Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasound-Assisted Extraction of Procyanidins
2.3. Drying Conditions
2.4. Energy Consumption of Drying Methods
2.5. Storage Stability Test of GSS Extract Powder
2.6. Determination of Phytochemicals and Antioxidant Properties
2.6.1. Phytochemical Analysis
Total Phenolic Contents (TPC)
Total Flavonoid Contents (TFC)
Determination of Procyanidins
2.6.2. Antioxidant Analysis
DPPH Radical Scavenging Capacity Assay
Ferric-Reducing Antioxidant Power (FRAP) Assay
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. Optimization Process Conditions for UAE Extraction of Bioactive Compounds from GSS
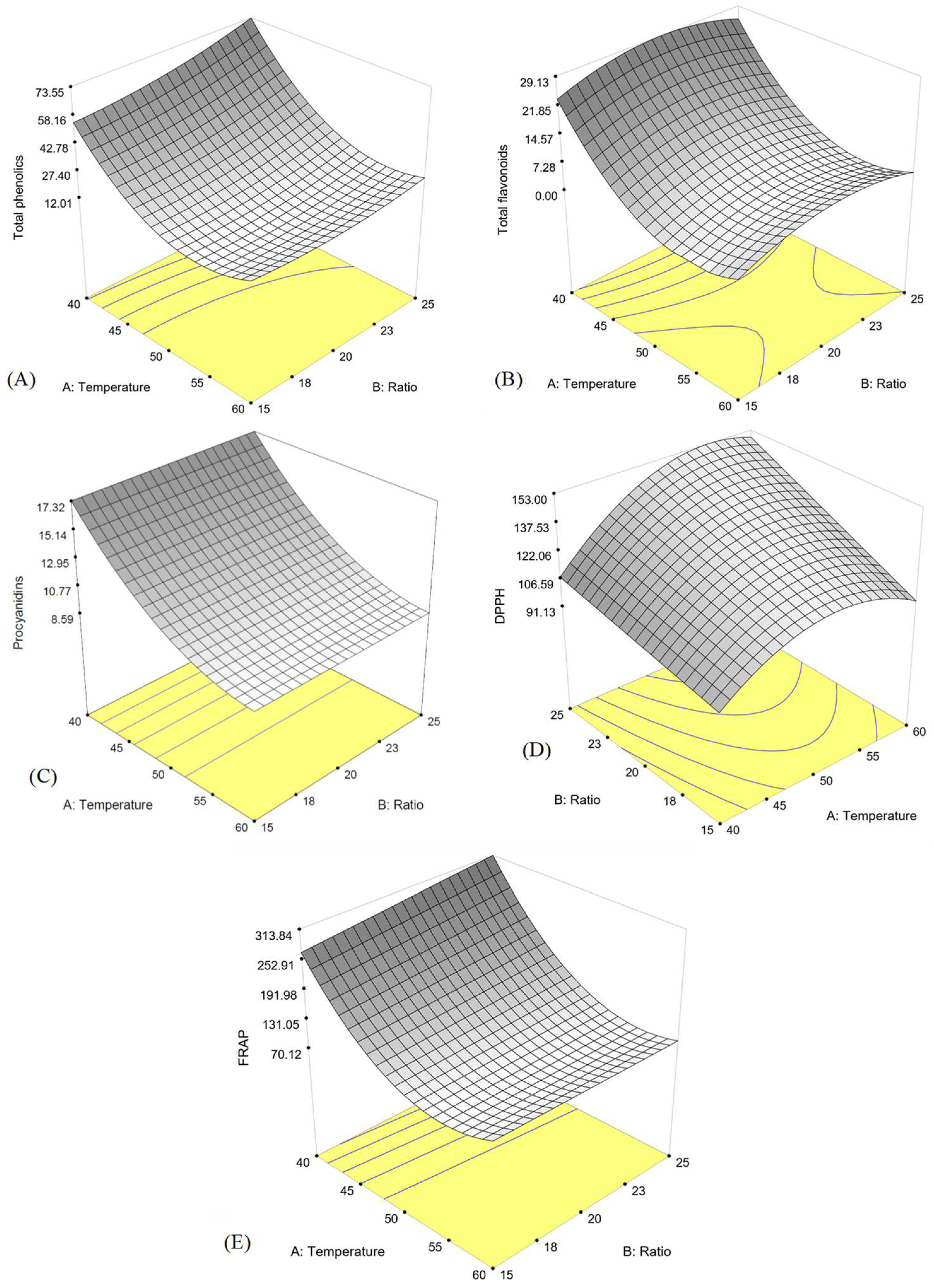
3.2. Response Surface Methodology (RSM) Analysis
3.3. Effect of Drying Methods on Procyanidin Content and Antioxidant Properties of GSS Extract
3.4. Effect of Storage Temperature and Time on the Stability of Retained Procyanidins of Dry GSS Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GSS | green soybean seeds |
| UAE | ultrasound-assisted extraction |
| RSM | response surface method |
| TPC | total phenolic contents |
| TFC | total flavonoid contents |
| PC | procyanidin |
| FRAP | ferric-reducing antioxidant power |
References
- Leksawasdi, N.; Taesuwan, S.; Prommajak, T.; Techapun, C.; Khonchaisri, R.; Sittilop, N.; Halee, A.; Jantanasakulwong, K.; Phongthai, S.; Nunta, R.; et al. Ultrasonic extraction of bioactive compounds from green soybean pods and application in green soybean milk antioxidants fortification. Foods 2022, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Weidner, S.; Król, A.; Karamać, M.; Amarowicz, R. Phenolic compounds and the antioxidant properties in seeds of green-and yellow-podded bean (Phaseolus vulgaris L.) varieties. CyTA-Journal Food 2018, 16, 373–380. [Google Scholar] [CrossRef]
- Li, H.Z.; Tan, Y.L.; Zhang, Z.J.; Xia, Y.Y.; Li, X.J.; Cui, L.X.; Chen, T. Optimization of ultrasound-assisted extraction of procyanidins from perilla seed hull and their antioxidant activities in vitro. Food Sci. Technol. 2018, 39, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, R.; Güell, C.; Ferrando, M. A procyanidin-rich extract encapsulated in water-in-oil-in-water emulsions produced by premix membrane emulsification. Food Hydrocoll. 2015, 43, 636–648. [Google Scholar] [CrossRef]
- Sun, X.; Jin, Z.; Yang, L.; Hao, J.; Zu, Y.; Wang, W.; Liu, W. Ultrasonic-assisted extraction of procyanidins using ionic liquid solution from Larix gmelinii bark. J. Chem. 2013, 2013, 541037. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, Y.; Zhao, C.; Ni, Y.; Wang, K.; Zhang, J.; Zhao, W. Ultrasonic assisted-reflux synergistic extraction of camptothecin and betulinic acid from Camptotheca acuminata Decne. Fruits. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.M.; Gouda, M.; Zhu, Y.Y.; Ye, X.Q.; Chen, J.C. Ultrasound-assisted extraction optimization of proanthocyanidins from kiwi (Actinidia chinensis) leaves and evaluation of its antioxidant activity. Antioxidants 2021, 10, 1–18. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 1–11. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.Q.; Vuong, Q.V.; Nguyen, M.H.; Roach, P.D. The effects of drying conditions on bioactive compounds and antioxidant activity of the Australian maroon bush, Scaevola spinescens. J. Food Process. Preserv. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Khemacheewakul, J.; Prommajak, T.; Leksawasdi, N.; Techapun, C.; Nunta, R.; Hanprom, N. Production and storage stability of antioxidant fiber from Pigeon pea (Cajanus cajan) pod. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 293–297. [Google Scholar] [CrossRef]
- Hii, C.L.; Ong, S.P.; Yap, J.Y.; Putranto, A.; Mangindaan, D. Hybrid drying of food and bioproducts: A review. Drying Technol. 2021, 39, 1554–1576. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Pham, N.M.Q.; Van Vuong, Q.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Phytochemical retention and antioxidant capacity of Xao tam phan (Paramignya trimera) root as prepared by different drying methods. Drying Technol. 2016, 34, 324–334. [Google Scholar] [CrossRef]
- Khodaie, L.; Bamdad, S.; Delazar, A.; Nazemiyeh, H. Antioxidant, total phenol and flavonoid contents of two Pedicularis L. species from Eastern Azerbaijan, Iran. Bioimpacts 2012, 2, 47–53. [Google Scholar]
- Liu, T.; Cao, Y.; Zhao, M. Extraction optimization, purification and antioxidant activity of procyanidins from hawthorn (C. pinnatifida Bge. var. major) fruits. Food Chem. 2010, 119, 1656–1662. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Tylewicz, U.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Romani, S. Design of sonotrode ultrasound-assisted extraction of phenolic compounds from Psidium guajava L. leaves. Food Anal. Methods 2017, 10, 2781–2791. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Wang, W.D. Optimization of ultrasonic-assisted extraction and in vitro antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 315–323. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 1–23. [Google Scholar] [CrossRef]
- Settharaksa, S.; Hmadhlu, P.; Chansuwan, W.; Siripongvutikorn, S. Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. Int. Food Res. J. 2012, 19, 1581–1587. [Google Scholar]
- Aourabi, S.; Sfaira, M.; Mahjoubi, F. Optimization of ultrasound-assisted extraction of polyphenol content from Zea mays Hairs (Waste). Sci. World J. 2020, 2020, 5072938. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Effects of drying conditions on physicochemical and antioxidant properties of banana (Musa cavendish) peels. Dry. Technol. 2017, 35, 1141–1151. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Buratto, A.P.; Carpes, S.T.; Pereira, E.A.; Diedrich, C.; Oldoni, T.L.C.; da Silva, L.D. Effect of drying method in the maintenance of bioactive compounds and antioxidant activity of Feijoa pulp (Acca sellowiana). Orbital 2019, 11, 386–393. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef]
- Ramírez, M.J.; Giraldo, G.I.; Orrego, C.E. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 2015, 277, 89–96. [Google Scholar] [CrossRef]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary flavonoids for immunoregulation and cancer: Food design for targeting disease. Antioxidants 2019, 8, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Langrish, T.A.G.; Harrington, J.; Huang, X.; Zhong, C. Using CFD simulations to guide the development of a new spray dryer design. Processes 2020, 8, 1–22. [Google Scholar] [CrossRef]
- Chávez, B.E.; Ledeboer, A.M. Drying of probiotics: Optimization of formulation and process to enhance storage survival. Dry. Technol. 2007, 25, 1193–1201. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J. Appl. Microbiol. 2008, 105, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, A.N.; Mrmošanin, J.M.; Krstić, J.N.; Mitić, S.S.; Tošić, S.B.; Mitić, M.N.; Arsić, B.B.; Micić, R.J. Effect of storage temperature on the decay of catechins and procyanidins in dark chocolate. Czech J. Food Sci. 2017, 35, 360–366. [Google Scholar]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on procyanidin composition and concentration of processed blueberry products. J. Agric. Food Chem. 2009, 57, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Widyaningsih, T.D.; Akbar, S.M.; Wijayanti, N. Optimization of maltodextrin concentration, drying temperature and drying time on total flavonoid content and antioxidant activity of black garlic (Allium sativum L.) aqueous extract powder using response surface methodology. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 1–12. [Google Scholar] [CrossRef]
- Dallas, C.; Hipólito-Reis, P.; Ricardo-da-Silva, J.M.; Laureano, O. Influence of acetaldehyde, pH, and temperature on transformation of procyanidins in model wine solutions. Am. J. Enol. Vitic. 2003, 542, 1–7. [Google Scholar]
- Mrmošanin, J.M.; Pavlović, A.N.; Veljković, J.N.; Mitić, S.S.; Tošić, S.B.; Mitić, M.N. The effect of storage temperature and thermal processing on catechins, procyanidins and total flavonoid stability in commercially available cocoa powders. Chem. Technol. 2015, 13, 39–49. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Gutiérrez-Lomelí, M.; Lugo-Cervantes, E.; Zurita, F.; Robles-García, M.A.; Ruiz-Cruz, S.; Aguilar, J.A.; Morales-Del Rio, J.A.; Guerrero-Medina, P.J. Storage effect on phenols and on the antioxidant activity of extracts from Anemopsis californica and inhibition of elastase enzyme. J. Chem. 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zorić, Z.; Pelaić, Z.; Pedisić, S.; Elez Garofulić, I.; Bursać Kovačević, D.; Dragović–Uzelac, V. Effect of storage conditions on phenolic content and antioxidant capacity of spray dried sour cherry powder. LWT—Food Sci. Technol. 2017, 79, 251–259. [Google Scholar] [CrossRef]

| Temperature (°C) | Liquid-to-Solid Ratio | Bioactive Compounds | Antioxidant Activity | |||
|---|---|---|---|---|---|---|
| TPC (mg GAE/g) | TFC (mg CAE/g) | Procyanidins (mg PC/g) | DPPH (µM Trolox eq/g) | FRAP (µM Trolox eq/g) | ||
| 15:1 | 55.2 ± 0.45 b | 18.2 ± 0.73 c | 14.6 ± 0.09 d | 112 ± 0.05 g | 255 ± 0.41 c | |
| 40 | 20:1 | 56.2 ± 0.63 b | 28.6 ± 0.67 b | 16.2 ± 0.07 b | 150 ± 0.05 d | 299 ± 0.28 b |
| 25:1 | 76.8 ± 0.46 a | 30.9 ± 0.58 a | 21.1 ± 0.05 a | 192 ± 0.09 a | 318 ± 0.71 a | |
| 15:1 | 17.1 ± 0.45 e | 6.09 ± 0.10 d | 15.8 ± 0.09 c | 106 ± 0.27 i | 107 ± 0.38 d | |
| 50 | 20:1 | 17.4 ± 0.28 e | 3.86 ± 0.11 e | 8.54 ± 0.07 e,f | 145 ± 0.10 f | 105 ± 0.46 e |
| 25:1 | 26.6 ± 0.38 c | 4.06 ± 0.06 e | 8.39 ± 0.03 e,f | 186 ± 0.07 b | 92.7 ± 0.33 f | |
| 15:1 | 15.2 ± 0.96 f | 3.55± 0.03 e | 6.88 ± 0.04 f | 108 ± 0.09 h | 81.8 ± 0.89 g | |
| 60 | 20:1 | 23.9 ± 0.47 d | 5.39 ± 0.07 d | 9.43 ± 0.03 e | 149 ± 0.10 e | 92.1 ± 0.6 f |
| 25:1 | 25.1 ± 0.97 c,d | 6.07 ± 0.06 d | 9.45 ± 0.03 e | 184 ± 0.03 c | 91.7 ± 0.48 f | |
| Parameters | Total Phenolics | Total Flavonoids | Procyanidin | DPPH | FRAP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Model | ||||||||||
| Constant | 634.08 | 266.69 | 82.81 | 49.64 | 2497.37 | |||||
| Temperature | −22.59 | <0.001 | −10.82 | <0.001 | −2.08 | <0.001 | −2.64 | <0.001 | −92.62 | <0.001 |
| Ratio | −1.33 | <0.001 | 3.01 | 0.001 | −1.04 | 0.617 | 8.91 | <0.001 | 15.31 | <0.001 |
| Temperature2 | 0.22 | <0.001 | 0.11 | <0.001 | 0.02 | 0.046 | 0.03 | <0.001 | 0.88 | <0.001 |
| Ratio2 | 0.14 | 0.006 | −0.05 | 0.186 | ||||||
| Temp × Ratio | −0.06 | 0.002 | −0.05 | <0.001 | −0.02 | 0.156 | −0.02 | <0.001 | −0.27 | <0.001 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| R2 | 0.9785 | 0.9523 | 0.7005 | 0.9991 | 0.9871 | |||||
| Response Variables | Predicted Values | Actual Values | p-Value |
|---|---|---|---|
| Total phenolics (mg GAE/g sample) | 73.6 | 76.6 ± 0.54 | 0.16 |
| Total flavonoids (mg catechin/g sample) | 30.7 | 28.9 ± 0.55 | 0.37 |
| Procyanidin (mg/g sample) | 19.0 | 19.2 ± 0.33 | 0.89 |
| DPPH (µg Trolox eq/g sample) | 191 | 194 ± 3.53 | 0.79 |
| FRAP (µmol Trolox eq/g sample) | 314 | 320 ± 4.88 | 0.72 |
| Drying Method | Procyanidins (mg PC/g) | DPPH (µM Trolox eq/g) | FRAP (µM Trolox eq/g) | Energy Consumption (kWh) |
|---|---|---|---|---|
| Freeze drying | 17.3 ± 0.14 b | 198 ± 0.65 a | 238 ± 0.3 b | 184 |
| Hot air drying | 12.1 ± 0.51 c | 127 ± 0.48 b | 122 ± 0.31 d | 5.00 |
| Spray drying | 21.4 ± 0.37 a | 199 ± 0.85 a | 243 ± 0.26 a | 7.88 |
| Vacuum drying | 20.8 ± 0.23 a | 200 ± 0.85 a | 207 ± 0.19 c | 6.57 |
| Storage Temperature (°C) | Time (Days) | % Retention | ||
|---|---|---|---|---|
| Procyanidins | DPPH | FRAP | ||
| 0 | 100 | 100 | 100 | |
| 7 | 94.1 ± 0.38 | 97.9 ± 0.19 | 99.2 ± 1.42 | |
| 25 | 14 | 73.8 ± 1.22 | 90.0 ± 0.51 | 97.4 ± 0.40 |
| 21 | 70.0 ± 0.15 | 87.5 ± 1.40 | 91.9 ± 0.11 | |
| 28 | 68.6 ± 0.13 | 72.7 ± 0.89 | 80.2 ± 0.35 | |
| 0 | 100 | 100 | 100 | |
| 7 | 74.2 ± 0.16 | 98.9 ± 0.38 | 99.8 ± 0.17 | |
| 35 | 14 | 64.2 ± 1.13 | 88.2 ± 1.66 | 98.9 ± 1.03 |
| 21 | 61.9 ± 1.65 | 85.4 ± 1.61 | 92.9 ± 0.99 | |
| 28 | 49.6 ± 0.39 | 83.9 ± 0.70 | 79.5 ± 0.89 | |
| 0 | 100 | 100 | 100 | |
| 7 | 60.3 ± 0.85 | 78.1 ± 0.83 | 93.8 ± 0.29 | |
| 45 | 14 | 59.8 ± 0.93 | 77.8 ± 0.26 | 90.4 ± 1.17 |
| 21 | 51.5 ± 0.39 | 69.6 ± 0.32 | 80.1 ± 0.13 | |
| 28 | 35.6 ± 0.47 | 67.4 ± 0.38 | 74.1 ± 0.18 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khonchaisri, R.; Sumonsiri, N.; Prommajak, T.; Rachtanapun, P.; Leksawasdi, N.; Techapun, C.; Taesuwan, S.; Halee, A.; Nunta, R.; Khemacheewakul, J. Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods 2022, 11, 1775. https://doi.org/10.3390/foods11121775
Khonchaisri R, Sumonsiri N, Prommajak T, Rachtanapun P, Leksawasdi N, Techapun C, Taesuwan S, Halee A, Nunta R, Khemacheewakul J. Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods. 2022; 11(12):1775. https://doi.org/10.3390/foods11121775
Chicago/Turabian StyleKhonchaisri, Rattanaporn, Nutsuda Sumonsiri, Trakul Prommajak, Pornchai Rachtanapun, Noppol Leksawasdi, Charin Techapun, Siraphat Taesuwan, Anek Halee, Rojarej Nunta, and Julaluk Khemacheewakul. 2022. "Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract" Foods 11, no. 12: 1775. https://doi.org/10.3390/foods11121775
APA StyleKhonchaisri, R., Sumonsiri, N., Prommajak, T., Rachtanapun, P., Leksawasdi, N., Techapun, C., Taesuwan, S., Halee, A., Nunta, R., & Khemacheewakul, J. (2022). Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods, 11(12), 1775. https://doi.org/10.3390/foods11121775










