public Health Center–based Prospective study. Eur. J. Clin. Nutr. 2021, 75, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Iso, H.; Ishihara, J.; Okada, K.; Inoue, M.; Tsugane, S. Association of dietary intake of soy, beans, and isoflavones. Circulation 2007, 116, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, X.; Li, C.; Jiao, S.; Dong, W. Association between consumption of soy and risk of cardiovascular disease: A meta-analysis of observational studies. Eur. J. Prev. Cardiol. 2017, 24, 735. [Google Scholar] [CrossRef]
- Yong, P.; Yang, X.; Zhang, Y. Microbial fibrinolytic enzymes: An overview of source, production, properties, and thrombolytic activity in vivo. Appl. Microbiol. Biotechnol. 2005, 69, 126–132. [Google Scholar] [CrossRef]
- Dabbagh, F.; Negahdaripour, M.; Berenjian, A.; Behfar, A.; Mohammadi, F.; Zamani, M.; Irajie, C.; Ghasemi, Y. Nattokinase: Production and application. Appl. Microbiol. Biotechnol. 2014, 98, 9199–9206. [Google Scholar] [CrossRef]
- Weng, Y.; Jian, Y.; Sawyer, S.; Wang, K. Nattokinase: An oral antithrombotic agent for the prevention of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 523. [Google Scholar] [CrossRef]
- Chen, H.; Mcgowan, E.M.; Ren, N.; Lal, S.; Lin, Y. Nattokinase: A promising alternative in prevention and treatment of cardiovascular diseases. Biomark. Insights 2018, 13, 117727191878513. [Google Scholar] [CrossRef]
- Yatagai, C.; Maruyama, M.; Kawahara, T.; Sumi, H. Nattokinase-promoted tissue plasminogen activator release from human cells. Pathophysiol. Haemost. Thromb. 2008, 36, 227–232. [Google Scholar] [CrossRef]
- Chien-Hsun, H.; Ming-Ching, S.; Jen-Shiou, L.; Yao-Ke, W.; Kai-Lin, H.; Thau-Ming, C.; Nae-Cherng, Y. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. sciencedirect. Nutr. Res. 2009, 29, 190–196. [Google Scholar] [CrossRef]
- You, W.K.; Sohn, Y.D.; Kim, K.Y.; Park, D.H.; Jang, Y.; Chung, K.H. Purification and molecular cloning of a novel serine protease from the centipede, scolopendra subspinipes mutilans. Insect Biochem. Mol. Biol. 2004, 34, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yamanaka, N.; Ohnishi, K.; Fukayama, M.; Yoshino, M. Inhibition of angiotensin I converting enzyme by Subtilisin NAT (nattokinase) in natto, a Japanese traditional fermented food. Food Funct. 2012, 3, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.-Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Keitarou, K.; Satoshi, Y. Trends in the application of Bacillus in fermented foods. Curr. Opin. Biotechnol. 2018, 56, 36–42. [Google Scholar] [CrossRef]
- Syahbanu, F.; Giriwono, P.E.; Tjandrawinata, R.R.; Suhartono, M.T. Molecular analysis of a fibrin-degrading enzyme from Bacillus subtilis K2 isolated from the indonesian soybean–based fermented food moromi. Mol. Biol. Rep. 2020, 47, 8553–8563. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Ryu, M.S.; Yang, H.J.; Park, S. γ-PGA-rich chungkookjang, short-term fermented soybeans: Prevents memory impairment by modulating brain insulin sensitivity, neuro-inflammation, and the gut–microbiome–brain axis. Foods 2021, 10, 221. [Google Scholar] [CrossRef]
- Ohta, T. Natto: In Legume-Based Fermented Foods; Reddy, N.R., Pierson, M.D., Salunkhe, D.K., Eds.; CRC Press: Boca Raton, FL, USA, 1986; pp. 85–93. [Google Scholar]
- Mine, Y.; Wong, A.H.K.; Jiang, B. Fibrinolytic enzymes in Asian traditional fermented foods. Food Res. Int. 2005, 38, 243–250. [Google Scholar] [CrossRef]
- Miyake, S.; Wantanabe, K.; Yoshikawa, M.; Nonoguchi, Y. Studies on Bacillus natto protease: The constituting amino acids of the crystalline protease of Bac. Natto. Sawamura. Sci. Rep. Hyogo Univ. Agric. 1956, 28, 527–529. [Google Scholar] [CrossRef]
- Oshima, K. The properties of protease A, the proteolytic enzyme of natto bacteria. J. Soc. Agric. For. Sapporo 1925, 71, 387–403. [Google Scholar]
- Miyake, S.; Shimizu, J. The studies on Bacillus natto protease (1): On crystallization of protease. Sci. Rep. Hyogo Univ. Agric. 1953, 32, 260–262. [Google Scholar] [CrossRef]
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese natto; a typical and popular soybean food in the Japanese diet. Experientia 1987, 43, 1110. [Google Scholar] [CrossRef] [PubMed]
- Ito, K. Review of the health benefits of habitual consumption of miso soup: Focus on the effects on sympathetic nerve activity, blood pressure, and heart rate. Environ. Health Prev. Med. 2020, 25, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Tatsumi, E.; Ding, C.H.; Li, L.T. Angiotensin I–converting enzyme inhibitory peptides in douchi, a chinese traditional fermented soybean product. Food Chem. 2006, 98, 551–557. [Google Scholar] [CrossRef]
- Guan, R.F.; Liu, Z.F.; Zhang, J.J.; Wei, Y.X.; Said, W.; Liu, D.H.; Ye, X.Q. Investigation of biogenic amines in sufu (furu): A Chinese traditional fermented soybean food product. Food Control 2013, 31, 345–352. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Chung, K.R.; Yang, H.J.; Jang, D.J. Gochujang (Korean red pepper paste): A Korean ethnic sauce, its role and history. J. Ethn. Foods 2015, 2, 29–35. [Google Scholar] [CrossRef]
- Shin, D.; Jeong, D. Korean traditional fermented soybean products: Jang. J. Ethn. Foods 2015, 2, 2–7. [Google Scholar] [CrossRef]
- Kim, D.C.; Quang, T.H.; Yoon, C.S.; Ngan, N.; Lim, S.I.; Lee, S.Y.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory activities of indole alkaloids from kanjang (Korean fermented soy source) in lipopolysaccharide-induced BV2 microglial cells. Food Chem. 2016, 213, 69–75. [Google Scholar] [CrossRef]
- Tamang, J.P. Native microorganisms in the fermentation of kinema. Indian J. Microbiol. 2003, 43, 127–130. [Google Scholar]
- Chettri, R.; Tamang, J.P. Bacillus species isolated from tungrymbai and bekang, naturally fermented soybean foods of india. Int. J. Food Microbiol. 2015, 197, 72–76. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, S.K. Probiotic potential of Lactobacillus plantarum ID1 isolated from batter of dosa, a south indian fermented food. Probiotics Antimicrob. Proteins 2014, 6, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Syahbanu, F.; Giriwono, P.E.; Tjandrawinata, R.R.; Suhartono, M.T. Molecular docking of Subtilisin K2, a fibrin-degrading enzyme from indonesian moromi, with its substrates. Ciência E Tecnol. Aliment. 2021, 42, e61820. [Google Scholar] [CrossRef]
- Hachmeister, K.A.; Fung, D.Y.C. Tempeh: A mold-modified indigenous fermented food made from soybeans and/or cereal grains. Crit. Rev. Microbiol. 1993, 19, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Press, C. Fermented Foods and Beverages of the World; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Inatsu, Y.; Nakamura, N.; Yuriko, Y.; Fushimi, T.; Kawamoto, S. Characterization of Bacillus subtilis strains in thua nao, a traditional fermented soybean food in northern Thailand. Lett. Appl. Microbiol. 2010, 43, 237–242. [Google Scholar] [CrossRef]
- Antai, S.P.; Ibrahim, M.H. Micro-organisms associated with African locust bean (parkia filicoidea welw) fermentation for ‘dawadawa’ production. J. Appl. Bacteriol. 2010, 61, 145–148. [Google Scholar] [CrossRef]
- Selvarajan, E.; Bhatnagar, N. Nattokinase: An updated critical review on challenges and perspectives. Cardiovasc. Hematol. Agents Med. Chem. 2017, 16, CD012175. [Google Scholar] [CrossRef]
- Tamang, J.P.; Koichi, W.; Holzapfel, W.H. Review: Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Parkouda, C.; Adewumi, G.A.; Ouoba, L.I.I.; Jespersen, L. Technologically relevant Bacillus species and microbial safety of west african traditional alkaline fermented seed condiments. Crit. Rev. Food Sci. Nutr. 2020, 62, 871–888. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Gong, M.; Zhang, H.; Liu, L. Production of proteins and commodity chemicals using engineered Bacillus subtilis platform strain. Essays Biochem. 2021, 65, 173–185. [Google Scholar] [CrossRef]
- Xiang, M.; Kang, Q.; Zhang, D. Advances on systems metabolic engineering of Bacillus subtilis as a chassis cell. Synth. Syst. Biotechnol. 2020, 5, 245–251. [Google Scholar] [CrossRef]
- Vignesh, H.; Eajas, B.M.; Ramesh, B.; Saravanan, N. Production, optimization and characterization of nattokinase from Bacillus subtilis REVS 12 isolated from natto. Int. J. Sci. Eng. Res. 2014, 5, 423. [Google Scholar]
- Man, L.L.; Xiang, D.J.; Zhang, C.L. Strain screening from traditional fermented soybean foods and induction of nattokinase production in Bacillus subtilis MX–6. Probiotics Antimicro. Proteins 2019, 11, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, H.; Chen, Z.; Lv, Z.; Xie, Y.; Lu, F. Profiling of dynamic changes in the microbial community during the soy sauce fermentation process. Appl. Microbiol. Biotechnol. 2013, 97, 9111–9119. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Hayato, I.; Kazuo, U.; Yasuhiro, S.; Masaki, O.; Sumio, A.; Yoshinori, T.; Isao, S.; Akikazu, T. The profibrinolytic enzyme Subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J. Biol. Chem. 2021, 276, 24690–24696. [Google Scholar] [CrossRef]
- Wang, C.; Du, M.; Zheng, D.; Kong, F.; Zu, G.; Feng, Y. Purification and characterization of nattokinase from Bacillus subtilis natto B-12. J. Agric. Food Chem. 2009, 57, 9722–9729. [Google Scholar] [CrossRef]
- Wei, X.T.; Luo, M.F.; Xu, L.; Zhang, Y.W.; Lin, X.; Kong, P.; Liu, H.Z. Production of fibrinolytic enzyme from Bacillus amyloliquefaciens by fermentation of chickpeas, with the evaluation of the anticoagulant and antioxidant properties of chickpeas. J. Agric. Food Chem. 2011, 59, 3957–3963. [Google Scholar] [CrossRef]
- Cheng, T.; Bao, P.; Bo, L.; Nout, R.; Ping, L.; Hong, J.; Chen, L. Purification and characterization of a fibrinolytic enzyme of Bacillus subtilis DC33, isolated from Chinese traditional douchi. J. Ind. Microbiol. Biotechnol. 2006, 33, 750–758. [Google Scholar] [CrossRef]
- Liang, X.; Jia, S.; Sun, Y.; Chen, M.; Chen, X.; Zhong, J.; Huan, L. Secretory Expression of Nattokinase from Bacillus subtilis YF38 in Escherichia coli. Mol. Biotechnol. 2007, 37, 187–194. [Google Scholar] [CrossRef]
- Zhang, Z. Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional chinese soybean food. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 134, 45–52. [Google Scholar] [CrossRef]
- Kim, W. Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11-4 screened from chungkook–jang. Appl. Environ. Microbiol. 1996, 62, 2482. [Google Scholar] [CrossRef]
- Chang, S.P.; Dong, H.K.; Lee, W.Y.; Kang, D.O.; Song, J.J.; Choi, N.S. Identification of fibrinogen-induced nattokinase WRL101 from Bacillus subtilis WRL101 isolated from doenjang. Afr. J. Microbiol. Res. 2013, 7, 1983–1992. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, N.S. Purification and characterization of subtilisin DJ-4 secreted by Bacillus sp. strain DJ-4 screened from doen-jang. J. Agric. Chem. Soc. Jpn. 2020, 64, 1722–1725. [Google Scholar] [CrossRef]
- Afifah, D.N.; Sulchan, M.; Syah, D.; Yanti Suhartono, M.T. Purification and characterization of a fibrinolytic enzyme from Bacillus pumilus 2.g isolated from gembus, an indonesian fermented food. Prev. Nutr. Food Sci. 2014, 19, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Cao, Z.; Wong, C.; Liu, Y.; Li, J. Isolation and optimal fermentation condition of the Bacillus subtilis subsp. natto strain WTC016 for nattokinase production. Fermentation 2019, 5, 92. [Google Scholar] [CrossRef]
- Kumar, D.; Rakshitha, R.; Vidhya, M.A.; Jennifer, P.S.; Kalaichelv, P.T. Production, optimization and characterization of fibrinolytic enzyme by Bacillus subtilis RJAS19. Pak. J. Biol. Sci. PJBS 2014, 17, 529–534. [Google Scholar] [CrossRef]
- Chang, C.T.; Fan, M.H.; Kuo, F.C.; Sung, H.Y. Potent fibrinolytic enzyme from a mutant of Bacillus subtilisimr NK1. J. Agric. Food Chem. 2020, 48, 3210–3216. [Google Scholar] [CrossRef]
- Wang, S.L.; Wu, Y.Y.; Liang, T.W. Purification and biochemical characterization of a nattokinase by conversion of shrimp shell with Bacillus subtilis TKU007. New Biotechnol. 2011, 28, 196–202. [Google Scholar] [CrossRef]
- Uppuluri, S. Screening, production, purification, characterization and evaluation of nattokinase from Bacillus subtilis isolated from blood soil Andhra Pradesh. Am. Res. Thoughts 2016, 2, 3691–3698. [Google Scholar]
- Wang, S.L.; Chen, H.J.; Liang, T.W.; Lin, Y.D. A novel nattokinase produced by pseudomonas sp. TKU015 using shrimp shells as substrate. Process Biochem. 2009, 44, 70–76. [Google Scholar] [CrossRef]
- Borah, D.; Shahin, L.; Sangra, A. Production, purification and characterization of nattokinase from Bacillus subtilis, isolated from sewage water. Int. J. Pharm. Arch. 2012, 1, 21–24. [Google Scholar]
- Mahajan, P.M.; Nayak, S.; Lele, S.S. Fibrinolytic enzyme from newly isolated marine bacterium Bacillus subtilis ICTF-1: Media optimization, purification and characterization. J. Biosci. Bioeng. 2012, 113, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.V.; Wu, G.J.; Hsieh, M.C.; Chang, S.H.; Tsai, G.J. Purification and characterization of nattokinase from cultural filtrate of red alga porphyra dentata fermented by Bacillus subtilis n1. J. Mar. Sci. Technol. 2015, 23, 240–248. [Google Scholar] [CrossRef]
- Lu, F.; Sun, L.; Lu, Z.; Bie, X.; Fang, Y.; Shu, L. Isolation and identification of an endophytic strain EJS-3 producing novel fibrinolytic enzymes. Curr. Microbiol. 2007, 54, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.D.; Vaithilingam, M.; Shanker, R.; Kumar, S.; Thiyur, S.; Babu, V.; Selvakumar, J.N.; Prakash, S. Exploring the in vitro thrombolytic activity of nattokinase from a new strain Pseudomonas aeruginosa CMSS. Jundishapur J. Microbiol. 2015, 8, e23567. [Google Scholar] [CrossRef] [PubMed]
- Keziah, S.M.; Devi, C.S. Fibrinolytic and ACE Inhibitory Activity of Nattokinase Extracted from Bacillus subtilis VITMS 2: A Strain Isolated from Fermented Milk of Vigna unguiculata. Protein J. 2021, 40, 876–890. [Google Scholar] [CrossRef]
- Vaithilingam, M.; Chandrasekaran, S.D.; Gupta, S.; Paul, D.; Sahu, P.; Selvaraj, J.N.; Babu, V. Extraction of Nattokinase Enzyme from Bacillus cereus Isolated from Rust. Natl. Acad. Sci. Lett. 2016, 39, 263–267. [Google Scholar] [CrossRef]
- Ponnuswamy, V.; Arumugaperumal, A.; Prakash, V.; Valan, A.M.; Abdullah, A. Cow dung is a novel feedstock for fibrinolytic enzyme production from newly isolated Bacillus sp. IND7 and its application in in vitro clot lysis. Front. Microbiol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, S.; Liu, M.; Gao, C.; Yang, J.; Zhang, X.; Ding, B. Ultra–small and anionic starch nanospheres: Formation and vitro thrombolytic behavior study. Carbohydr. Polym. 2013, 96, 426–434. [Google Scholar] [CrossRef]
- Cai, D.; Zhu, C.; Chen, S. Microbial production of nattokinase: Current progress, challenge and prospect. World J. Microbiol. Biotechnol. 2017, 33, 84. [Google Scholar] [CrossRef]
- Li, S.Y. Screening of strains with the high activity and thermostability nattokinase by 60co γ–ray irradiation. J. Nucl. Agric. Sci. 2013, 6, 782–785. [Google Scholar]
- Liu, S.; Jiang, M.J.; Chen, X.H.; Chen, G.; Dong, M.S. Effect of the 36th amino acid site mutagenesis on the activity and thermostability of nattokinase. J. Nanjing Agric. Univ. 2008, 31, 130–136. [Google Scholar] [CrossRef]
- Guan, Z.; Li, Z.; Sun, H. Research on the breeding of Bacillus subtilis natto highly producing nattokinase by the compound mutation of hydroxylamine hydrochloride and UV radiation. Food Ferment. Ind. 2009, 35, 71–74. [Google Scholar] [CrossRef]
- Wei, X.; Luo, M.; Xie, Y.; Yang, L.; Li, H.; Xu, L.; Liu, H. Strain Screening, Fermentation, Separation, and Encapsulation for Production of Nattokinase Functional Food. Appl. Biochem. Biotechnol. 2012, 168, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Shen, J.; Li, Y.; Wang, Z.; Zhao, Y.; Chen, Y.; Zhao, J. High and Economical Nattokinase Production with Acetoin as a Useful Byproduct from Soybean Milk and Glucose. Probiotics Antimicro. Proteins 2021. [Google Scholar] [CrossRef]
- Aydin, B.; Raja, M.; John, K.; Fariba, D.; Younes, G. Nattokinase production: Medium components and feeding strategy studies. Chem. Ind. Chem. Eng. Q. 2014, 20, 541–547. [Google Scholar] [CrossRef]
- Meng, F.Y.; Xue, F.; Shi, H. Effects of natto kinase on blood lipid and blood rheology in atherosclerosis model rats. Chin. J. Lab. Diagn. 2013, 17, 1567–1569. [Google Scholar]
- Nie, G.; Zhu, Z.; Fang, L.; Nie, Z.; Ye, Y.; Yue, W. Co-production of nattokinase and poly (γ-glutamic acid) under solid-state fermentation using soybean and rice husk. Braz. Arch. Biol. Technol. 2015, 58, 718–724. [Google Scholar] [CrossRef]
- Pan, S.; Chen, G.; Zeng, J.; Cao, X.; Zheng, X.; Zeng, W.; Liang, Z. Fibrinolytic enzyme production from low-cost substrates by marine Bacillus subtilis: Process optimization and kinetic modeling. Biochem. Eng. J. 2018, 141, 268–277. [Google Scholar] [CrossRef]
- Guo, N.; Jiang, Y.W.; Song, X.R.; Li, Y.Y.; Liu, Z.M.; Fu, Y.J. Effect of Bacillus natto solid-state fermentation on the functional constituents and properties of ginkgo seeds. J. Food Biochem. 2019, 43, e12820. [Google Scholar] [CrossRef]
- Dong, M.Z.; An, J.Y.; Wang, L.T.; Fan, X.H.; Fu, Y.J. Development of fermented chestnut with Bacillus natto: Functional and sensory properties. Food Res. Int. 2020, 130, 108941. [Google Scholar] [CrossRef]
- Sahoo, A.; Mahanty, B.; Daverey, A.; Dutta, K. Nattokinase production from Bacillus subtilis using cheese whey: Effect of nitrogen supplementation and dynamic modelling. J. Water Process Eng. 2020, 38, 101533. [Google Scholar] [CrossRef]
- Zeng, W.; Li, W.; Shu, L.; Yi, J.; Chen, G.; Liang, Z. Non-sterilized fermentative co–production of poly (γ–glutamic acid) and fibrinolytic enzyme by a thermophilic Bacillus subtilis GXA-28. Bioresour. Technol. 2013, 142, 697–700. [Google Scholar] [CrossRef]
- Zhou, H.M.; Zhang, H.X.; Xie, Y.H.; Zhou, T.T.; Liu, H.; Luo, Y.B. Optimization of liquid fermentation conditions and encapsulation for nattokinase production. Adv. Mater. Res. 2013, 781–784, 1403–1409. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Thuan, D.T.H.; Tran, T.M.T.; Nguyen, T.H. Determination the optimum fermentation in obtaining nattokinase by Bacillus subtilis natto. ISSR J. 2015, 13, 663–668. [Google Scholar]
- Zhang, J.; Cui, Q.; Qian, B.; Sun, X. High Cell Density Cultivation of A Recombinant Bacillus Subtilis for Nattokinase Production. Authorea 2020. [Google Scholar] [CrossRef]
- Li, T.; Zhan, C.; Guo, G.; Liu, Z.; Ouyang, P. Tofu processing wastewater as a low-cost substrate for high activity nattokinase production using Bacillus subtilis. BMC Biotechnol. 2021, 21, 57. [Google Scholar] [CrossRef]
- Lee, B.H.; Lai, Y.S.; Wu, S.C. Antioxidation, angiotensin converting enzyme inhibition activity, nattokinase, and antihypertension of Bacillus subtilis (natto)-fermented pigeon pea. J. Food Drug Anal. 2015, 23, 750–757. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, X.; Tong, Y.; Wang, J.; Ye, J.; Yang, R. Chitosan/casein based microparticles with a bilayer shell–core structure for oral delivery of nattokinase. Food Funct. 2020, 11, 10799–10816. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xiong, S.; Zhang, J.; Yang, C.Y. Expression and purification of recombinant nattokinase in Spodoptera frugiperda cells. Biotechnol. Lett. 2007, 29, 1459–1464. [Google Scholar] [CrossRef]
- Wang, K.; Tull, L. Expression of blood clot-dissolving proteins in transgenic plant (LB852). FASEB J. 2007, 28, LB852. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Quyen, T.D.; Le, H.T. Cloning and enhancing production of a detergent- and organic-solvent-resistant nattokinase from Bacillus subtilis VTCC-DVN-12-01 by using an eight-protease-gene-deficient Bacillus subtilis WB800. Microb. Cell Fact. 2013, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.M.; Zhao, R.L.; Jin, T.T.; Zhang, X.M.; Chen, X.D. Deleting multiple lytic genes enhances biomass yield and production of recombinant proteins by Bacillus subtilis. Microb. Cell Fact. 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhou, Y.; Chen, J.; Cai, D.; Wang, D.; Qi, G.; Chen, S. Efficient expression of nattokinase in Bacillus licheniformis: Host strain construction and signal peptide optimization. J. Ind. Microbiol. Biotechnol. 2015, 42, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.F.; Chen, B.; Li, Y.; He, Z.; Huo, J. Cloning of nattokinase gene and expression in pichia pastoris. J. Biol. 2011, 28(05), 55–57. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, B.; Rao, Y.; Li, L.; Zhu, J.; Li, J.; Ma, X.; Chen, S. Improving the utilization rate of soybean meal for efficient production of bacitracin and heterologous proteins in the aprA-deficient strain of Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2019, 103, 4789–4799. [Google Scholar] [CrossRef]
- Ni, H.; Guo, P.C.; Jiang, W.L.; Fan, X.M.; Luo, X.Y.; Li, H.H. Expression of nattokinase in Escherichia coli and renaturation of its inclusion body. J. Biotechnol. 2016, 231, 65–71. [Google Scholar] [CrossRef]
- Li, C.; Du, Z.; Qi, S.; Zhang, X.; Tian, H. Food-grade expression of nattokinase in Lactobacillus delbrueckii subsp. bulgaricus and its thrombolytic activity in vitro. Biotechnol. Lett. 2020, 42, 2179–2187. [Google Scholar] [CrossRef]
- Yan, G.; Shu, M.; Shen, W.; Ma, L.; Zhai, C.; Wang, Y.; H, Z. Heterologous expression of nattokinase from B. subtilis natto using Pichia pastoris GS115 and assessment of its thrombolytic activity. BMC Biotechnol. 2021, 21, 49. [Google Scholar] [CrossRef]
- Han, L.; Zhang, L.; Liu, J.; Li, H.; Wang, Y.; Hasi, A. Transient expression of optimized and synthesized nattokinase gene in melon (Cucumis melo L.) fruit by agroinfiltration. Plant Biotechnol. 2015, 32, 175–180. [Google Scholar] [CrossRef]
- Han, L.; Ju, L.F.; Niu, Y.D.; Wang, Y.C.; Agula, H. Optimization, synthesis and transient expression of nattokinase gene in tobacco(Nicotiana tabacum L.) leaves. China Biotechnol. 2015, 2, e25271. [Google Scholar] [CrossRef]
- Kyoko, H.T.; Hiroshi, E. Molecular breeding to create optimized crops: From genetic manipulation to potential applications in plant factories. Front. Plant Sci. 2016, 7, 539. [Google Scholar] [CrossRef]
- Shahid, N.; Daniell, H. Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant Biotechnol. J. 2016, 14, 2079–2099. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015, 33, 902–913. [Google Scholar] [CrossRef]
- Yao, J.; Weng, Y.; Alexia, D.; Wang, K. Plants as factories for human pharmaceuticals: Applications and challenges. Int. J. Mol. Sci. 2015, 16, 26122. [Google Scholar] [CrossRef]
- Guan, C.; Cui, W.; Cheng, J.; Liu, Z.; Zhou, Z. Development of an efficient autoinducible expression system by promoter engineering in Bacillus subtilis. Microb. Cell Fact. 2016, 15, 66. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Jin, Z.; Huan, L. Secretory expression of a heterologous nattokinase in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2007, 75, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zhongmei, L.; Wenhui, Z.; Chunlei, G.; Wenjing, C.; Li, Z.; Zhemin, Z. High-level extracellular production of recombinant nattokinase in Bacillus subtilis WB800 by multiple tandem promoters. BMC Microbiol. 2019, 19, 89. [Google Scholar] [CrossRef]
- Wu, S.M.; Feng, C.; Zhong, J.; Huan, L.D. Roles of S3 site residues of nattokinase on its activity and substrate specificity. J. Biochem. 2007, 142, 357–364. [Google Scholar] [CrossRef]
- Weng, M.Z.; Zheng, Z.L.; Wei, B.; Cai, Y.J.; Yin, Y.; Zou, G.L. Enhancement of oxidative stability of the subtilisin nattokinase by site-directed mutagenesis expressed in Escherichia coli. BBA Proteins Proteom. 2009, 1794, 1566–1572. [Google Scholar] [CrossRef]
- Weng, M.Z.; Deng, X.W.; Bao, W.; Zhu, L.; Wu, J.; Cai, Y.; Jia, Y.; Zheng, Z.; Zou, G. Improving the activity of the subtilisin nattokinase by site–directed mutagenesis and molecular dynamics simulation. Biochem. Biophys. Res. Commun. 2015, 465, 580–586. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, H.; Han, L.; Cui, W.; Zhou, L.; Zhou, Z. Improvement of the acid resistance, catalytic efficiency, and thermostability of nattokinase by multisite-directed mutagenesis. Biotechnol. Bioeng. 2019, 116, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Vianney, Y.M.; Tjoa, S.E.E.; Aditama, R.; Putra, S.E.D. Designing a less immunogenic nattokinase from Bacillus subtilis subsp. natto: A computational mutagenesis. J. Mol. Model. 2019, 25, 337. [Google Scholar] [CrossRef] [PubMed]
- Purwaeni, E.; Riani, C.; Retnoningrum, D.S. Molecular Characterization of Bacterial Fibrinolytic Proteins from Indonesian Traditional Fermented Foods. Protein J. 2020, 39, 258–267. [Google Scholar] [CrossRef]
- Rasagnya, P.S.; Vangalapati, A.M. Studies on Optimization of Process Parameters for Nattokinase Production by Bacillus subtilis NCIM 2724 and Purification by Liquid-Liquid Extraction. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 4516–4521. [Google Scholar]
- Garg, R.; Thorat, B.N. Nattokinase purification by three phase partitioning and impact of t-butanol on freeze drying. Sep. Purif. Technol. 2014, 131, 19–26. [Google Scholar] [CrossRef]
- Liu, J.G.; Xing, J.M.; Chang, T.S.; Liu, H.Z. Purification of nattokinase by reverse micelles extraction from fermentation broth: Effect of temperature and phase volume ratio. Bioprocess Biosyst. Eng. 2006, 28, 267. [Google Scholar] [CrossRef]
- Yang, C.; Xing, J.; Guan, Y.; Liu, H. Superparamagnetic poly (methyl methacrylate) beads for nattokinase purification from fermentation broth. Appl. Microbiol. Biotechnol. 2006, 72, 616–622. [Google Scholar] [CrossRef]
- Ero, M.P.; Ng, C.M.; Mihailovski, T.; Harvey, N.R.; Lewis, B.H. A pilot study on the serum pharmacokinetics of nattokinase in humans following a single, oral, daily dose. Altern. Ther. Health Med. 2013, 19, 16–19. [Google Scholar]
- Arsalan, A.; Younus, H. Enzymes and nanoparticles: Modulation of enzymatic activity via nanoparticles. Int. J. Biol. Macromol. 2018, 118 Pt B, 1833–1847. [Google Scholar] [CrossRef]
- Deepak, V.; Pandian, S.; Kalishwaralal, K.; Gurunathan, S. Purification, immobilization, and characterization of nattokinase on PHB nanoparticles. Bioresour. Technol. 2009, 100, 6644–6646. [Google Scholar] [CrossRef]
- Chen, C.; Duan, H.; Gao, C.; Liu, M.; Wu, X.; Wei, Y.; Zhang, X.; Liu, Z. Non-covalent modification of thrombolytic agent nattokinase: Simultaneous improvement of fibrinolysis activity and enzymatic stability. RSC Adv. 2014, 4, 27422–27429. [Google Scholar] [CrossRef]
- Dong, X.Y.; Kong, F.P.; Yuan, G.Y.; Wei, F.; Jiang, M.L.; Li, G.M.; Wang, Z.; Zhao, Y.D.; Chen, H. Optimisation of preparation conditions and properties of phytosterol liposome-encapsulating nattokinase. Nat. Prod. Res. 2012, 26, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, O.M.; Ali, S.S.; Matter, I.A.; Elsamahy, T.; Mahmoud, Y.A. Enzymes immobilization onto magnetic nanoparticles to improve industrial and environmental applications. Methods Enzymol. 2019, 630, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, N.; Zhong, X.; Jiang, Y. Metal–organic frameworks: A potential platform for enzyme immobilization and related applications. Front. Bioeng. Biotechnol. 2020, 8, 695. [Google Scholar] [CrossRef]
- Wen, N.; Lü, S.; Xu, X.; Ning, P.; Wang, Z.; Zhang, Z.; Gao, C.; Liu, Y.; Liu, M. A polysaccharide-based micelle-hydrogel synergistic therapy system for diabetes and vascular diabetes complications treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 94–103. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, S.; Lü, S.; Qi, T.; Yan, J. Synthesis of mesoporous silica/polyglutamic acid peptide dendrimer with dual targeting and its application in dissolving thrombus. J. Biomed. Mater. Res. Part A 2019, 107, 1824–1831. [Google Scholar] [CrossRef]
- Ren, L.; Wang, X.; Wu, H.; Shang, B.; Wang, J. Conjugation of nattokinase and lumbrukinase with magnetic nanoparticles for the assay of their thrombolytic activities. J. Mol. Catal. B Enzym. 2010, 62, 190–196. [Google Scholar] [CrossRef]
- Law, D.; Zhang, Z. Stabilization and target delivery of nattokinase using compression coating. Drug Dev. Ind. Pharm. 2007, 33, 495. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, S.; Wang, X.; Jie, L.; Yin, Z. Preparation and evaluation of nattokinase-loaded self-double-emulsifying drug delivery system. Asian J. Pharm. Sci. 2015, 10, 386–395. [Google Scholar] [CrossRef]
- Prakash, B.; Amita, V.; Fahad, A.A.; Firoz, A.; Vikas, K.; Bibhu, P. Development of surface-engineered plga nanoparticulate-delivery system of Tet-1-conjugated nattokinase enzyme for inhibition of Aβ40 plaques in Alzheimer’s disease. Int. J. Nanomed. 2017, 12, 8749–8768. [Google Scholar] [CrossRef]
- Fadl, N.; Ahmed, H.; Booles, H.; Sayed, A. Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum. Exp. Toxicol. 2013, 32, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-Y.; Liu, L.-H.; Chen, C.-Y.; Lin, I.-F.; Ali, D.; Lee, A.Y.-L.; Wang, H.-M.D. Bifunctional mechanisms of autophagy and apoptosis regulations in melanoma from Bacillus subtilis natto fermentation extract. Food Chem. Toxicol. 2021, 150, 112020. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.; Belur, P.D.; Subramanya, S.B. Methods available to assess therapeutic potential of fibrinolytic enzymes of microbial origin: A review. J. Anal. Sci. Technol. 2018, 9, 10. [Google Scholar] [CrossRef]
- Guo, H.; Ban, Y.H.; Cha, Y.; An, E.S.; Choi, J.; Seo, D.W.; Park, D.; Choi, E.K.; Kim, Y.B. Comparative anti–thrombotic activity and haemorrhagic adverse effect of nattokinase and tissue–type plasminogen activator. Food Sci. Biotechnol. 2019, 28, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, Y.; Nirengi, S.; Homma, T.; Esaki, K.; Ohta, M.; Clark, J.F.; Hamaoka, T. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci. Rep. 2015, 5, 11601. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chen, K.T.; Lee, T.H.; Wang, C.H.; Kuo, Y.W.; Chiu, Y.H.; Hsieh, C.L.; Wu, C.J.; Chang, Y.L. Effects of natto extract on endothelial injury in a rat model. Acta Med. Okayama 2010, 64, 399–406. [Google Scholar] [CrossRef]
- Ren, N.N.; Chen, H.J.; Li, Y.; Mcgowan, E.; Lin, Y.G. A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia. Zhonghua Yi Xue Za Zhi 2017, 97, 2038–2042. [Google Scholar] [CrossRef]
- Wu, D.J.; Lin, C.S.; Lee, M.Y. Lipid-lowering effect of nattokinase in patients with primary hypercholesterolemia. Acta Cardiol. Sin. 2009, 25, 26–30. [Google Scholar]
- Yang, N.C.; Chou, C.W.; Chen, C.Y.; Hwang, K.L.; Yang, Y.C. Combined nattokinase with red yeast rice but not nattokinase alone has potent effects on blood lipids in human subjects with hyperlipidemia. Asia Pac. J. Clin. Nutr. 2009, 18, 310–317. [Google Scholar] [CrossRef]
- Kang, S.J.; Lim, Y.; Kim, A.J. Korean red ginseng combined with nattokinase ameliorates dyslipidemia and the area of aortic plaques in high cholesterol-diet fed rabbits. Food Sci. Biotechnol. 2014, 23, 283–287. [Google Scholar] [CrossRef]
- Kim, J.; Gum, S.; Paik, J.; Lim, H.; Kim, K.C.; Ogasawara, K.; Inoue, K.; Park, S.; Jang, Y.; Lee, J. Effects of Nattokinase on Blood Pressure: A Randomized, Controlled Trial. Hypertens. Res. 2008, 31, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Gitte, J.; Miki, L.; Ero, M.P.; Kathleen, B. Consumption of nattokinase is associated with reduced blood pressure and von willebrand factor, a cardiovascular risk marker: Results from a randomized, double–blind, placebo–controlled, multicenter north american clinical trial. Integr. Blood Press. Control 2016, 9, 95–104. [Google Scholar] [CrossRef]
- Ji, H.; Yu, L.; Liu, K.; Yu, Z.; Zhang, Q.; Zou, F.; Liu, B. Mechanisms of nattokinase in protection of cerebral ischemia. Eur. J. Pharmacol. 2014, 745C, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Kim, M.H.; Kim, J.; An, G.; Kim, D.; Kwon, Y.R.; Lee, A.M.; Kim, Y.B.; Kim, H.H. Abstract W P 262: Neuroprotective effect of nattokinase mediated by inhibition of platelet aggregation and thrombosis in photothrombotic stroke. Stroke 2015, 46, AWP262. [Google Scholar] [CrossRef]
- Bhatt, P.C.; Pathak, S.; Kumar, V.; Panda, B. Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nanonutraceutical. Inflammopharmacology 2018, 26, 105–118. [Google Scholar] [CrossRef]
- Huang, Z.; Ng, T.K.; Chen, W.; Sun, X.; Chen, S. Nattokinase attenuates retinal neovascularization via modulation of Nrf2/HO-1 and glial activation. Investig. Ophthalmol. Vis. Sci. 2021, 62, 25. [Google Scholar] [CrossRef]
- Takano, A.; Hirata, A.; Ogasawara, K.; Sagara, N.; Inomata, Y.; Kawaji, T.; Tanihara, H. Posterior vitreous detachment induced by nattokinase (Subtilisin NAT): A novel enzyme for pharmacologic vitreolysis. Investig. Opthalmology Vis. Sci. 2006, 47, 2075–2079. [Google Scholar] [CrossRef]
- Takabayashi, T.; Imoto, Y.; Sakashita, M.; Kato, Y.; Tokunaga, T.; Yoshida, K.; Narita, N.; Ishizuka, T.; Fujieda, S. Nattokinase, profibrinolytic enzyme, effectively shrinks the nasal polyp tissue and decreases viscosity of mucus. Allergol. Int. 2017, 66, 594–602. [Google Scholar] [CrossRef]
- Yan, Y.M.; Wang, Y.J.; Qian, J.L.; Wu, S.; Ji, Y.; Liu, Y.; Zeng, J.; Gong, A. Nattokinase crude extract inhibits Hepatocellular Carcinoma growth in mice. J. Microbiol. Biotechnol. 2003, 29, 1281–1287. [Google Scholar] [CrossRef]
- Lampe, B.J.; English, J.C. Toxicological assessment of nattokinase derived from bacillus subtilis var. natto. Food Chem. Toxicol. 2016, 88, 87–99. [Google Scholar] [CrossRef]
- Hsieh, C.W.; Lu, W.C.; Hsieh, W.C.; Huang, Y.P.; Lai, C.H.; Ko, W.C. Improvement of the stability of nattokinase using γ-polyglutamic acid as a coating material for microencapsulation. LWT-Food Sci. Technol. 2009, 42, 144–149. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).