Effects of Commercial Polysaccharides Stabilizers with Different Charges on Textural, Rheological, and Microstructural Characteristics of Set Yoghurts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Set Yoghurts Preparation
2.3. Physical Properties, Water-Holding Capacity (WHC), and Texture of Set Yoghurts
2.4. LF-NMR Determination
2.5. Characterization of Rheological Properties
2.6. Microstructure Observation by Cryo-SEM and CLSM
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties, WHC, and Texture of Different Set Yoghurts
3.2. LF-NMR Analysis
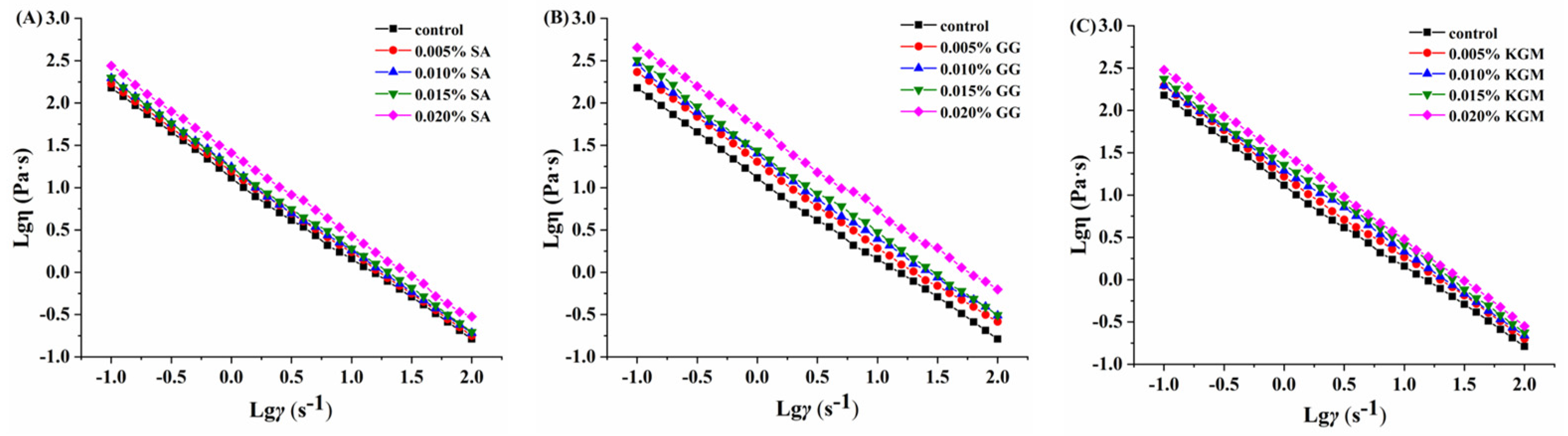
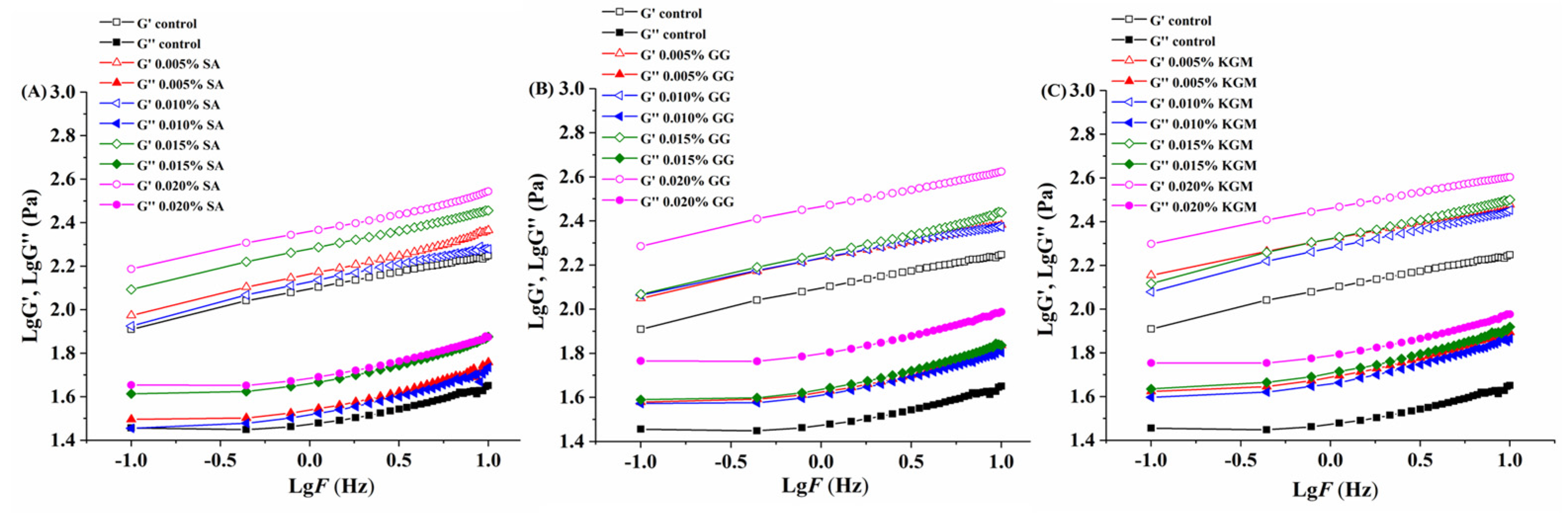
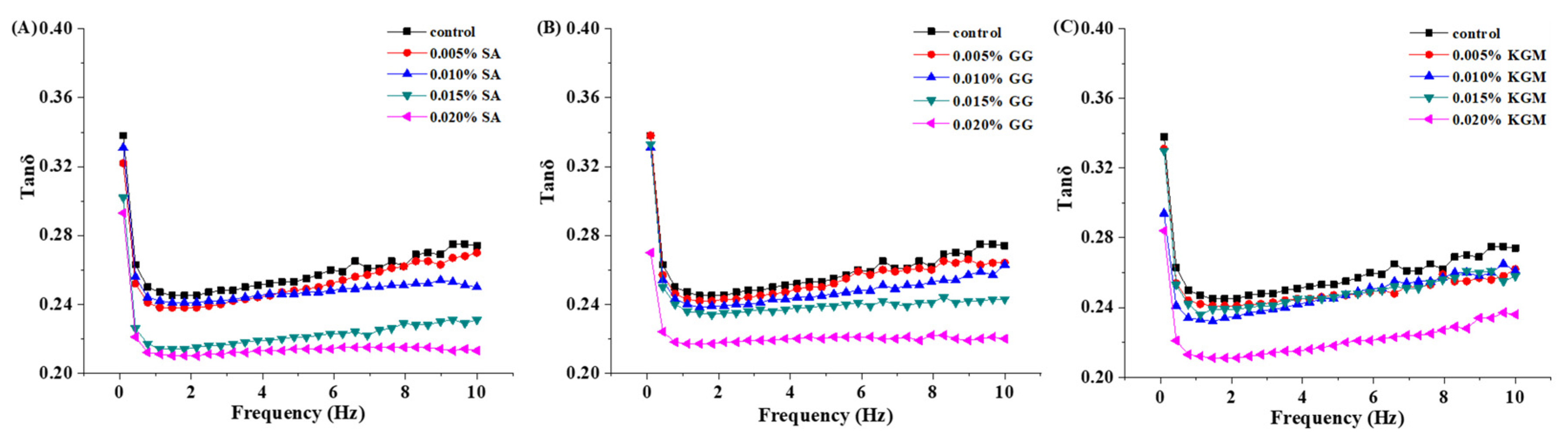
3.3. Rheological Analysis
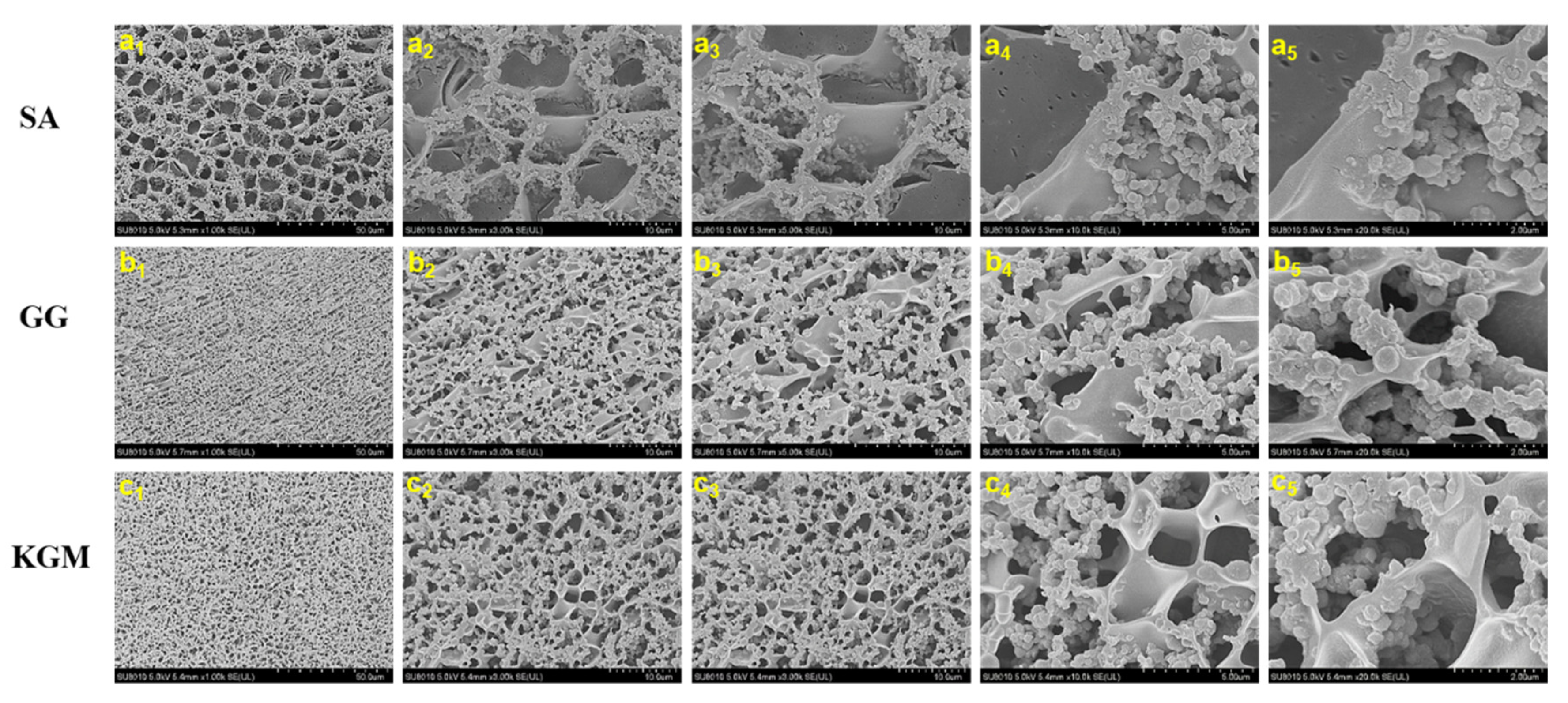
3.4. Microstructure Observation and Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, S.; Corke, H.; Shah, N.P. Utilization of konjac glucomannan as a fat replacer in low-fat and skimmed yogurt. J. Dairy Sci. 2016, 99, 7063–7074. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Halady, P.S. Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Hemar, Y.; Cui, B. Improvement of the rheological and textural properties of calcium sulfate- induced soy protein isolate gels by the incorporation of different polysaccharides. Food Chem. 2020, 310, 125983. [Google Scholar] [CrossRef]
- Kiani, H.; Mousavi, M.E.; Razavi, H.; Morris, E.R. Effect of gellan, alone and in combination with high-methoxy pectin, on the structure and stability of doogh, a yogurt-based Iranian drink. Food Hydrocoll. 2010, 24, 744–754. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C.; Zhang, H.; Hossen, M.A.; Hossen, D.E.; Dai, J.; Liu, Y.; Li, S. In vitro digestion of sodium alginate/pectin co-encapsulated Lactobacillus bulgaricus and its application in yogurt bilayer beads. Int. J. Biol. Macromol. 2021, 193, 1050–1058. [Google Scholar] [CrossRef]
- Khubber, S.; Chaturvedi, K.; Thakur, N.; Sharma, N.; Yadav, S.K. Low-methoxyl pectin stabilizes low-fat set yoghurt and improves their physicochemical properties, rheology, microstructure and sensory liking. Food Hydrocoll. 2020, 111, 106240. [Google Scholar] [CrossRef]
- Zhang, T. Effects of stabilizers and exopolysaccharides on physiochemical properties of fermented skim milk by Streptococcus thermophilus ST1. Afr. J. Biotechnol. 2012, 11, 6123–6130. [Google Scholar]
- Pang, Z.; Deeth, H.; Bansal, N. Effect of polysaccharides with different ionic charge on the rheological, microstructural and textural properties of acid milk gels. Food Res. Int. 2015, 72, 62–73. [Google Scholar] [CrossRef]
- Khanal, B.S.; Bhandari, B.; Prakash, S.; Liu, D.; Zhou, P.; Bansal, N. Modifying textural and microstructural properties of low fat Cheddar cheese using sodium alginate. Food Hydrocoll. 2018, 83, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Krzeminski, A.; Prell, K.A.; Busch-Stockfisch, M.; Weiss, J.; Hinrichs, J. Whey protein–pectin complexes as new texturising elements in fat-reduced yoghurt systems. Int. Dairy J. 2014, 36, 118–127. [Google Scholar] [CrossRef]
- Salomonsen, T.; Sejersen, M.T.; Viereck, N.; Ipsen, R.; Engelsen, S.B. Water mobility in acidified milk drinks studied by low-field 1H NMR. Int. Dairy J. 2007, 17, 294–301. [Google Scholar] [CrossRef]
- Torres, I.C.; Mutaf, G.; Larsen, F.H.; Ipsen, R. Effect of hydration of microparticulated whey protein ingredients on their gelling behaviour in a non-fat milk system. J. Food Eng. 2016, 184, 31–37. [Google Scholar] [CrossRef]
- Peters, J.P.C.M.; Vergeldt, F.J.; As, H.V.; Luyten, H.; Boom, R.M.; Van der Goot, A.J. Time domain nuclear magnetic resonance as a method to determine and characterize the water-binding capacity of whey protein microparticles. Food Hydrocoll. 2016, 54, 170–178. [Google Scholar] [CrossRef]
- Ozel, B.; Uguz, S.S.; Kilercioglu, M.; Grunin, L.; Oztop, M.H. Effect of different polysaccharides on swelling of composite whey protein hydrogels: A low field (LF) NMR relaxometry study. J. Food Eng. 2017, 40, e12465. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, S.; Lai, S.; Chen, F.; Yang, H. Combined effects of ultrasound and calcium on the chelate-soluble pectin and quality of strawberries during storage. Carbohydr. Polym. 2018, 200, 427–435. [Google Scholar] [CrossRef]
- Ong, L.; Dagastine, R.R.; Kentish, S.E.; Gras, S.L. Microstructure of milk gel and cheese curd observed using cryo scanning electron microscopy and confocal microscopy. LWT 2011, 44, 1291–1302. [Google Scholar] [CrossRef]
- Ribero, G.G.; Rubiolo, A.C.; Zorrilla, S.E. Microstructure of Mozzarella cheese as affected by the immersion freezing in NaCl solutions and by the frozen storage. J. Food Eng. 2009, 91, 516–520. [Google Scholar] [CrossRef]
- Bai, M.; Huang, T.; Guo, S.; Wang, Y.; Bilige, M. Probiotic Lactobacillus casei Zhang improved the properties of stirred yogurt. Food Biosci. 2020, 37, 100718. [Google Scholar] [CrossRef]
- Ge, Z.; Bao, X.; Li, Z.; Chen, X.; Li, W.; Dong, M. In situ exopolysaccharides produced by Lactobacillus helveticus MB2-1 and its effect on gel properties of Sayram ketteki yoghurt. Int. J. Biol. Macromol. 2022, 208, 314–323. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z. Okra polysaccharide: Effect on the texture and microstructure of set yoghurt as a new natural stabilizer. Int. J. Biol. Macromol. 2019, 133, 117–126. [Google Scholar] [CrossRef]
- Jana, P.; Liptakova, D.; Lubomir, V. Suitability of lactic acid bacteria for fermentation of maize and amaranth. J. Food Nutr. Res. 2015, 54, 354–364. [Google Scholar]
- Sert, D.; Mercan, E.; Dertli, E. Characterization of lactic acid bacteria from yogurt-like product fermented with pine cone and determination of their role on physicochemical, textural and microbiological properties of product. LWT 2017, 78, 70–76. [Google Scholar] [CrossRef]
- Yildiz, E.; Ozcan, T. Functional and textural properties of vegetable-fibre enriched yoghurt. Int. J. Dairy Technol. 2018, 72, 199–207. [Google Scholar] [CrossRef]
- Ren, F.; Yu, B.; Dong, D.; Hou, Z.; Cui, B. Rheological, thermal and microstructural properties of whey protein isolate-modified cassava starch mixed gels at different pH values. Int. J. Food Sci. Tech. 2017, 52, 2445–2454. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramaswamy, H.S. Dynamic rheology and thermal transitions in meat-based strained baby foods. J. Food Eng. 2007, 78, 1274–1284. [Google Scholar] [CrossRef]
- Cui, B.; Lu, Y.; Tan, C.; Wang, G.; Li, G.H. Effect of cross-linked acetylated starch content on the structure and stability of set yoghurt—ScienceDirect. Food Hydrocoll. 2014, 35, 576–582. [Google Scholar] [CrossRef]
- Prasanna, P.; Grandison, A.S.; Charalampopoulos, D. Microbiological, chemical and rheological properties of low-fat set yoghurt produced with exopolysaccharide (EPS) producing Bifidobacterium strains. Food Res. Int. 2013, 51, 15–22. [Google Scholar] [CrossRef]
- Laiho, S.; Williams, R.P.W.; Poelman, A.; Appelqvist, I.; Logan, A. Effect of whey protein phase volume on the tribology, rheology and sensory properties of fat-free stirred yoghurts. Food Hydrocoll. 2017, 67, 166–177. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Qi, Y.; Wu, D.; Liu, X.; Fang, L.; Min, W. Impact of Auricularia cornea var. Li polysaccharides on the physicochemical, textual, flavor, and antioxidant properties of set yogurt. Int. J. Biol. Macromol. 2022, 206, 148–158. [Google Scholar] [CrossRef]
- Benmeziane, F.; Raigar, R.K.; Ayat, E.H.; Aoufi, D.; Chala, A. Lentil (Lens culinaris) flour addition to Yogurt: Impact on physicochemical, microbiological and sensory attributes during refrigeration storage and microstructure changes. LWT Food Sci. Technol. 2020, 140, 110793. [Google Scholar] [CrossRef]
- Srisuvor, N.; Chinprahast, N.; Prakitchaiwattana, C.; Subhimaro, S. Effects of inulin and polydextrose on physicochemical and sensory properties of low-fat set yoghurt with probiotic-cultured banana purée. LWT 2013, 51, 30–36. [Google Scholar] [CrossRef]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. Int. J. Dairy 2010, 59, 216–221. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends Food Sci. Technol. 2016, 56, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Andic, S.; Boran, G.; Tunçtürk, Y. Effects of carboxyl methyl cellulose and edible cow gelatin on physico-chemical, textural and sensory properties of yoghurt. Int. J. Agric. Biol. 2013, 15, 245–251. [Google Scholar]
- Sahan, N.; Yasar, K.; Hayaloglu, A.A. Physical, chemical and flavour quality of non-fat yogurt as affected by a β-glucan hydrocolloidal composite during storage. Food Hydrocoll. 2008, 22, 1291–1297. [Google Scholar] [CrossRef]
- Sekhavatizadeh, S.; Sadeghzadehfar, S. The effect of guar gum as the fat substitute on some sensory and chemical properties of low-fat yogurt. Neuropharmacology. 2013, 66, 242–252. [Google Scholar]
- Pang, Z.; Xu, R.; Luo, T.; Che, X.; Bansal, N.; Liu, X. Physiochemical properties of modified starch under yogurt manufacturing conditions and its relation to the properties of yogurt. J. Food Eng. 2019, 245, 11–17. [Google Scholar] [CrossRef]
- Rosseto, H.C.; Toledo, L.; Santos, R.; Francisco, L.; Bruschi, M.L. Design of propolis-loaded film forming systems for topical administration: The effect of acrylic acid derivative polymers. J. Mol. Liq. 2020, 322, 114514. [Google Scholar] [CrossRef]
- Akkaya, S.; Ozel, B.; Oztop, M.H.; Yanik, D.K.; Gogus, F. Physical characterization of high methoxyl pectin and sunflower oil wax emulsions: A low-field 1H NMR relaxometry study. J. Food Sci. 2020, 86, 120–128. [Google Scholar] [CrossRef]
- Choi, H.M.; Yoo, B. Steady and dynamic shear rheology of sweet potato starch-xanthan gum mixtures. Food Chem. 2009, 116, 638–643. [Google Scholar] [CrossRef]
- Zhao, Z.; Corredig, M. Colloidal properties of casein micelles suspensions as a function of pH during concentration by osmotic stressing. Food Hydrocoll. 2016, 60, 445–452. [Google Scholar] [CrossRef]
- Zhao, Z.; Corredig, M. Influence of sodium chloride on the colloidal and rennet coagulation properties of concentrated casein micelle suspensions. J. Dairy Sci. 2016, 99, 6036–6045. [Google Scholar] [CrossRef]
- Zhi, Y.; Yang, H.; Yang, H. Effects of sucrose addition on the rheology and microstructure of κ-carrageenan gel. Food Hydrocoll. 2018, 99, 164–173. [Google Scholar]
- Zhang, Y.; Li, Y.; Wang, P.; Tian, Y.; Liang, Q.; Ren, F. Rennet-induced coagulation properties of yak casein micelles: A comparison with cow casein micelles. Food Res. Int. 2017, 102, 25. [Google Scholar] [CrossRef]
- Zhang, S.; Hsieh, F.H.; Vardhanabhuti, B. Acid-induced gelation properties of heated whey protein–pectin soluble complex (Part I): Effect of initial pH. Food Hydrocoll. 2014, 36, 76–84. [Google Scholar] [CrossRef]
- Tudorica, C.M.; Jones, T.E.R.; Kuri, V.; Brennan, C.S. The effects of refined barley β-glucan on the physico-structural properties of low-fat dairy products: Curd yield, microstructure, texture and rheology. J. Sci. Food Agr. 2010, 84, 1159–1169. [Google Scholar] [CrossRef]
- Lee, W.J.; Lucey, J.A. Rheological properties, whey separation, and microstructure in set-style yoghurt: Effects of heating temperature and incubation temperature. J. Texture Stud. 2007, 34, 515–536. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, R.; Li, J. Effects of the β-glucan, curdlan, on the fermentation performance, microstructure, rheological and textural properties of set yogurt. LWT 2020, 128, 109449. [Google Scholar] [CrossRef]
- Marcano, J.; Hernando, I.; Fiszman, S. In vitro measurements of intragastric rheological properties and their relationships with the potential satiating capacity of cheese pies with konjac glucomannan. Food Hydrocoll. 2015, 51, 16–22. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, Q. Encapsulation of konjac glucomannan in oil droplets to reduce viscosity of aqueous suspensions and gradually increase viscosity during simulated gastric digestion. J. Food Eng. 2016, 175, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Goff, H.D.; Chen, M.; Zhong, F. The hydration rate of konjac glucomannan after consumption affects its in vivo glycemic response and appetite sensation and in vitro digestion characteristics. Food Hydrocoll. 2021, 122, 107102. [Google Scholar] [CrossRef]
- Sow, L.C.; Peh, Y.R.; Pekerti, B.N.; Fu, C.; Bansal, N.; Yang, H. Nanostructural analysis and textural modification of tilapia fish gelatin affected by gellan and calcium chloride addition. LWT 2017, 85, 137–145. [Google Scholar] [CrossRef]

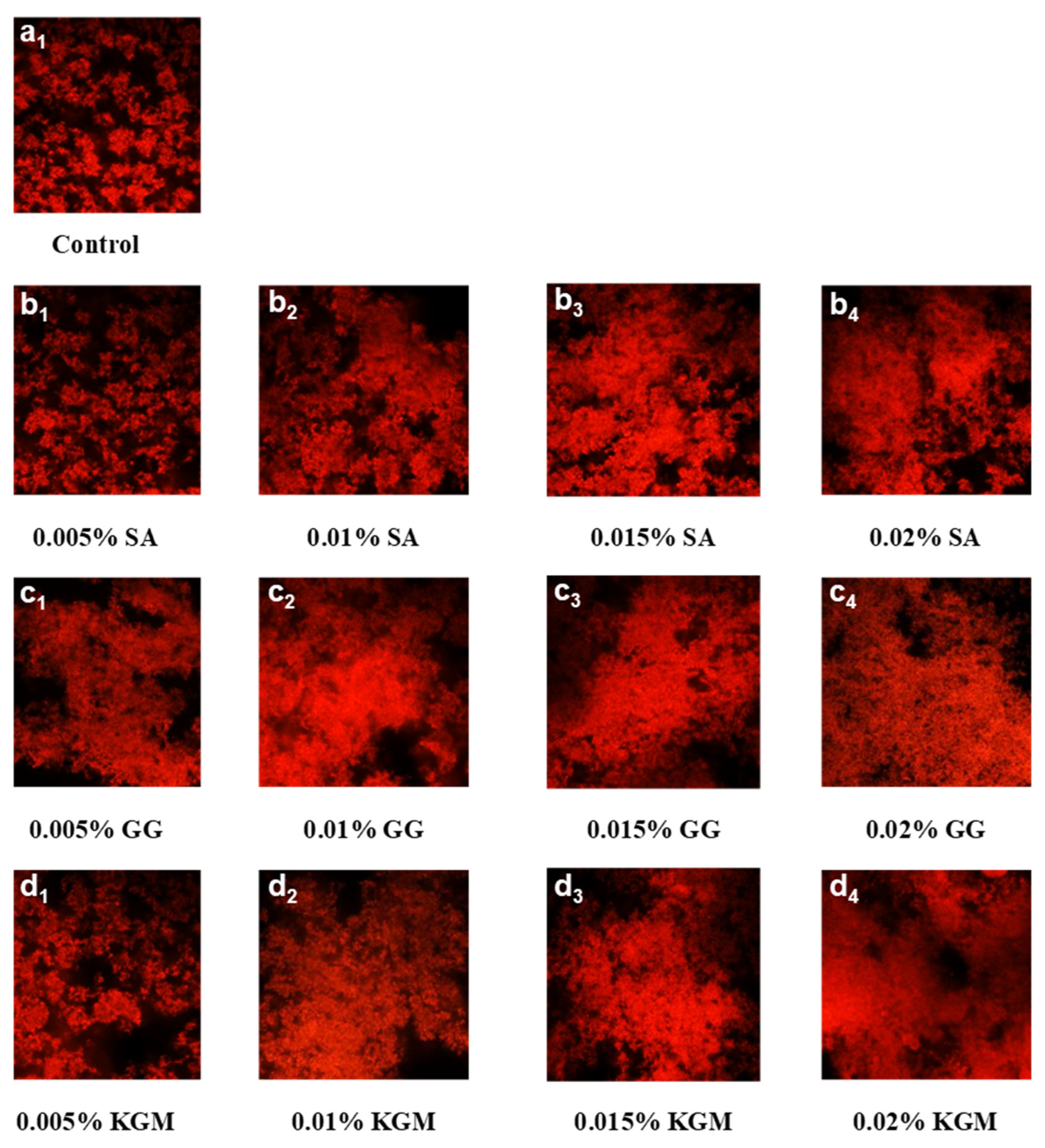
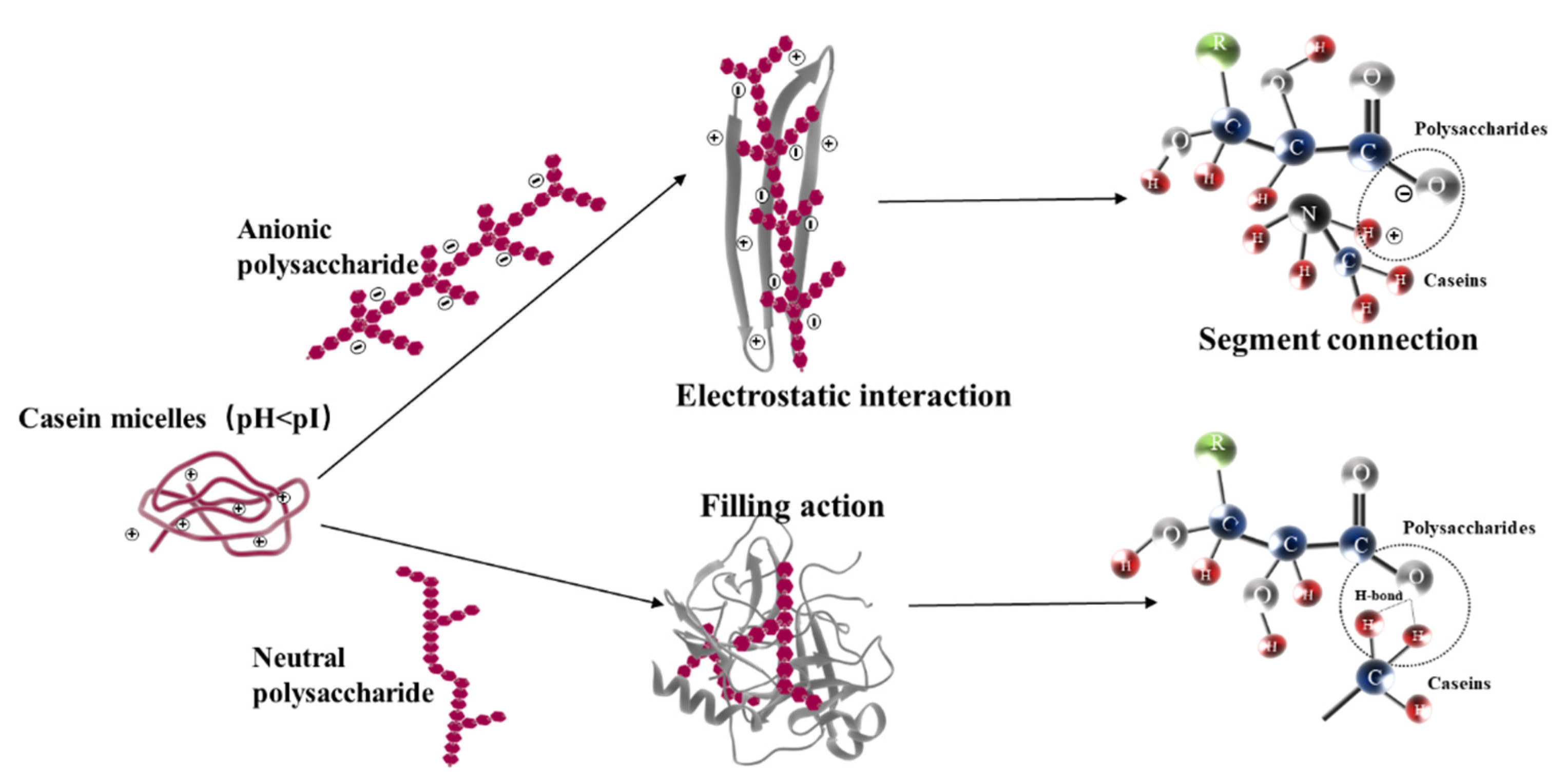
| Samples | Titratable Acidity (°T) | pH | TS (%) | TSS ns (°Brix) | WHC (%) | Firmness (N) | Rupture Distance (mm) | |
|---|---|---|---|---|---|---|---|---|
| Control | 96.79 ± 0.55 | 4.51 ± 0.03 c | 11.95 ± 0.09 b | 7.37 ±0.15 | 77.98 ± 0.89 c | 1.09 ± 0.08 | 2.23 ± 0.07 c | |
| SA | 0.005% | 96.84 ± 0.45 A | 4.64 ± 0.03 Aa | 11.99 ± 0.11 Bb | 7.31 ± 0.21 | 78.21 ± 0.57 bc | 1.11 ± 0.09 | 2.27 ± 0.07 Bc |
| 0.010% | 96.32 ± 0.33 | 4.60 ± 0.01 Aab | 12.07 ± 0.06 Bab | 7.35 ± 0.92 | 79.29 ± 0.32 b | 1.13 ± 0.10 | 2.39 ± 0.11 Bc | |
| 0.015% | 96.73 ± 0.51 A | 4.55 ± 0.03 bc | 12.16 ± 0.05 Ba | 7.38 ± 0.23 | 80.14 ± 0.98 ab | 1.14 ± 0.11 | 2.76 ± 0.03 Cb | |
| 0.020% | 96.71 ± 0.77 | 4.59 ± 0.02 b | 12.20 ± 0.03 Ba | 7.39 ± 0.10 | 80.85 ± 0.61 a | 1.12 ± 0.12 | 2.96 ± 0.13 Ba | |
| Control | 96.79 ± 0.55 | 4.51 ± 0.03 b | 11.95 ± 0.09 c | 7.37 ± 0.15 | 77.98 ± 0.89 c | 1.09 ± 0.08 | 2.23 ± 0.07 e | |
| GG | 0.005% | 95.89 ± 0.62 AB | 4.56 ± 0.03 Ba | 12.21 ± 0.06 Ab | 7.38 ± 0.25 | 78.37 ± 0.13 c | 1.17 ± 0.11 | 2.75 ± 0.04 Ad |
| 0.010% | 96.12 ± 0.78 | 4.54 ± 0.01 Bab | 12.25 ± 0.10 Aab | 7.40 ± 0.01 | 79.87 ± 0.27 b | 1.21 ± 0.17 | 2.99 ± 0.02 Ac | |
| 0.015% | 96.38 ± 0.41 AB | 4.57 ± 0.03 a | 12.29 ± 0.02 Aa | 7.43 ± 0.35 | 80.35 ± 0.45 b | 1.28 ± 0.35 | 3.21 ± 0.08 Ab | |
| 0.020% | 96.06 ± 0.57 | 4.58 ± 0.04 a | 12.34 ± 0.04 Aa | 7.45 ± 0.12 | 81.94 ± 1.18 a | 1.32 ± 0.10 | 3.48 ± 0.15 Aa | |
| Control | 96.79 ± 0.55 a | 4.51 ± 0.03 c | 11.95 ± 0.09 c | 7.37 ± 0.15 | 77.98 ± 0.89 c | 1.09 ± 0.08 b | 2.23 ± 0.07 c | |
| KGM | 0.005% | 95.21 ± 0.87 Bb | 4.56 ± 0.01 Bb | 12.01 ± 0.01 Bbc | 7.40 ± 0.45 | 78.01 ± 0.58 c | 1.14 ± 0.10 ab | 2.73 ± 0.01 Ab |
| 0.010% | 95.60 ± 0.59 b | 4.59 ± 0.01 Aab | 12.10 ± 0.04 Bb | 7.42 ± 0.95 | 79.54 ± 0.43 b | 1.16 ± 0.09 a | 2.82 ± 0.27 Aab | |
| 0.015% | 95.71 ± 0.04 Bb | 4.61 ± 0.04 a | 12.23 ± 0.05 ABa | 7.45 ± 0.31 | 79.87 ± 0.40 b | 1.20 ± 0.09 a | 2.97 ± 0.06 Ba | |
| 0.020% | 95.58 ± 0.28 b | 4.54 ± 0.02 bc | 12.28 ± 0.03 Aa | 7.47 ± 0.26 | 81.01 ± 0.61 a | 1.26 ± 0.08 a | 3.05 ± 0.12 Ba | |
| Sample | K | nns | R2 | K′ | n′ ns | R2 | K″ | n″ ns | R2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 14.09 ± 0.27 e | 0.98 ± 0.03 | 0.999 | 123.20 ± 6.98 d | 0.16 ± 0.08 | 0.993 | 31.54 ± 1.08 d | 0.12 ± 0.03 | 0.859 | |
| SA | 0.005% | 16.25 ± 0.13 Cd | 0.98 ± 0.05 | 0.999 | 144.64 ± 8.63 Bc | 0.19 ± 0.01 | 0.993 | 36.65 ± 1.16 Cc | 0.15 ± 0.01 | 0.888 |
| 0.010% | 17.85 ± 0.21 Cc | 0.99 ± 0.06 | 0.999 | 132.25 ± 9.13 Ccd | 0.17 ± 0.09 | 0.991 | 34.58 ± 1.21 Cc | 0.16 ± 0.04 | 0.913 | |
| 0.015% | 18.39 ± 0.14 Cb | 0.98 ± 0.10 | 0.999 | 188.80 ± 7.86 Bb | 0.18 ± 0.06 | 0.999 | 48.51 ± 1.56 Bb | 0.16 ± 0.08 | 0.897 | |
| 0.020% | 26.27 ± 0.32 Ba | 0.98 ± 0.13 | 0.999 | 227.51 ± 6.88 Ba | 0.17 ± 0.10 | 0.993 | 51.38 ± 1.79 Ba | 0.14 ± 0.05 | 0.885 | |
| Control | 14.09 ± 0.27 d | 0.98 ± 0.03 | 0.999 | 123.20 ± 6.98 c | 0.16 ± 0.08 | 0.993 | 31.54 ± 1.08 c | 0.12 ± 0.03 | 0.859 | |
| GG | 0.005% | 20.93 ± 0.28 Ac | 0.99 ± 0.05 | 0.998 | 167.88 ± 3.15 Bbc | 0.16 ± 0.08 | 0.997 | 44.39 ± 1.57 Bb | 0.15 ± 0.02 | 0.894 |
| 0.010% | 25.28 ± 0.24 Abc | 0.98 ± 0.14 | 0.999 | 169.51 ± 4.98 Bbc | 0.15 ± 0.04 | 0.997 | 43.27 ± 1.34 Bb | 0.15 ± 0.08 | 0.890 | |
| 0.015% | 29.05 ± 0.16 Ab | 0.99 ± 0.09 | 0.999 | 177.30 ± 3.09 Bb | 0.18 ± 0.04 | 0.997 | 45.88 ± 1.42 Bb | 0.16 ± 0.05 | 0.900 | |
| 0.020% | 50.59 ± 0.41 Aa | 0.96 ± 0.04 | 0.999 | 288.74 ± 6.08 Aa | 0.16 ± 0.05 | 0.998 | 66.76 ± 2.99 Aa | 0.14 ± 0.03 | 0.892 | |
| Control | 14.09 ± 0.27 e | 0.98 ± 0.03 | 0.999 | 123.20 ± 6.98 d | 0.16 ± 0.08 | 0.993 | 31.54 ± 1.08 d | 0.12 ± 0.03 | 0.859 | |
| KGM | 0.005% | 18.02 ± 0.11 Bd | 0.98 ± 0.05 | 0.999 | 208.31 ± 6.46 Ab | 0.16 ± 0.09 | 0.999 | 51.48 ± 2.03 Ab | 0.16 ± 0.02 | 0.923 |
| 0.010% | 20.38 ± 0.19 Bc | 0.98 ± 0.02 | 0.999 | 187.97 ± 5.65 Ac | 0.18 ± 0.03 | 0.997 | 48.24 ± 1.98 Ac | 0.16 ± 0.05 | 0.923 | |
| 0.015% | 23.07 ± 0.20 Bb | 0.97 ± 0.08 | 0.999 | 207.01 ± 7.82 Ab | 0.18 ± 0.02 | 0.998 | 53.48 ± 2.11 Ab | 0.16 ± 0.07 | 0.933 | |
| 0.020% | 29.46 ± 0.23 Ba | 0.99 ± 0.05 | 0.999 | 287.67 ± 8.33 Aa | 0.15 ± 0.05 | 0.998 | 64.95 ± 2.54 Aa | 0.14 ± 0.06 | 0.891 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Z.; Yin, D.; Li, Z.; Chen, X.; Dong, M. Effects of Commercial Polysaccharides Stabilizers with Different Charges on Textural, Rheological, and Microstructural Characteristics of Set Yoghurts. Foods 2022, 11, 1764. https://doi.org/10.3390/foods11121764
Ge Z, Yin D, Li Z, Chen X, Dong M. Effects of Commercial Polysaccharides Stabilizers with Different Charges on Textural, Rheological, and Microstructural Characteristics of Set Yoghurts. Foods. 2022; 11(12):1764. https://doi.org/10.3390/foods11121764
Chicago/Turabian StyleGe, Zhiwen, Dongjie Yin, Zhiyu Li, Xiaohong Chen, and Mingsheng Dong. 2022. "Effects of Commercial Polysaccharides Stabilizers with Different Charges on Textural, Rheological, and Microstructural Characteristics of Set Yoghurts" Foods 11, no. 12: 1764. https://doi.org/10.3390/foods11121764
APA StyleGe, Z., Yin, D., Li, Z., Chen, X., & Dong, M. (2022). Effects of Commercial Polysaccharides Stabilizers with Different Charges on Textural, Rheological, and Microstructural Characteristics of Set Yoghurts. Foods, 11(12), 1764. https://doi.org/10.3390/foods11121764





