Abstract
Protein stability in bottled white wine is an essential organoleptic property considered by consumers. In this paper, the effectiveness of an early enzymatic treatment was investigated by adding a food-grade microbial protease at two different stages of winemaking: (i) at cold settling, for a short-term and low temperature (10 °C) action prior to alcoholic fermentation (AF); (ii) at yeast inoculum, for a long-lasting and medium temperature (18 °C) action during AF. The results reveal that protease sufficiently preserved its catalytic activity at both operational conditions: 10 °C (during cold settling) and 18 °C (during AF). Furthermore, protease addition (dosage 50–150 μL/L) raised the alcoholic fermentation rate. The treatment at yeast inoculum (dosage 50 μL/L) had a remarkable effect in preventing haze formation, as revealed by its impact on protein instability and haze-active proteins. This minimally invasive, time and resource-saving enzymatic treatment, integrated into the winemaking process, could produce stable white wine without affecting color quality and phenol content.
1. Introduction
The formation of protein haze in bottled white wine is affected by several known factors, including the presence of phenolic compounds, polysaccharides, sulfate and metal ions, ionic strength as well as by the pH and the wine storage temperature. However, other important factors remain unidentified [1]. Although the exact mechanism of protein haze formation is not very clear, it may be assumed that it is mainly associated with hydrophobic protein–phenolic interactions [2]. Furthermore, it has been observed that pathogenesis-related (PR) proteins (e.g., thaumatin-like proteins TLP and chitinase CH) derived from grapes are mainly responsible for haze formation in bottled white wine [3]. Van Sluyter et al. [4] have proposed a revised mechanism of protein haze formation involving three different stages. Such mechanism is based on the discovery that TLP and CH have different unfolding temperatures and unfolding/aggregation behavior, as well as the interaction of non-proteaceous components (salts, sulphate and phenolics) in the late phase.
The most used method to treat white wine instability is the batch addition of bentonite at the end of wine ageing before bottling. This required treatment, in addition to being an additional time- and resource-consuming step, is associated with negative environmental impact (disposal of spent materials), product loss (3–10%) and quality degradation of wine at the end of its maturation [1,3], estimated to be about USD 1 billion per year [5]. To overcome these criticisms, several approaches have been studied to solve the question of protein instability in white wine, all of them suggesting their application on finished wine. Among the innovative methods based on a physical approach, it is possible to include the use of ultrasound [6]. In recent years, countless works have been included in the research of stabilization methods, always as an additional process step based on the use of porous adsorbent materials [5]: acrylic acid plasma-coated magnetic nanoparticles [7], zeolites [8], grape seeds powder [9] and zirconium oxide. Research is needed to determine if the components of these adsorbent materials (e.g., magnetic nanoparticles and acrylic acid) are released into the wine or if the compounds in the wash may permeate the material and leach into the wine. Furthermore, improved regeneration methods should be associated with the use of porous adsorbents in order to lower waste and water needed to clean the materials [5]. Additive stabilizing approaches to prevent haze formation have been suggested and consist in using agents of animal/vegetable origins, such as mannoproteins (50% potential instability reduction [10]), carrageenan/pectin (75–90% protein reduction [11]) and chitosan (14% protein reduction [12]). Most of these techniques diminish the protein content and do not affect the sensory characteristics of the wine, but some issues must be considered (e.g., alteration of the metal ion content and generation of precipitates/residues that could affect other stages of wine production, such as filtration) [5]. Unstable protein fractions in white wine have been the target of studies applying proteolytic enzymes instead of traditional bentonite fining to avoid protein haziness [13]. Proteases must be able to work under restricted wine conditions of pH, alcohol content and temperature. The use of a fungal endoprotease (aspergilloglutamic protease) following flash pasteurization was tested in must and white wine by Marangon et al. [14]. Plant-based proteases have also been extensively studied [1]: papain from papaya [15,16] and bromelain from pineapple [17] have been tested concerning their ability in the degradation of heat-unstable white wine proteins. Bromelain exhibited effectiveness in the degradation of wine proteins (approximately 70%) in model wine as well as in real white wine, also when immobilized on chitosan beads and applied in a laboratory-scale stirred reactor [18,19].
The novelty and significant contribution of this study consists of providing an enzymatic treatment to be applied in must of white grape, since it naturally already contains the targeted proteins extracted from grape (TLP and CH) and its content of endogenous/exogenous inhibitors is still limited. A microbial protease was therefore applied in must of white grape at two different stages at the beginning of winemaking: (i) at cold settling, for a short-term and low temperature (10 °C) action prior to alcoholic fermentation (AF); (ii) at yeast inoculum, for a long-lasting and medium temperature (18 °C) action during AF. Such early protein stabilization, performed on grape must at the initial stage of winemaking and integrated into the production process (not as an additional step), is deemed to be minimally invasive given that wine is still evolving.
2. Materials and Methods
2.1. Enzymes, Chemicals and Must
A commercial food-grade protease from Aspergillus niger was kindly provided by IMCD Italia SpA (Milan, Italy); the chromogenic substrate (Z-Gly-Pro-pNA) was purchased from Bachem (Bubendorf, Switzerland) and the active dry yeast (Saccharomyces cerevisiae, Zymaflore®) used for the experimental fermentation was purchased by Laffort (Tortona, Italy). All other reagents were from Merck Group (Milan, Italy). The must from Vitis vinifera L. cv Riesling italic grape was kindly supplied by Castello delle Regine (Terni, Italy) and its main oenological parameters were determined according to European official methods of analysis [20].
2.2. Enzymatic Activity Assay and Kinetic Study
Protease activity was spectrophotometrically assessed (λ: 410 nm, Shimadzu UV 2450, Milan, Italy) at two different temperatures representing the operational conditions (10 °C (cold settling prior to AF) and 18 °C (AF)). The assay was performed in model wine must (tartaric buffer 0.3 M, pH 3.2) to which was added the synthetic chromogenic substrate Z-Gly-Pro-pNA, prepared as described by Lopez et al. [21]. For the kinetic characterization, the substrate concentration was varied from 0.03 to 1.0 mM and the kinetic parameters Vmax (maximum initial velocity) and KM (Michaelis–Menten constant, corresponding to the substrate concentration when the initial velocity is one-half of the Vmax) were estimated according to the Michaelis–Menten equation by a non-linear regression (GraphPad Prism 5.01, GraphPad Software, Inc., La Jolla, CA, USA). kcat (turnover number, indicating the number of moles of substrate converted into the product per number of moles of catalyst per minute) and Ka (affinity constant, kcat/KM, suggesting the affinity of the enzyme toward the substrate) were calculated as reported by Benucci et al. [18].
2.3. Enzymatic Treatment and Laboratory-Scale Fermentation Tests
Protease was applied in the range of 2–150 μL/L at two different winemaking phases: (i) at cold settling, for a short-term and low temperature (10 °C) action prior to alcoholic fermentation (AF) (treatment I); (ii) at yeast inoculum, for a long-lasting and medium temperature (18 °C, temperature usually used in white wine AF) action during AF (treatment II). The effectiveness of proteolytic dosage was evaluated at racking after 24 h settling (treatment I) and at middle/end of AF (treatment II). A detailed description of the treatments applied is summarized in Table 1. Treatment I was conducted dividing the must into 200 mL aliquots inside graduated cylinders, adding the different doses of proteolytic enzyme (2, 5, 10, 30, 50, 70, 100 and 150 μL/L). Then, the samples were subjected to cold settling prior to AF (10 °C, overnight) after also adding the pectinolytic preparation (3 mL/hL). Samples were compared with the grape juice settled with only pectinolytic enzyme (Ctrl). Treatment II was conducted on clear must (after cold settling with pectinolytic enzyme) adding protease (2, 5, 10, 30, 50, 70, 100 and 150 μL/L) at yeast inoculum and compared with the clear must fermented without any enzyme addition (Ctrl). The laboratory-scale fermentation tests were carried out as reported by Benucci et al. [22] using commercial active dry yeast (Saccharomyces cerevisiae Zymaflore® 20 g/hL). Treatments were performed in triplicate. AF was performed at 18 °C for about 16 days and weight loss was used as parameter for monitoring the evolution of fermentation process. The dynamics of weight loss were fitted by means of a sigmoid or altered Gompertz decay function as described by Benucci et al. [23]. The fermentation rate (K) and half-time (M) were estimated by a non-linear regression procedure (GraphPad Prism 5.0, GraphPad software, Inc., La Jolla, CA, USA).

Table 1.
Detailed frame of the applied treatments.
2.4. Heat Stability and Haze-Active (HA) Protein Determination
The haze potential (ΔNTU) of white wine must was calculated as the difference between sample turbidity (HD 25.2 turbidimeter, Delta OHM, Padua, Italy) after heat test (80 °C for 6 h and 4 °C for 16 h [24]) and its initial turbidity [18]. HA proteins were detected as described by Siebert et al. [25] and Benucci et al. [26] using tannic acid (1 mg/mL) at 25 °C after 30 min. The haze difference between the sample with and without added tannic acid was reported as the HA proteins.
2.5. Total Protein Content and Electrophoretic Separation (SDS-PAGE)
Total protein content of the samples was determined using the potassium dodecyl sulphate method [24] and protein quantification was carried out using the bicinchoninic acid (BCA) protein assay kit (Sigma-Aldrich, Saint Louis, MO, USA) [18]. Electrophoretic separation (SDS-PAGE) was performed using a vertical electrophoresis apparatus (Mini-Protean Tetra cell, Bio-Rad, Richmond, CA, USA) and standard molecular weight (Precision Plus Protein Standards, Kaleidoscope, Bio-Rad, Richmond, CA, USA) [18].
2.6. Effect of Enzymatic Treatment on Chromatic Characteristics and Phenolics
The concentration of total phenols was determined as reported by Becchetti et al. [27]. In brief, 10 mL of Na2CO3 (20% w/v) and 5 mL of Folin–Ciocalteau reagent were added to 1 mL of wine must and lead to 100 mL with distilled water. After 30 min, the absorbance at 700 nm was registered and results were expressed as gallic acid equivalent (mg/L). CIELAB parameters (hue (h), chroma (C*) and total color differences (ΔE*)) were also assessed using a CR-5 colorimeter (Konica Minolta, Tokyo, Japan) by a D65 illumining and CIELAB uniform color space [28]. ΔE* was calculated referring to Ctrl sample.
2.7. Statistical Analysis
Data provided are the mean of triplicate analytical determinations. One-way ANOVA (p < 0.01) and Tukey’s HSD test (p < 0.05) were applied to determine any statistical differences among samples (EXCEL® Add-in macro DSAASTAT program) [29]. The 95% confidence intervals presented in Tables were estimated by using GraphPad Prism 5.0.
3. Results and Discussion
3.1. Enzyme Kinetic Study
Before testing the effectiveness of protease in preventing wine haze, its kinetic behavior was studied in model wine must with the addition of a synthetic chromogenic substrate at two different temperatures representing the operational conditions: 10 °C (cold settling prior to AF) and 18 °C (during AF). As expected, the protease showed the hyperbolic behavior of the Michaelis–Menten equation (Figure 1) and the corresponding kinetic parameters are reported in Table 2.
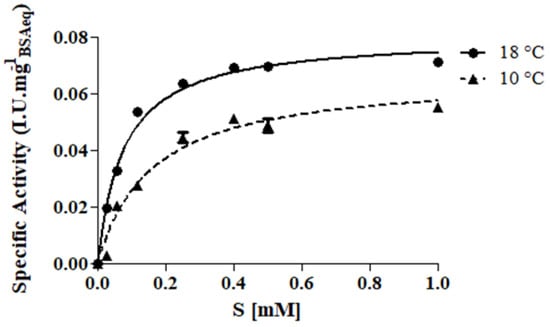
Figure 1.
Kinetic curves of protease tested at two different temperatures: 10 °C (▲, cold settling prior to AF) and 18 °C (●, AF). Assay was performed in model wine-must (tartaric buffer 0.3 M, pH 3.2) added with the synthetic chromogenic substrate Z-Gly-Pro-pNA (0–1 mM).

Table 2.
Kinetic parameters of protease tested at two different temperatures: 10 °C (cold settling prior to AF) and 18 °C (AF). Assay was performed in model wine must (tartaric buffer 0.3 M, pH 3.2) added with the synthetic chromogenic substrate Z-Gly-Pro-pNA (0–1 mM).
Although the similar values of Vmax, the other parameters (KM, kcat and Ka) proved a better catalytic efficiency at 18 °C with respect to 10 °C. However, also at low temperature (10 °C), protease sufficiently preserved its catalytic activity (Table 2). These results are in line with Kang et al. [30] who found a 10% relative activity loss for prolyl endopeptidase purified from A. oryzae in the temperature range 15–30 °C using the same substrate (Z-Gly-Pro-pNA) in phosphate buffer (pH 5.0).
3.2. Alcoholic Fermentation Kinetic
The kinetic of sugar consumption in wine must (24° Brix corresponding to 20.7° Babo; pH = 3.47 ± 0.01; total acidity = 5.24 ± 0.01 gtartaric acid/L; potential alcohol content = 14.0% v/v) suggests remarkable differences among samples (Figure 2). The lowest fermentation rate (K) was observed for Ctrl, whereas in all the treated samples it increased as the enzyme amount was raised, especially for enzyme dosages above 50 μL/L.
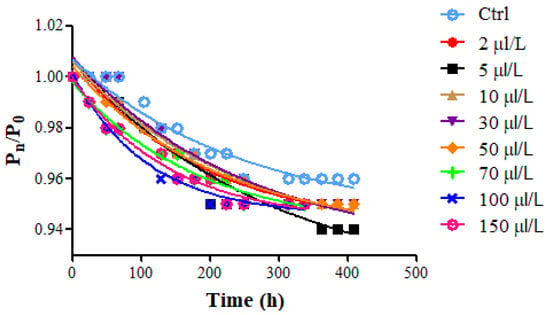
Figure 2.
Time course of weight loss (ratio between the current and initial weight (Pn/P0) against time (t)) representing sugar consumption during the alcoholic fermentation (18 °C) of Riesling Italic must (treatment II, at yeast inoculum) at different protease dosages (2–150 μL/L).
Considering K and M values (Table 3), it is possible to notice an increase in the K values and a corresponding decrease in M values at the highest protease dosage (from 50 to 150 μL/L). This trend could be explained considering that the proteolytic enzyme treatment may have affected yeast vitality and consequently the velocity of sugar consumption during alcoholic fermentation. This phenomenon may be ascribed to the enzymatic hydrolysis of the proteins in wine must and the consequent increase in the peptides/amino acids available as an assimilable nitrogen source for yeast metabolism. Lei et al. [31] found that protease (Flavorzyme) supplementation to high gravity beer was beneficial for the success of alcoholic fermentation, both in terms of wort fermentability and higher ethanol yield. Mathias et al. [32] demonstrated that the protease addition promoted the increase in total nitrogen and amino acid content in the sweet wort and a higher fermentation efficiency in brewing.

Table 3.
Parameters obtained by fitting the altered Gompertz equation to the experimental data of weight loss. K: alcoholic fermentation rate; M: half-time of weight loss.
3.3. Effect of Enzymatic Treatment on Protein Instability
In order to prevent haze formation in white wine, a microbial protease was applied at two different early winemaking phases: (i) at cold settling and (ii) at yeast inoculum.
Concerning treatment I, a significant reduction in protein instability was found adding protease (Figure 3a) at dosages above 10 μL/L. The lowest ΔNTU (5.1) was observed at 50 μL/L, whereas no further stabilizing effect was revealed at highest dosages. The cold settling in presence of the pectinolytic enzyme alone (Ctrl) lowered the protein instability (ΔNTU = 15) as compared to the turbid pressed grape juice (ΔNTU = 21.3, data not shown). Similar results were observed for the treatment II (evaluated at the middle/end of AF), with the lowest ΔNTU values (5.9 and 4.1, respectively) at 50 μL/L, corresponding to a 75–83% instability removal (Figure 3b). No further ΔNTU reduction was found at increasing proteolytic enzyme dosage (Figure 3b). Several studies have investigated the effectiveness of different proteolytic enzymes in free or immobilized form as an alternative treatment to bentonite fining for white wine protein stabilization. Benucci et al. [17] tested the feasibility of stem bromelain, free or immobilized on chitosan support, to reduce white wine protein haze potential (approximately 70%). Marangon et al. [14] demonstrated that the simple addition of AGP (a mixture of Aspergillopepsins I and II) during fermentation yielded a protein hydrolysis of about 20%.
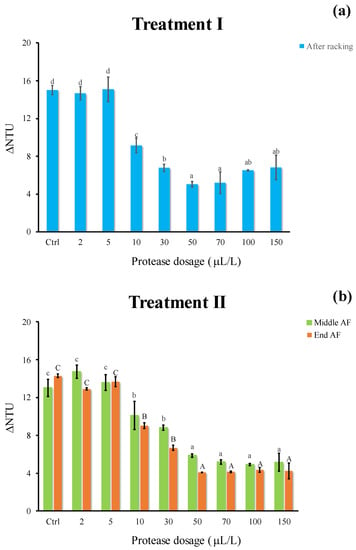
Figure 3.
Haze potential of white must wine (ΔNTU) subjected to enzymatic addition (protease in the range of 2–150 μL/L) after (a) treatment I (cold settling prior to AF) and (b) treatment II (at yeast inoculum). Ctrl is clarified must with the only pectinolytic enzyme. For each sample, means with different roman letters are significantly different (p ≤ 0.05).
Similar to that observed for protein instability, the decrease in HA proteins appeared remarkable adding protease (Figure 4). Irrespective of the oenological phase, the proteolytic enzyme had a significant effect in breaking down HA proteins (Figure 4a,b) and the most effective dosage resulted as 50 μL/L (0.6 NTU). The efficacy of protease and pectinase in beverages has been highlighted by the literature. Both enzymes significantly decreased the amount of HA proteins (−75%) in pomegranate juice treated in fluidized bed reactor [26].
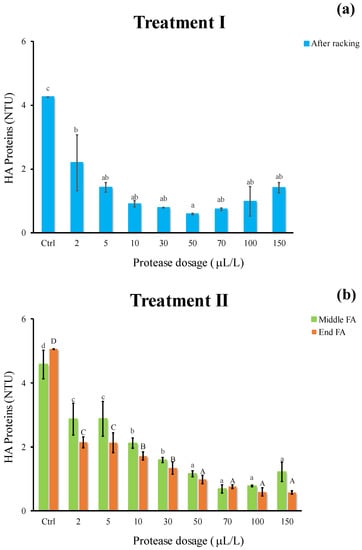
Figure 4.
Haze active (HA) proteins of white must wine (NTU) subjected to enzymatic addition (protease in the range of 2–150 μL/L) after (a) treatment I (cold settling prior to AF) and (b) treatment II (at yeast inoculum). Ctrl is clarified must with the only pectinolytic enzyme. For each sample, means with different roman letters are significantly different (p ≤ 0.05).
In both enzymatic processes, a significant difference in total protein appeared between the Ctrl and treated samples (−20% and −32% for treatment I and II, respectively), whereas no remarkable discrepancy among dosages were revealed (Figure 5a,b). Total protein amount decreased to a greater extent when protease was added at yeast inoculum and left in wine must during all the AF duration (treatment II). Therefore, it is possible to hypothesize that this protein depletion may be attributed to the combined action exerted by the proteolytic enzyme throughout a long-lasting contact time at higher temperature, as well as by fermenting yeast metabolism and co-precipitation phenomena. It may be presumed that, at cold settling (treatment I), the most unfavorable conditions both in terms of temperature (10 °C compared to 18 °C of AF) and time (24 h as compared to 16 days) were detrimental for enzyme activity.
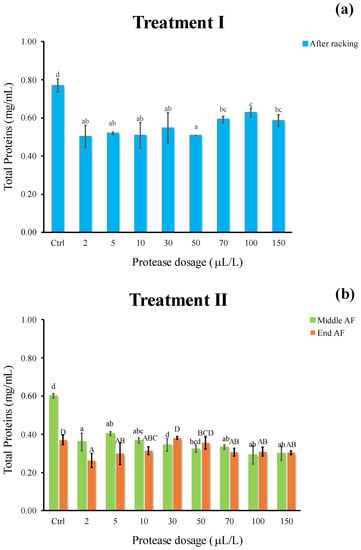
Figure 5.
Total proteins (mg/mL) of white must wine subjected to enzymatic addition (protease in the range of 2–150 μL/L) after (a) Treatment I (cold settling prior to AF) and (b) Treatment II (at yeast inoculum). Ctrl is clarified must with the only pectinolytic enzyme. For each sample, means with different roman letters are significantly different (p ≤ 0.05).
Before and after the enzymatic treatment, the wine samples were subjected to the SDS-page and the relative electrophoretic profiles, obtained under denaturing conditions, are depicted in Figure 6. The reduction in the bands between 20–25 kDa, corresponding to CH (25 kDa) and TLP (22 kDa) [14,33], after enzymatic treatment proved its effectiveness in both winemaking phases (Figure 6).
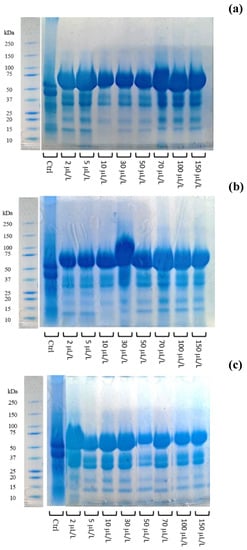
Figure 6.
Electrophoretic profile under denaturing conditions of white must-wine subjected to enzymatic addition (protease in the range of 2–150 μL/L) after (a) treatment I (cold settling prior to AF, evaluated at racking after 24 h settling), (b) treatment II (at yeast inoculum, evaluated at middle AF) and (c) treatment II (at yeast inoculum, evaluated at the end AF). Ctrl is clarified must with the only pectinolytic enzyme.
3.4. Effect of Enzymatic Treatment on Chromatic Characteristics and Phenolics
Table 4 and Table 5 show the colorimetric parameters L*, hue (h*), chroma (C*) and total color difference (ΔE) (obtained on the basis of the CIELAB space coordinates (L*, a*, b*)). Irrespective of the oenological phase of enzyme addition, L* showed no significant differences (Table 4 and Table 5), while h* values were in the range of 81.7–85.9, confirming that the main shade of wine samples was in the yellow portion. No relevant differences were revealed either with respect to Ctrl or among the enzymatically treated samples. For both treatments, C* parameter (Table 4 and Table 5) was similar among the samples and Ctrl. All values indicated that the color of the samples fallen into the “pale-yellow” color category [34]. ΔE parameter in all treated samples was around 3, thus suggesting the lack of a remarkable difference in color between samples and Ctrl (Table 4 and Table 5). As reported by Lukić et al. [34], to have an appreciable difference in color between white wine samples, the ΔE must be greater than 3.5.

Table 4.
Visual color attributes (L*, hue (h*), chroma value (C*) and total color difference (ΔE)) of white must wine subjected to enzymatic addition (protease in the range of 2–150 μL/L) after treatment I (cold settling prior to AF). Ctrl is clarified must with the only pectinolytic enzyme.

Table 5.
Visual color attributes (L*, hue (h*), chroma value (C*) and total color difference (ΔE)) of white must wine subjected to enzymatic addition (protease in the range of 2–150 μL/L) after treatment II (at yeast inoculum, evaluated at middle AF and at the end of AF). Ctrl is clarified must with the only pectinolytic enzyme.
The phenolic content of wine must produced by Riesling Italic grapes (Figure 7) resulted in the range of 200–280 mg/L, in line with what is reported in the literature [35]. Concerning treatment I (Figure 7a), no appreciable differences were observed among samples, whereas a slight decrease in phenol content was revealed as compared to the Ctrl following treatment II in all samples at the end of AF (Figure 7b). It is reasonable to assume the phenomenon underlying this decline is the precipitation of plant residues, tartaric salts, as well as the sorption of grape high molecular weight phenols by yeast cell walls [36], also related to ΔE changes.
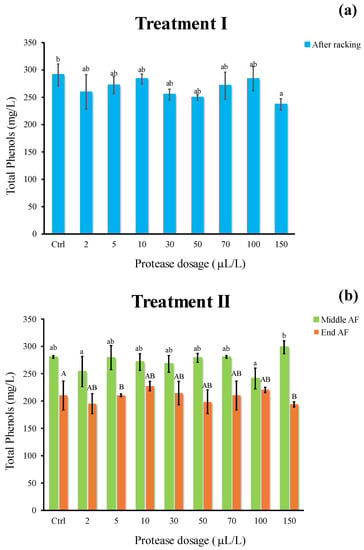
Figure 7.
Total phenols (mg/L) of white must wine subjected to enzymatic addition (protease in the range of 2–150 μL/L) after (a) treatment I (cold settling prior to AF) and (b) treatment II (at yeast inoculum). Ctrl is clarified must with the only pectinolytic enzyme. For each sample, means with different roman letters are significantly different (p ≤ 0.05).
Overall, these results suggest that the novel protein stabilization treatment did not significantly affect the phenolic composition of wine, as already demonstrated by applying other biocatalysts [19].
4. Conclusions
According to the results of this study, the early protein stabilization treatment, performed at the initial stages of winemaking, was useful in lowering the protein instability and the amount of HA proteins in wine must from white grape, without affecting color quality and phenol content. Such early treatment could contribute to the protein stabilization naturally occurring later throughout the wine ageing on lees. In summary, the addition of microbial protease at yeast inoculum (performed at 50 μL/L dosage) appeared to be the most useful in preventing protein haze in comparison with the enzymatic treatment at cold settling, probably due to the most favorable conditions for protease activity both in terms of temperature (18 °C of AF as compared to 10 °C of settling) and time (16 days as compared to 24 h). Despite the fact there are more sustainable approaches for winemaking, there should also be further investigation. This minimally invasive enzymatic treatment integrated into the production process could represent a valuable alternative to conventional bentonite fining as well as to the most recently available stabilization methods, always intended as an additional process step.
Author Contributions
Conceptualization, C.L. and I.B.; methodology, C.L. and M.M.; validation, C.L., I.B. and M.E.; formal analysis, C.L., M.M. and C.M.; investigation, I.B., M.M., C.L. and I.B.; resources, I.B. and M.E.; data curation, C.L., C.M. and I.B.; writing—original draft preparation, C.L. and I.B.; writing—review and editing, M.E.; supervision, I.B. and M.E.; project administration, I.B. and M.E. funding acquisition, M.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by LazioInnova Spa, Lazio Region (Italy), StaBirVino project “Enzimi immobilizzati per la stabilizzazione sostenibile di birra e vino” (Grant A0375-2020-36649, Progetti Gruppi di Ricerca 2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We are thankful to Rover Pompe Snc, Chiarello Enzo (Italy) for providing the oenological materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White wine protein instability: Mechanism, quality control and technological alternatives for wine stabilisation—An overview. Beverages 2020, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Marangon, M.; Vincenzi, S.; Lucchetta, M.; Curioni, A. Heating and reduction affect the reaction with tannins of wine protein fractions differing in hydrophobicity. Anal. Chim. Acta 2010, 660, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Harrison, R. Pathogenesis-Related Proteins in Wine and White Wine Protein Stabilization. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: Vienna, Austria, 2020. [Google Scholar]
- Van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine protein haze: Mechanisms of formation and advances in prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef] [PubMed]
- Silva-Barbieri, D.; Salazar, F.N.; López, F.; Brossard, N.; Escalona, N.; Pérez-Correa, J.R. Advances in White Wine Protein Stabilization Technologies. Molecules 2022, 27, 1251. [Google Scholar] [CrossRef]
- Celotti, E.; Barahona, M.S.O.; Bellantuono, E.; Cardona, J.; Roman, T.; Nicolini, G.; Natolino, A. High-power ultrasound on the protein stability of white wines: Preliminary study of amplitude and sonication time. LWT 2021, 147, 111602. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Qi, G.; Smith, P.; Bindon, K.; Vasilev, K. Regeneration of magnetic nanoparticles used in the removal of pathogenesis-related proteins from white wines. Foods 2020, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Mierczynska-Vasilev, A.; Wahono, S.K.; Smith, P.A.; Bindon, K.; Vasilev, K. Using zeolites to protein stabilize white wines. ACS Sustain. Chem. Eng. 2019, 7, 12240–12247. [Google Scholar] [CrossRef]
- Romanini, E.; McRae, J.M.; Bilogrevic, E.; Colangelo, D.; Gabrielli, M.; Lambri, M. Use of grape seeds to reduce haze formation in white wines. Food Chem. 2021, 341, 128250. [Google Scholar] [CrossRef]
- Millarini, V.; Ignesti, S.; Cappelli, S.; Ferraro, G.; Adessi, A.; Zanoni, B.; Fratini, E.; Domizio, P. Protection of Wine from Protein Haze Using Schizosaccharomyces japonicus Polysaccharides. Foods 2020, 9, 1407. [Google Scholar] [CrossRef]
- Ratnayake, S.; Stockdale, V.; Grafton, S.; Munro, P.; Robinson, A.L.; Pearson, W.; McRae, J.M.; Bacic, A. Carrageenans as heat stabilisers of white wine. Aust. J. Grape Wine Res. 2019, 25, 439–450. [Google Scholar] [CrossRef]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2021, 58, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Van Sluyter, S.C.; Robinson, E.M.; Muhlack, R.A.; Holt, H.E.; Haynes, P.A.; Godden, P.W.; Smith, P.A.; Waters, E.J. Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem. 2012, 135, 1157–1165. [Google Scholar] [CrossRef]
- Esti, M.; Benucci, I.; Lombardelli, C.; Liburdi, K.; & Garzillo, A.M.V. Papain from papaya (Carica papaya L.) fruit and latex: Preliminary characterization in alcoholic–acidic buffer for wine application. Food Bioprod. Process. 2013, 91, 595–598. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M.; Liburdi, K. Effect of wine inhibitors on the proteolytic activity of papain from Carica papaya L. latex. Biotechnol. Prog. 2015, 31, 48–54. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M.; Liburdi, K. Effect of free and immobilised stem bromelain on protein haze in white wine. Aust. J. Grape Wine Res. 2014, 20, 347–352. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Liburdi, K.; Acciaro, G.; Zappino, M.; Esti, M. Immobilised native plant cysteine proteases: Packed-bed reactor for white wine protein stabilisation. J. Food Sci. Technol. 2016, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EEC) No 2676/90 of 17 September 1990 Determining Community Methods for the Analysis of Wines; European Commission: Brussels, Belgium, 1990; Volume 272, pp. 1–192. [Google Scholar]
- Lopez, M.; Edens, L. Effective prevention of chill-haze in beer using an acid proline-specific endoprotease from Aspergillus niger. J. Agric. Food Chem. 2005, 53, 7944–7949. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M. Arginase Activity Characterization During Alcoholic Fermentation by Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeast. Food Bioprocess Technol. 2021, 14, 1996–2003. [Google Scholar] [CrossRef]
- Benucci, I.; Fiorelli, V.; Lombardelli, C.; Liburdi, K.; Esti, M. Kinetic characterization of arginase from Saccharomyces cerevisiae during alcoholic fermentation at different temperatures. LWT 2017, 82, 268–273. [Google Scholar] [CrossRef]
- Vincenzi, S.; Marangon, M.; Tolin, S.; Curioni, A. Protein evolution during the early stages of white winemaking and its relations with wine stability. Aust. J. Grape Wine Res. 2011, 17, 20–27. [Google Scholar] [CrossRef]
- Siebert, K.J.; Carrasco, A.; Lynn, P.Y. Formation of protein− polyphenol haze in beverages. J. Agric. Food Chem. 1996, 44, 1997–2005. [Google Scholar] [CrossRef]
- Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Cacciotti, I.; Esti, M. Multi-enzymatic systems immobilized on chitosan beads for pomegranate juice treatment in fluidized bed reactor: Effect on haze-active molecules and chromatic properties. Food Bioprocess Technol. 2019, 12, 1559–1572. [Google Scholar] [CrossRef]
- Becchetti, R.; Sanvito, M. Metodi di Analisi dei Vini e delle Bevande Spiritose; Gibertini Elettronica: Milan, Italy, 1999. [Google Scholar]
- de Esteban, M.L.G.; Ubeda, C.; Heredia, F.J.; Catania, A.A.; Assof, M.V.; Fanzone, M.L.; Jofre, V.P. Impact of closure type and storage temperature on chemical and sensory composition of Malbec wines (Mendoza, Argentina) during aging in bottle. Food Res. Int. 2019, 125, 108553. [Google Scholar] [CrossRef] [PubMed]
- Onofri, A. Enhancing Excel capability to perform statistical analyses in agriculture applied research. In Computational Statistics and Data Analysis–Statistical Software Newsletters; International Association for Statistical Computing: The Hague, The Netherlands, 2006. [Google Scholar]
- Kang, C.; Yu, X.W.; Xu, Y. Purification and characterization of a prolyl endopeptidase isolated from Aspergillus oryzae. J. Ind. Microbiol. Biotechnol. 2014, 41, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhao, H.; Zhao, M. Proteases supplementation to high gravity worts enhances fermentation performance of brewer’s yeast. Biochem. Eng. J. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Mathias, T.R.S.; Lopes, M.C.R.D.; Oliveira, C.A.; Carvalho, R.C.; Marques, F.F.C.; Sérvulo, E.F.C. Influence of mashing profile curve and addition of proteases on the composition of the wort and beer. MOJ Food Process. Technol. 2017, 5, 124. [Google Scholar]
- Comuzzo, P.; Voce, S.; Fabris, J.; Cavallaro, A.; Zanella, G.; Karpusas, M.; Kallithraka, S. Effect of the combined application of heat treatment and proteases on protein stability and volatile composition of Greek white wines. OENO One 2020, 54, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Lukić, K.; Brnčić, M.; Ćurko, N.; Tomašević, M.; Tušek, A.J.; Ganić, K.K. Quality characteristics of white wine: The short-and long-term impact of high power ultrasound processing. Ultrason. Sonochem. 2020, 68, 105194. [Google Scholar] [CrossRef]
- Valášek, P.; Mlček, J.; Adámková, A.; Křivánková, M.; Adámek, M.; Sedláčková, E. Comparison of contents of selected esters, higher alcohols and total content of polyphenolic substances in wines of the varieties’ Chardonnay’ and ‘Riesling’ by vintage. Mitt. Klosterneubg. Rebe Wein Obstb. Fruchteverwert. 2019, 69, 115–123. [Google Scholar]
- Ribéreau-Gayon, J.; Peynaud, E. Trattato di Enologia; Edagricole: Bologna, Italy, 1957. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).