Enzyme Activity and Physiochemical Properties of Flour after Supercritical Carbon Dioxide Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Extraction Process
2.2. scCO2 Medium Treatment
2.3. Flour Physicochemical Analyses
2.3.1. Moisture Content
2.3.2. Particle Size Analysis
2.3.3. Environmental Scanning Electron Microscope
2.3.4. Determination of CO2 Solubility in Flour with Magnetic Suspension Balance (MSB)
2.3.5. Fourier Transform Infrared Spectroscopy
2.3.6. Determination of the Total Lipids in the Flour
2.4. Monitoring of Enzyme Activity
Statistical Analysis
2.5. Baking Test
3. Results
3.1. Optimization of the Flour Protein Extraction Process and Determination of the Protein Concentration
3.2. Flour Specification
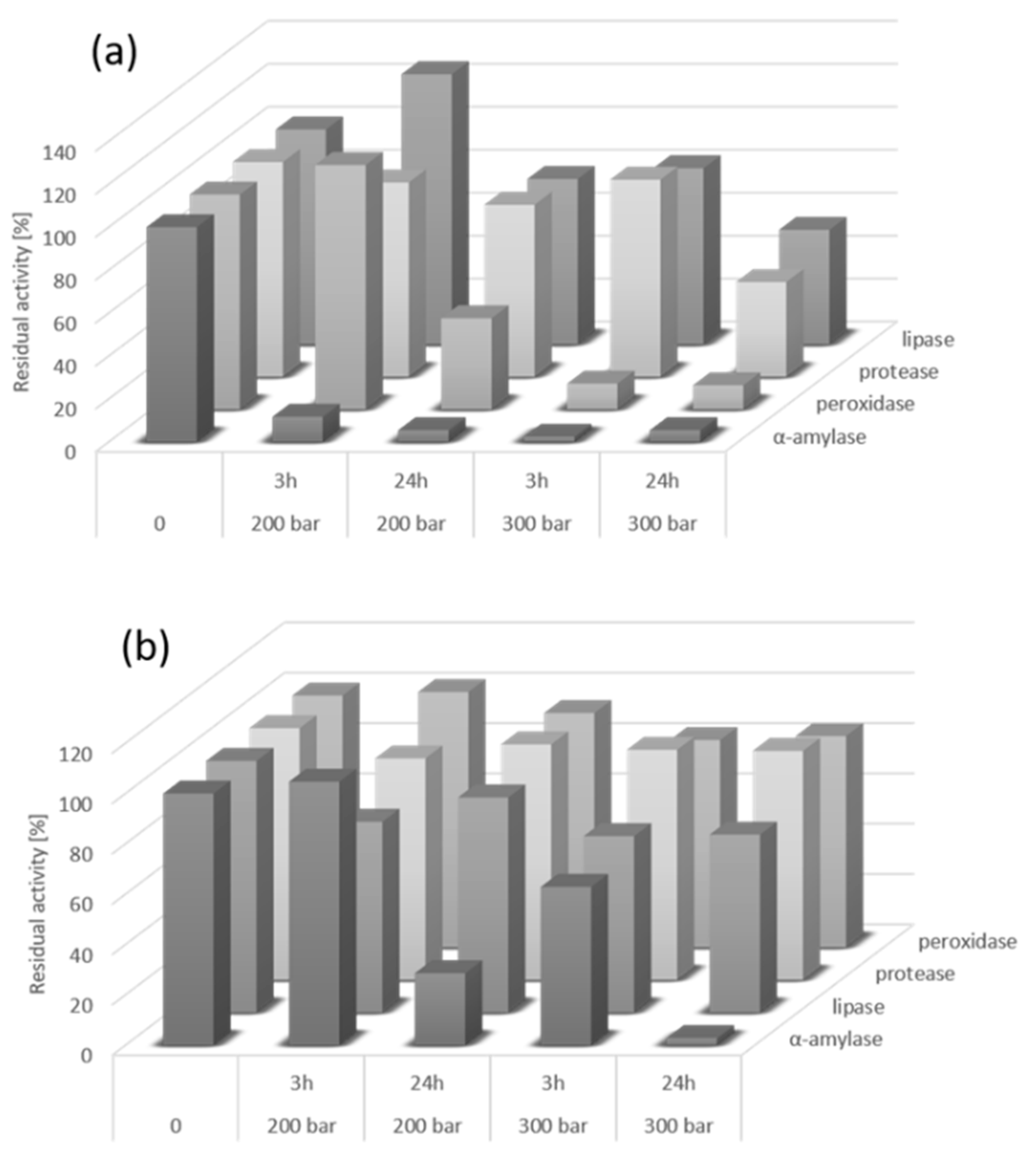
3.3. Effect of the scCO2 Medium on Enzyme Activity
3.4. Flour Particle Size Distribution
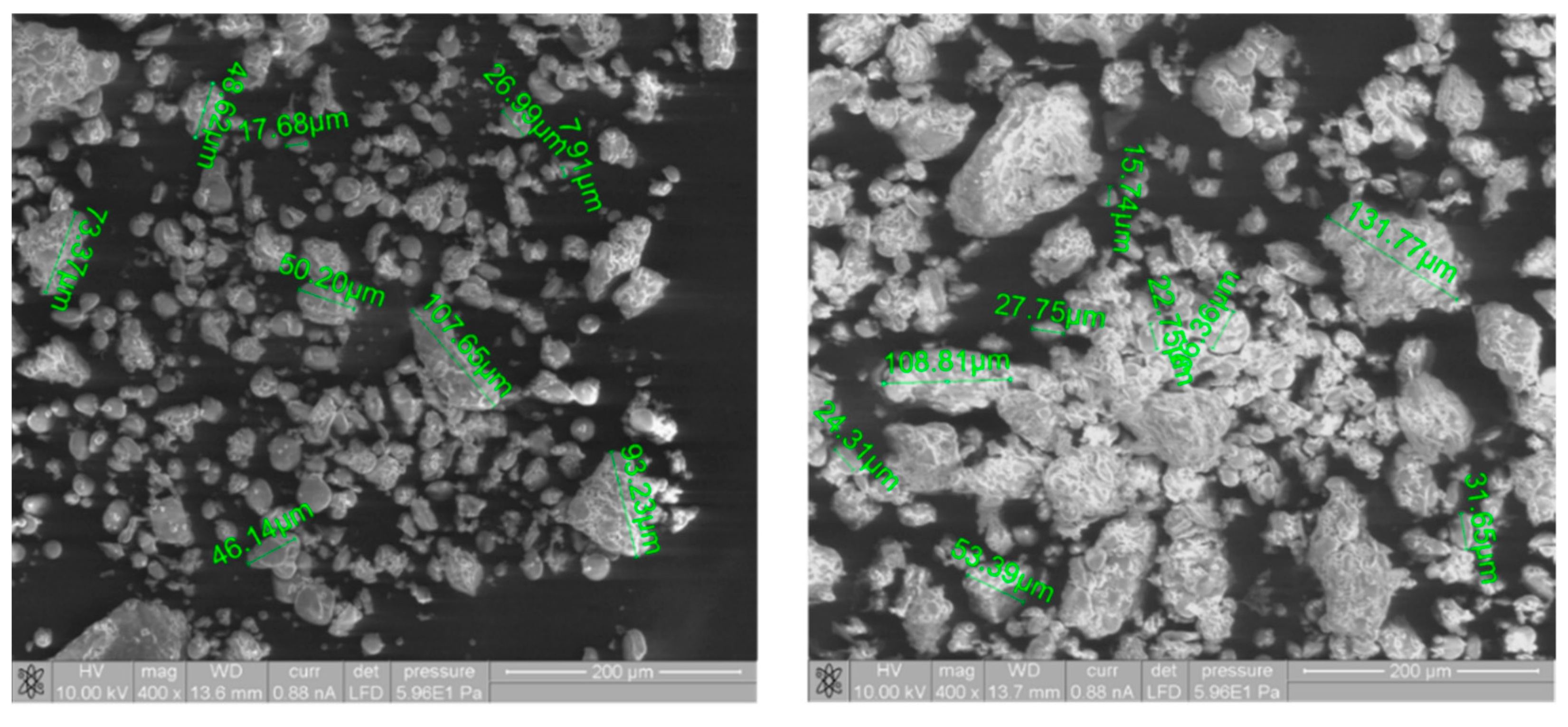
3.5. Environmental Scanning Electron Microscopy Studies
3.6. CO2 Solubility in Flour
3.7. Fourier Transform Infrared Spectroscopy
3.8. Changes in Wheat Lipid Content during the scCO2 Treatment
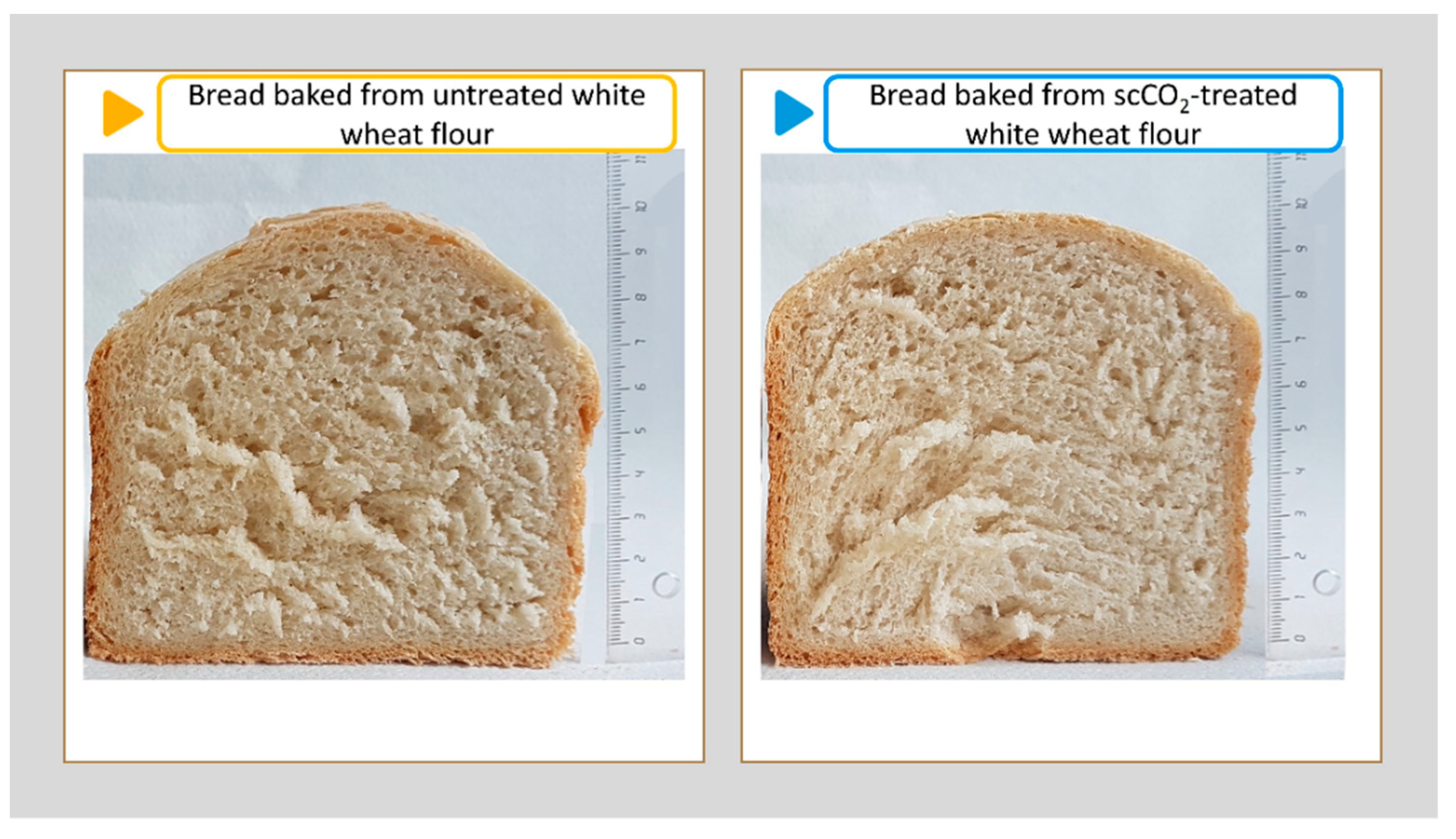
3.9. Sensory Evaluation of Baked Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, R.V.C.; Fernandes, Â.; Heleno, S.A.; Rodrigues, P.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Physicochemical Characterization and Microbiology of Wheat and Rye Flours. Food Chem. 2019, 280, 123–129. [Google Scholar] [CrossRef]
- Rani, K.U.; Prasada Rao, U.J.S.; Leelavathi, K.; Haridas Rao, P. Distribution of Enzymes in Wheat Flour Mill Streams. J. Cereal Sci. 2001, 34, 233–242. [Google Scholar] [CrossRef]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Le-Bail, A. Effect of Long-Term Storage Conditions on Wheat Flour and Bread Baking Properties. Food Chem. 2021, 346, 128902. [Google Scholar] [CrossRef]
- Bin, Q.; Peterson, D.G. Identification of Bitter Compounds in Whole Wheat Bread Crumb. Food Chem. 2016, 203, 8–15. [Google Scholar] [CrossRef]
- Hellwig, M. The Chemistry of Protein Oxidation in Food. Angew. Chem. Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef]
- Hatcher, D.W.; Barker, W. A Rapid Microassay for Determination of Peroxidase in Wheat and Flour. Cereal Chem. 2005, 82, 233–237. [Google Scholar] [CrossRef]
- Almeida, J.L.D.; Pareyt, B.; Gerits, L.R.; Delcour, J.A. Effect of Wheat Grain Steaming and Washing on Lipase Activity in Whole Grain Flour. Cereal Chem. 2014, 91, 321–326. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.; Chen, H.; Sun, D.; Deng, B.; Li, J.; Liu, Y.; Wang, F. Inactivation of Lipase and Lipoxygenase of Wheat Germ with Temperature-Controlled Short Wave Infrared Radiation and Its Effect on Storage Stability and Quality of Wheat Germ Oil. PLoS ONE 2016, 11, e0167330. [Google Scholar] [CrossRef]
- Poudel, R.; Rose, D.J. Changes in Enzymatic Activities and Functionality of Whole Wheat Flour Due to Steaming of Wheat Kernels. Food Chem. 2018, 263, 315–320. [Google Scholar] [CrossRef]
- McCleary, B.V.; McNally, M.; Monaghan, D.; Mugford, D.C. Collaborators: Measurement of α-Amylase Activity in White Wheat Flour, Milled Malt, and Microbial Enzyme Preparations, Using the Ceralpha Assay: Collaborative Study. J. AOAC Int. 2002, 85, 1096–1102. [Google Scholar] [CrossRef]
- Rana, N.; Verma, N.; Vaidya, D.; Ghabru, A. Application of Amylase Producing Bacteria Isolated from Hot Spring Water in Food Industry. Ann. Phytomed. Int. J. 2017, 6, 93–100. [Google Scholar] [CrossRef]
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.-X.; Sun, J.-P.; Xiao, H.; Shi, M.-W.; Long, J.-J. Investigation of the Influence of Supercritical Carbon Dioxide Treatment on Meta-Aramid Fiber: Thermal Decomposition Behavior and Kinetics. J. CO2 Util. 2020, 37, 85–96. [Google Scholar] [CrossRef]
- Calvignac, B.; Rodier, E.; Letourneau, J.-J.; dos Santos, P.M.A.; Fages, J. Cocoa Butter Saturated with Supercritical Carbon Dioxide: Measurements and Modelling of Solubility, Volumetric Expansion, Density and Viscosity. Int. J. Chem. React. Eng. 2010, 8, 1–29. [Google Scholar] [CrossRef][Green Version]
- Gui, F.; Wu, J.; Chen, F.; Liao, X.; Hu, X.; Zhang, Z.; Wang, Z. Inactivation of Polyphenol Oxidases in Cloudy Apple Juice Exposed to Supercritical Carbon Dioxide. Food Chem. 2007, 100, 1678–1685. [Google Scholar] [CrossRef]
- Ortuño, C.; Benedito, J. Microbial and Enzyme Inactivation by Ultrasound-Assisted Supercritical Fluids. In Ultrasound in Food Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 392–416. ISBN 978-1-118-96415-6. [Google Scholar]
- Harper, N.; Barreiros, S. Enhancement of Enzyme Activity in Supercritical Carbon Dioxide via Changes in Acid-Base Conditions. Biotechnol. Prog. 2002, 18, 1451–1454. [Google Scholar] [CrossRef]
- Baltacıoğlu, H.; Bayındırlı, A.; Severcan, M.; Severcan, F. Effect of Thermal Treatment on Secondary Structure and Conformational Change of Mushroom Polyphenol Oxidase (PPO) as Food Quality Related Enzyme: A FTIR Study. Food Chem. 2015, 187, 263–269. [Google Scholar] [CrossRef]
- Fan, J.; Mitchell, J.R.; Blanshard, J.M.V. A Model for the Oven Rise of Dough during Baking. J. Food Eng. 1999, 41, 69–77. [Google Scholar] [CrossRef]
- Lakzian, K.; Jalaei Salmani, H. An Extensive Thermodynamic Study on Amino Acids Aqueous Solutions and Their CO2 Solubility by Taking into Account Dipolar and Quadrupolar Contributions. J. Mol. Liq. 2021, 324, 114681. [Google Scholar] [CrossRef]
- Thirumdas, R.; Annapure, U.S. Chapter 7—Enzyme Inactivation in Model Systems and Food Matrixes by Cold Plasma. In Advances in Cold Plasma Applications for Food Safety and Preservation; Bermudez-Aguirre, D., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 229–252. ISBN 978-0-12-814921-8. [Google Scholar]
- Batista, V.F.; Galman, J.L.; GA Pinto, D.C.; Silva, A.M.S.; Turner, N.J. Monoamine Oxidase: Tunable Activity for Amine Resolution and Functionalization. ACS Catal. 2018, 8, 11889–11907. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hareland, G.A. Evaluation of Flour Particle Size Distribution by Laser Diffraction, Sieve Analysis and Near-Infrared Reflectance Spectroscopy. J. Cereal Sci. 1994, 20, 183–190. [Google Scholar] [CrossRef]
- Akoh, C.C. Food Lipids: Chemistry, Nutrition, and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-4487-4. [Google Scholar]
- Hubbard, J.D.; Downing, J.M.; Ram, M.S.; Chung, O.K. Lipid Extraction from Wheat Flour Using Supercritical Fluid Extraction. Cereal Chem. 2004, 81, 693–698. [Google Scholar] [CrossRef]
- Primožič, M.; Čolnik, M.; Knez, Ž.; Leitgeb, M. Advantages and Disadvantages of Using SC CO2 for Enzyme Release from Halophilic Fungi. J. Supercrit. Fluids 2019, 143, 286–293. [Google Scholar] [CrossRef]
- Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Development of Chitosan Functionalized Magnetic Nanoparticles with Bioactive Compounds. Nanomaterials 2020, 10, 1913. [Google Scholar] [CrossRef]
- Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. The Influence of Supercritical Carbon Dioxide on Graham Flour Enzyme Polyphenol Oxidase Activity. Molecules 2020, 25, 5981. [Google Scholar] [CrossRef]
- Renzyaeva, T. On the Role of Fats in Baked Flour Goods. Food Raw Mater. 2013, 1, 19–25. [Google Scholar] [CrossRef]
- Adebowale, Y.; Adeyemi, I.A.; Oshodi, A. Functional and Physicochemical Properties of Flours of Six Mucuna Species. Afr. J. Biotechnol. 2005, 4. [Google Scholar] [CrossRef]
- Silva, E.K.; Meireles, M.A.A.; Saldaña, M.D.A. Supercritical Carbon Dioxide Technology: A Promising Technique for the Non-Thermal Processing of Freshly Fruit and Vegetable Juices. Trends Food Sci. Technol. 2020, 97, 381–390. [Google Scholar] [CrossRef]
- Silva, E.K.; Arruda, H.S.; Eberlin, M.N.; Pastore, G.M.; Meireles, M.A.A. Effects of Supercritical Carbon Dioxide and Thermal Treatment on the Inulin Chemical Stability and Functional Properties of Prebiotic-Enriched Apple Juice. Food Res. Int. 2019, 125, 108561. [Google Scholar] [CrossRef]
- Yoshimura, T.; Shimoda, M.; Ishikawa, H.; Miyake, M.; Hayakawa, I.; Matsumoto, K.; Osajima, Y. Inactivation Kinetics of Enzymes by Using Continuous Treatment with Microbubbles of Supercritical Carbon Dioxide. J. Food Sci. 2001, 66, 694–697. [Google Scholar] [CrossRef]
- Bechtold, M.; Panke, S. 7.5 Reaction Engineering of Biotransformations. In Comprehensive Chirality; Carreira, E.M., Yamamoto, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 71–100. ISBN 978-0-08-095168-3. [Google Scholar]
- Bauer, C.; Steinberger, D.-J.; Schlauer, G.; Gamse, T.; Marr, R. Activation and Denaturation of Hydrolases in Dry and Humid Supercritical Carbon Dioxide (SC-CO2). J. Supercrit. Fluids 2000, 19, 79–86. [Google Scholar] [CrossRef]
- Lanza, M.; Priamo, W.L.; Oliveira, J.V.; Dariva, C.; Oliveira, D.D. The Effect of Temperature, Pressure, Exposure Time, and Depressurization Rate on Lipase Activity in SCCO2. Appl. Biochem. Biotechnol. 2004, 113, 181–187. [Google Scholar] [CrossRef]
- Primožič, M.; Kravanja, G.; Knez, Ž.; Crnjac, A.; Leitgeb, M. Immobilized Laccase in the Form of (Magnetic) Cross-Linked Enzyme Aggregates for Sustainable Diclofenac (Bio)Degradation. J. Clean. Prod. 2020, 275, 124121. [Google Scholar] [CrossRef]
- Bertoloni, G.; Bertucco, A.; Cian, V.D.; Parton, T. A Study on the Inactivation of Micro-Organisms and Enzymes by High Pressure CO2. Biotechnol. Bioeng. 2006, 95, 155–160. [Google Scholar] [CrossRef]
- Tedjo, W.; Eshtiaghi, M.N.; Knorr, D. Impact of Supercritical Carbon Dioxide and High Pressure on Lipoxygenase and Peroxidase Activity. J. Food Sci. 2000, 65, 1284–1287. [Google Scholar] [CrossRef]
- Bednarski, D.M.; Lantz, E.E.; Bobst, C.E.; Eisenhut, A.R.; Eyles, S.J.; Fey, J.P. Sterilization of Epidermal Growth Factor with Supercritical Carbon Dioxide and Peracetic Acid; Analysis of Changes at the Amino Acid and Protein Level. Biochim. Biophys. Acta BBA Proteins Proteom. 2020, 1868, 140334. [Google Scholar] [CrossRef]
- Weder, J.K.P. Studies on Proteins and Amino Acids Exposed to Supercritical Carbon Dioxide Extraction Conditions. Food Chem. 1984, 15, 175–190. [Google Scholar] [CrossRef]
- Takahashi, T.; Hiramoto, S.; Wato, S.; Nishimoto, T.; Wada, Y.; Nagai, K.; Yamaguchi, H. Identification of Essential Amino Acid Residues of an α-Amylase Inhibitor from Phaseolus Vulgaris White Kidney Beans1. J. Biochem. 1999, 126, 838–844. [Google Scholar] [CrossRef]
- Paul, J.S.; Gupta, N.; Beliya, E.; Tiwari, S.; Jadhav, S.K. Aspects and Recent Trends in Microbial α-Amylase: A Review. Appl. Biochem. Biotechnol. 2021, 193, 2649–2698. [Google Scholar] [CrossRef]
- Collins, D.P.; Dawson, J.H. 3.05—Recent History of Heme-Containing Proteins: Advances in Structure, Functions, and Reaction Intermediate Determination. In Comprehensive Inorganic Chemistry, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 65–102. ISBN 978-0-08-096529-1. [Google Scholar]
- Chapus, C.; Rovery, M.; Sarda, L.; Verger, R. Minireview on Pancreatic Lipase and Colipase. Biochimie 1988, 70, 1223–1233. [Google Scholar] [CrossRef]
- Lowe, M.E. The Catalytic Site Residues and Interfacial Binding of Human Pancreatic Lipase. J. Biol. Chem. 1992, 267, 17069–17073. [Google Scholar] [CrossRef]
- Okuda, M.; Ozawa, T.; Kawahara, A.; Takimura, Y. The Hydrophobicity of an Amino Acid Residue in a Flexible Loop of KP-43 Protease Alters Activity toward a Macromolecule Substrate. Appl. Microbiol. Biotechnol. 2020, 104, 8339–8349. [Google Scholar] [CrossRef]
- Impe, J.V.; Smet, C.; Tiwari, B.; Greiner, R.; Ojha, S.; Stulić, V.; Vukušić, T.; Jambrak, A.R. State of the Art of Nonthermal and Thermal Processing for Inactivation of Micro-Organisms. J. Appl. Microbiol. 2018, 125, 16–35. [Google Scholar] [CrossRef]
- Kravanja, G.; Hrnčič, M.K.; Škerget, M.; Knez, Ž. Interfacial Tension and Gas Solubility of Molten Polymer Polyethylene Glycol in Contact with Supercritical Carbon Dioxide and Argon. J. Supercrit. Fluids 2016, 108, 45–55. [Google Scholar] [CrossRef]
- Amir, R.M.; Anjum, F.M.; Khan, M.I.; Khan, M.R.; Pasha, I.; Nadeem, M. Application of Fourier Transform Infrared (FTIR) Spectroscopy for the Identification of Wheat Varieties. J. Food Sci. Technol. 2013, 50, 1018–1023. [Google Scholar] [CrossRef]
- Contreras-Jiménez, B.; Torres-Vargas, O.L.; Rodríguez-García, M.E. Physicochemical Characterization of Quinoa (Chenopodium Quinoa) Flour and Isolated Starch. Food Chem. 2019, 298, 124982. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Hernández-Sánchez, H.; Yee-Madeira, H.; Martín-Martínez, S.; Robles-Ramírez, M.D.C.; Rojas-López, M.; Berríos, J.D.J.; Mora-Escobedo, R. Physico-Chemical, Nutritional and Infrared Spectroscopy Evaluation of an Optimized Soybean/Corn Flour Extrudate. J. Food Sci. Technol. 2015, 52, 4066–4077. [Google Scholar] [CrossRef]
- Shin, W.-K.; Kim, W.; Kim, Y. Physicochemical and Sensory Characteristics of a Low-Fat Tofu Produced Using Supercritical CO2 Extracted Soy Flour. Food Sci. Biotechnol. 2013, 23, 43–48. [Google Scholar] [CrossRef]



| Flour Type | Moisture Content (%) | Fat Concentration (g/100 g Flour) | Protein Concentration (g/100 g Flour) |
|---|---|---|---|
| WHEAT FLOUR TYPE 500 | 13.1 ± 0.3 | 1.3 ± 0.5 | 12.0 ± 0.4 |
| WHEAT FLOUR TYPE 850 | 13.3 ± 0.4 | 1.5 ± 0.6 | 11.0 ± 0.6 |
| RYE FLOUR TYPE 1250 | 10.7 ± 0.3 | 1.3 ± 0.3 | 9.0 ± 0.3 |
| WHOLEGRAIN RYE FLOUR | 11.5 ± 0.2 | 1.7 ± 0.7 | 13.0 ± 0.5 |
| GRAHAM FLOUR | 11.0 ± 0.4 | 1.9 ± 0.5 | 13.4 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitgeb, M.; Knez, Ž.; Hojnik Podrepšek, G. Enzyme Activity and Physiochemical Properties of Flour after Supercritical Carbon Dioxide Processing. Foods 2022, 11, 1826. https://doi.org/10.3390/foods11131826
Leitgeb M, Knez Ž, Hojnik Podrepšek G. Enzyme Activity and Physiochemical Properties of Flour after Supercritical Carbon Dioxide Processing. Foods. 2022; 11(13):1826. https://doi.org/10.3390/foods11131826
Chicago/Turabian StyleLeitgeb, Maja, Željko Knez, and Gordana Hojnik Podrepšek. 2022. "Enzyme Activity and Physiochemical Properties of Flour after Supercritical Carbon Dioxide Processing" Foods 11, no. 13: 1826. https://doi.org/10.3390/foods11131826
APA StyleLeitgeb, M., Knez, Ž., & Hojnik Podrepšek, G. (2022). Enzyme Activity and Physiochemical Properties of Flour after Supercritical Carbon Dioxide Processing. Foods, 11(13), 1826. https://doi.org/10.3390/foods11131826








