Modification of Artichoke Dietary Fiber by Superfine Grinding and High-Pressure Homogenization and Its Protection against Cadmium Poisoning in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Chemical Analysis and Determination of Particle Size
2.4. Scanning Electron Microscopy
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Determination of Water-Holding Capacity (WHC)
2.7. Determination of Oil-Holding Capacity (OHC)
2.8. Total Negative Charge (TNC)
2.9. Determination of Heavy Metal Adsorption Capacity
2.10. Animals and Experimental Method
2.11. Determination of Plasma Transaminase (AST and ALT) Activities, CREA-S, UREA, Red Blood Cell Count, Hemoglobin Count, White Blood Cell Count and Neutrophil Ratio Levels
2.12. Determination of Glutathione Peroxidase (GSH-PX) Activity in Liver
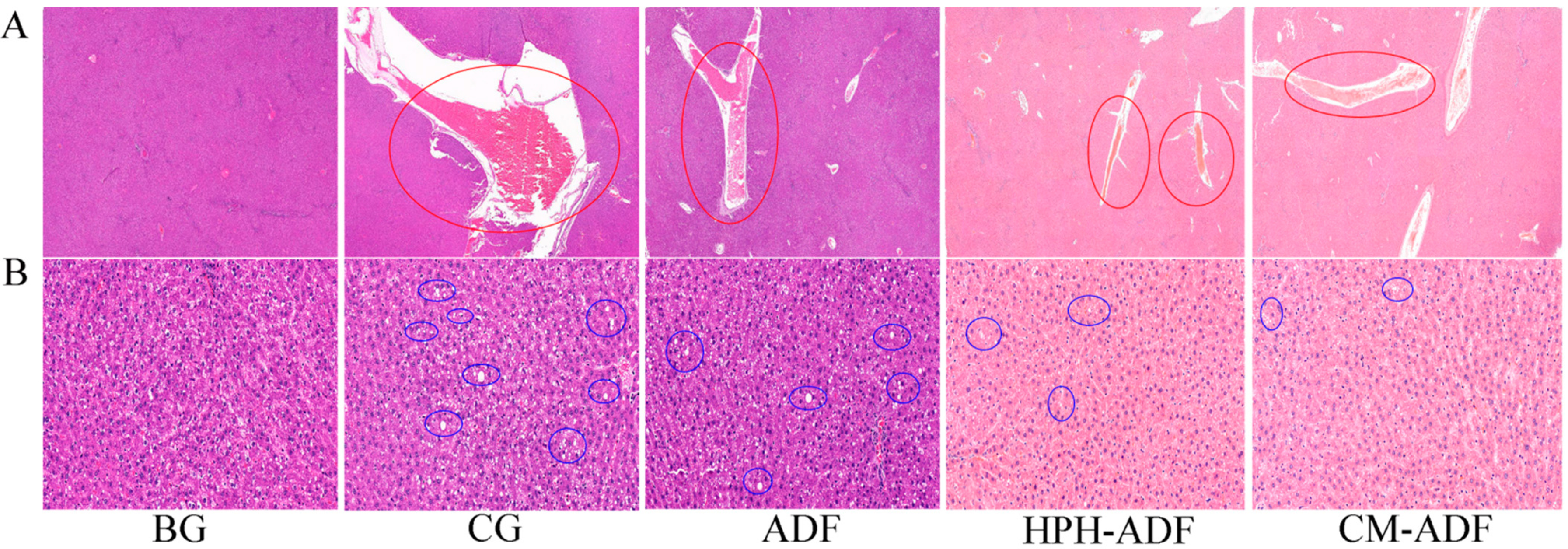
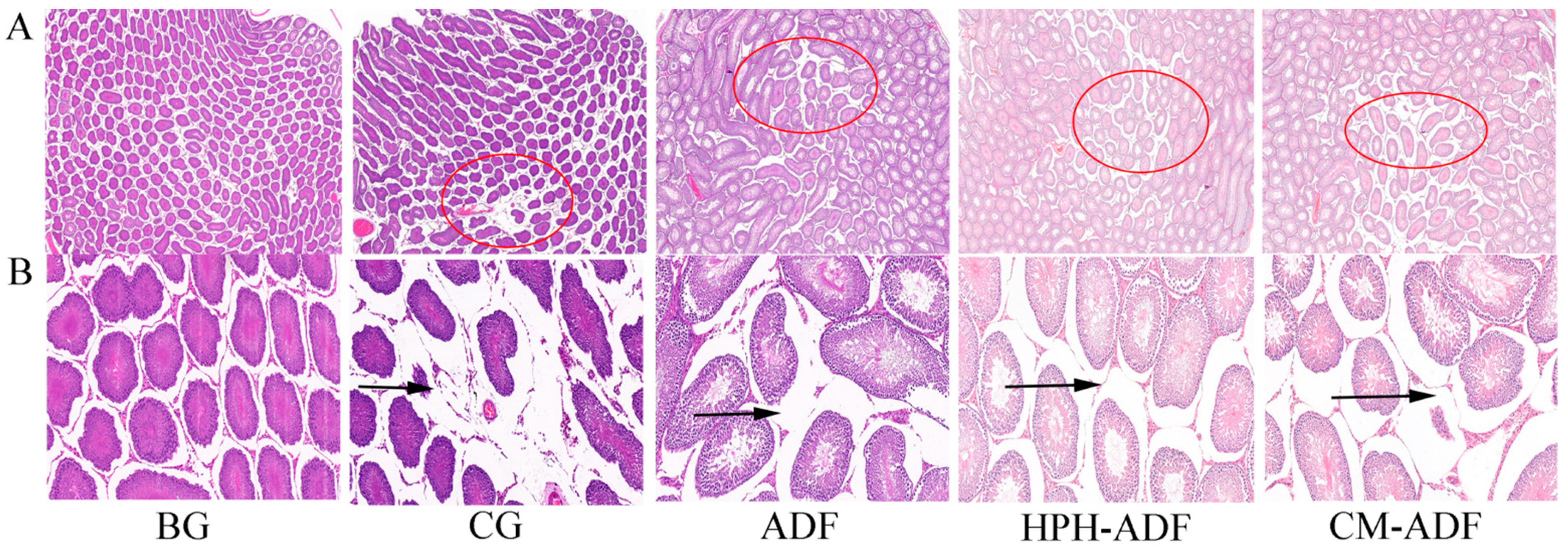
2.13. Histology and Case Analysis of Liver and Testis in Rats
2.14. Statistical Analysis
3. Results and Discussion
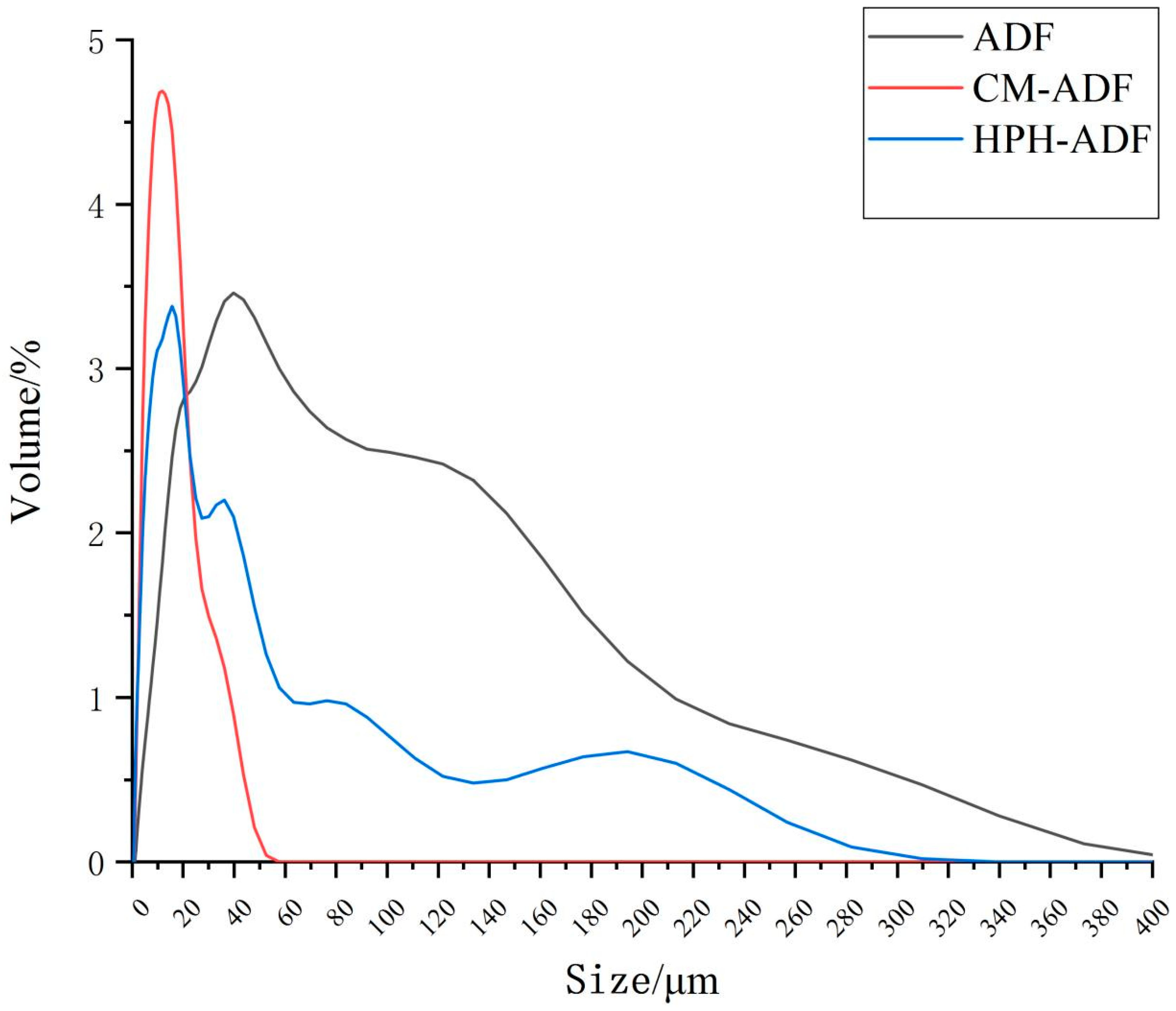
3.1. Chemical Analysis and Determination of ADF Particle Size
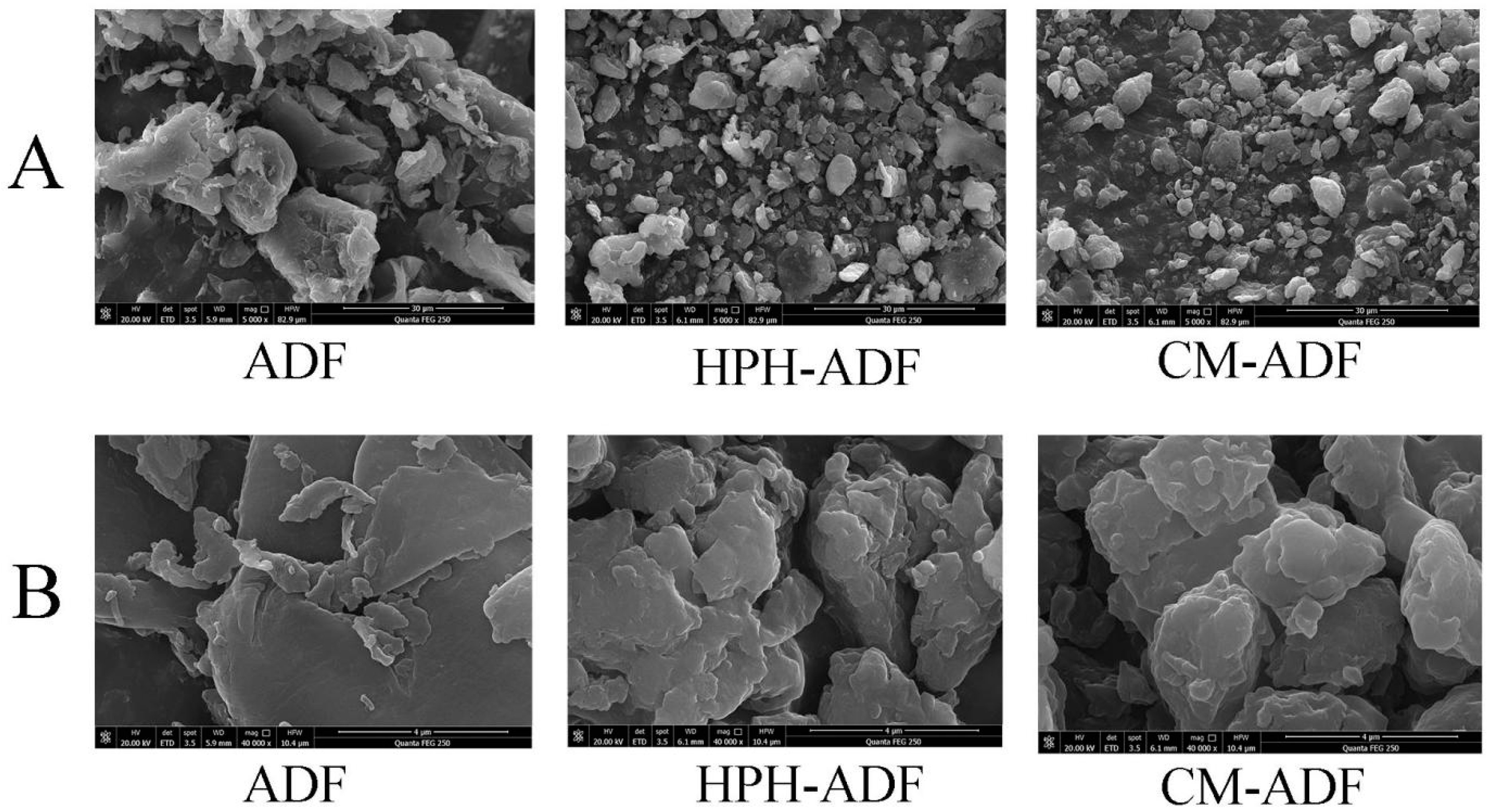
3.2. Scanning Electron Microscopy
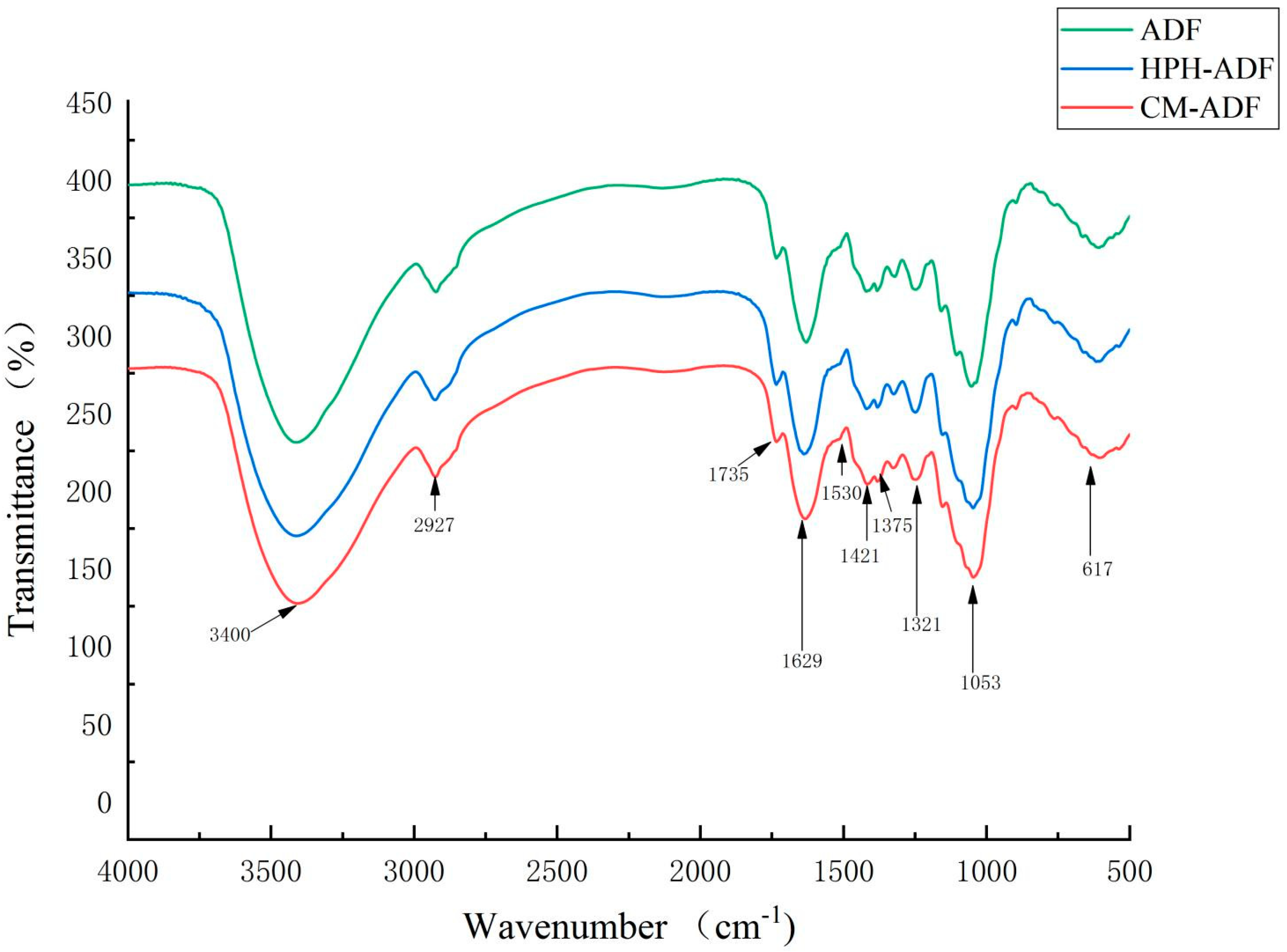
3.3. FTIR Spectra
3.4. WHC, OHC and TNC
3.5. Determination of Heavy Metal Adsorption Capacity
3.6. Effects of Artichoke Dietary Fiber on Serum AST and ALT Levels, Creatinine, Urea and Blood Routine Indices in Rats
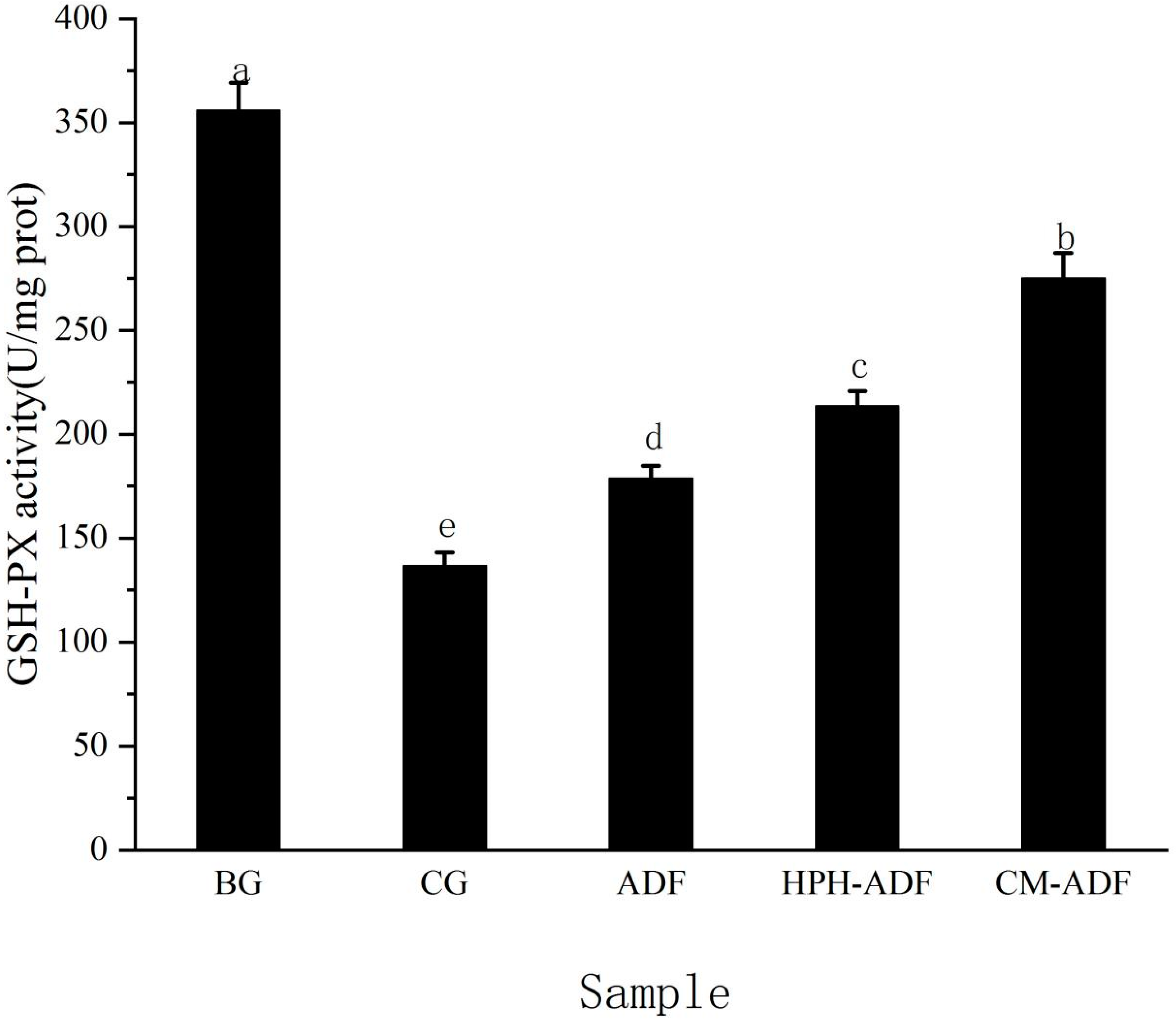
3.7. Effects of ADF on GSH-PX Activity in Rat Liver
3.8. Protective Effect of ADF on Liver Tissue in Rats
3.9. Protective Effect of ADF on Testicular Tissue in Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | Artichoke dietary fiber |
| SDF | Soluble dietary fiber |
| IDF | Insoluble dietary fiber |
| HPH-ADF | High-pressure homogenization of artichoke dietary fiber |
| CM-ADF | Compound modification of artichoke dietary fiber |
| HPH | High-pressure homogenization |
| SP | Superfine grinding |
| CM | Compound modification |
| BG | Blank group |
| CG | Control group |
| GSH-PX | Glutathione peroxidase |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| TNC | Total negative charge |
| OHC | Oil-holding capacity |
| WHC | Water-holding capacity |
References
- Grabowska, A.; Caruso, G.; Mehrafarin, A.; Kalisz, A.; Gruszecki, R.; Kunicki, E.; Sękara, A. Application of modern agronomic and biotechnological strategies to valorise worldwide globe artichoke (Cynara cardunculus L.) potential—An analytical overview. Ital. J. Agron. 2018, 13, 279–289. [Google Scholar] [CrossRef]
- Villanueva-Suárez, M.J.; Mateos-Aparicio, I.; Pérez-Cózar, M.L.; Yokoyama, W.; Redondo-Cuenca, A. Hypolipidemic effects of dietary fibre from an artichoke by-product in Syrian hamsters. J. Funct. Foods 2019, 56, 156–162. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Picciarelli, P.; Martelloni, L.; Sbrana, C.; Giovannetti, M. Globe artichoke as a functional food. Mediterr. J. Nutr. Metab. 2010, 3, 197–201. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Ntatsi, G.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and chemical composition of Greek artichoke genotypes. Food Chem. 2018, 267, 296–302. [Google Scholar] [CrossRef]
- Renna, M.; Signore, A.; Paradiso, V.M.; Santamaria, P. Faba Greens, Globe Artichoke’s Offshoots, Crenate Broomrape and Summer Squash Greens: Unconventional Vegetables of Puglia (Southern Italy) With Good Quality Traits. Front. Plant Sci. 2018, 9, 378. [Google Scholar] [CrossRef]
- Huang, S.; He, Y.; Zou, Y.; Liu, Z. Modification of insoluble dietary fibres in soya bean okara and their physicochemical properties. Int. J. Food Sci. Technol. 2015, 50, 2606–2613. [Google Scholar] [CrossRef]
- Chater, P.I.; Wilcox, M.D.; Pearson, J.P.; Brownlee, I.A. The impact of dietary fibres on the physiological processes governing small intestinal digestive processes. Bioact. Carbohydr. Diet. Fibre 2015, 6, 117–132. [Google Scholar] [CrossRef]
- Raninen, K.; Lappi, J.; Mykkänen, H.; Poutanen, K. Dietary fiber type reflects physiological functionality: Comparison of grain fiber, inulin, and polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef]
- Fissore, E.N.; Santo Domingo, C.; Gerschenson, L.N.; Giannuzzi, L. A study of the effect of dietary fiber fractions obtained from artichoke (Cynara cardunculus L. var. scolymus) on the growth of intestinal bacteria associated with health. Food Funct. 2015, 6, 1667–1674. [Google Scholar] [CrossRef]
- Wei, B.; Cai, C.; Xu, B.; Jin, Z.; Tian, Y. Disruption and molecule degradation of waxy maize starch granules during high pressure homogenization process. Food Chem. 2018, 240, 165–173. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Q.; Yang, Y.; He, W. Effect of high pressure homogenization on sugar beet pulp: Rheological and microstructural properties. LWT 2020, 125, 109245. [Google Scholar] [CrossRef]
- Spotti, M.J.; Campanella, O.H. Functional modifications by physical treatments of dietary fibers used in food formulations. Curr. Opin. Food Sci. 2017, 15, 70–78. [Google Scholar] [CrossRef]
- Xie, F.; Li, M.; Lan, X.; Zhang, W.; Gong, S.; Wu, J.; Wang, Z. Modification of dietary fibers from purple-fleshed potatoes (Heimeiren) with high hydrostatic pressure and high pressure homogenization processing: A comparative study. Innov. Food Sci. Emerg. 2017, 42, 157–164. [Google Scholar] [CrossRef]
- Hua, X.; Xu, S.; Wang, M.; Chen, Y.; Yang, H.; Yang, R. Effects of high-speed homogenization and high-pressure homogenization on structure of tomato residue fibers. Food Chem. 2017, 232, 443–449. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Superfine grinding improves functional properties and antioxidant capacities of bran dietary fibre from Qingke (hull-less barley) grown in Qinghai-Tibet Plateau, China. J. Cereal Sci. 2015, 65, 43–47. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Zhang, L.; Meng, Q. Effects of superfine grinding on asparagus pomace. Part I: Changes on physicochemical and functional properties. J. Food Sci. 2020, 85, 1827–1833. [Google Scholar] [CrossRef]
- Ma, S.; Ren, B.; Diao, Z.; Chen, Y.; Qiao, Q.; Liu, X. Physicochemical properties and intestinal protective effect of ultra-micro ground insoluble dietary fibre from carrot pomace. Food Funct. 2016, 7, 3902–3909. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.; Wu, J.; Wang, Z. Functional Properties and Morphological Characters of Soluble Dietary Fibers in Different Edible Parts ofAngelica Keiskei. J. Food Sci. 2016, 81, C2189–C2198. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, Y.; Pei, Y.; Gao, W.; Zhang, Z. Effect of Process on Physicochemical Properties of Oat Bran Soluble Dietary Fiber. J. Food Sci. 2009, 74, C628–C636. [Google Scholar] [CrossRef]
- Marshall, W.E.; Wartelle, L.H.; Boler, D.E.; Johns, M.M.; Toles, C.A. Enhanced metal adsorption by soybean hulls modified with citric acid. Bioresour. Technol. 1999, 69, 263–268. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef]
- Zhu, K.; Huang, S.; Peng, W.; Qian, H.; Zhou, H. Effect of ultrafine grinding on hydration and antioxidant properties of wheat bran dietary fiber. Food Res. Int. 2010, 43, 943–948. [Google Scholar] [CrossRef]
- Clarke, A.; Prescott, T.; Khan, A.; Olabi, A.G. Causes of breakage and disruption in a homogeniser. Appl. Energ. 2010, 87, 3680–3690. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Wang, X.; Meng, Q. Recent advances in processing food powders by using superfine grinding techniques: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2222–2255. [Google Scholar] [CrossRef]
- Yan, L.; Li, T.; Liu, C.; Zheng, L. Effects of high hydrostatic pressure and superfine grinding treatment on physicochemical/functional properties of pear pomace and chemical composition of its soluble dietary fibre. LWT 2019, 107, 171–177. [Google Scholar] [CrossRef]
- Ulbrich, M.; Flöter, E. Impact of high pressure homogenization modification of a cellulose based fiber product on water binding properties. Food Hydrocoll. 2014, 41, 281–289. [Google Scholar] [CrossRef]
- Wang, H.; Huang, T.; Tu, Z.C.; Ruan, C.Y.; Lin, D. The adsorption of lead (II) ions by dynamic high pressure micro-fluidization treated insoluble soybean dietary fiber. J. Food Sci. Technol. 2016, 53, 2532–2539. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Q.; Fang, D.; Zhuang, W.; Chen, C.; Jiang, W.; Zheng, Y. Modification of insoluble dietary fibers from bamboo shoot shell: Structural characterization and functional properties. Int. J. Biol. Macromol. 2018, 120, 1461–1467. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Ullah, I.; Yin, T.; Xiong, S.; Zhang, J.; Din, Z.; Zhang, M. Structural characteristics and physicochemical properties of okara (soybean residue) insoluble dietary fiber modified by high-energy wet media milling. LWT Food Sci. Technol. 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Chau, C.; Wang, Y.; Wen, Y. Different micronization methods significantly improve the functionality of carrot insoluble fibre. Food Chem. 2007, 100, 1402–1408. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Zeng, W.; Huang, Y.; Yang, X. Properties of dietary fiber from citrus obtained through alkaline hydrogen peroxide treatment and homogenization treatment. Food Chem. 2020, 311, 125873. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, J. Effect of ultrafine grinding on physicochemical and antioxidant properties of dietary fiber from wine grape pomace. Food Sci. Technol. Int. 2014, 20, 55–62. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, J.; Ren, M.; Zhang, X.; Wang, L. Modification of insoluble dietary fiber from rice bran with dynamic high pressure microfluidization: Cd(II) adsorption capacity and behavior. Innov. Food Sci. Emerg. 2021, 73, 102765. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, C.; Ou, S. In vitro binding capacities of three dietary fibers and their mixture for four toxic elements, cholesterol, and bile acid. J. Hazard Mater. 2011, 186, 236–239. [Google Scholar] [CrossRef]
- Koguchi, T.; Koguchi, H.; Nakajima, H.; Takano, S.; Yamamoto, Y.; Innami, S.; Maekawa, A.; Tadokoro, T. Dietary fiber suppresses elevation of uric acid and urea nitrogen concentrations in serum of rats with renal dysfunction induced by dietary adenine. Int. J. Vitam. Nutr. Res. 2004, 74, 253–263. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Mitchell, S.; Sahye-Pudaruth, S.; Coveney, J.; Olowoyeye, O.; Maraj, T.; Patel, D.; de Souza, R.J.; et al. Low-glycaemic index diet to improve glycaemic control and cardiovascular disease in type 2 diabetes: Design and methods for a randomised, controlled, clinical trial. BMJ Open 2016, 6, e12220. [Google Scholar] [CrossRef]
| Group | Number of Rats | Feeding | Drinking Water |
|---|---|---|---|
| BG | 5 | Basal diet | Deionized water |
| CG | 7 | Basal diet | 50 mg/L aqueous solution of CdCl2 |
| ADF | 7 | ADF special diet | 50 mg/L aqueous solution of CdCl2 |
| HPH-ADF | 7 | HPH-ADF special diet | 50 mg/L aqueous solution of CdCl2 |
| CM-ADF | 7 | CM-ADF special diet | 50 mg/L aqueous solution of CdCl2 |
| Sample | SDF (g/100 g) | IDF (g/100 g) | TDF (g/100 g) |
|---|---|---|---|
| ADF | 6.45 ± 1.26 d | 79.7 ± 1.36 a | 86.2 ± 1.33 b |
| HPH-ADF | 15.1 ± 1.38 b | 71 ± 0.98 c | 85.9 ± 1.25 d |
| CM-ADF | 21 ± 0.56 a | 66 ± 0.89 d | 86 ± 0.77 a |
| Sample | WHC (g/g) | OHC (g/g) | TNC (mmol/g) | SDF Content (%) |
|---|---|---|---|---|
| ADF | 5.8 ± 0.046 a | 2.3 ± 0.14 a | 2 ± 0.08 a | 7.6 ± 0.24 c |
| HPH-ADF | 3.6 ± 0.15 b | 2.8 ± 0.0.19 bc | 2.9 ± 0.15 b | 11 ± 0.32 b |
| CM-ADF | 2.9 ± 0.14 c | 3.2 ± 0.16 c | 5.3 ± 0.13 c | 14 ± 0.32 a |
| Sample | Cd (II)/(mmol/g) | Cu (II)/(mmol/g) | Pb (II)/(mmol/g) | Hg (II)/(mmol/g) | ||||
|---|---|---|---|---|---|---|---|---|
| pH2 | pH7 | pH2 | pH7 | pH2 | pH7 | pH2 | pH7 | |
| ADF | 3.5 ± 0.19 d | 12 ± 0.62 d | 2.9 ± 0.72 d | 12 ± 0.09 d | 12 ± 0.62 c | 24 ± 0.14 d | 3.1 ± 0.17 c | 10 ± 0.07 d |
| HPH-ADF | 4.0 ± 0.16 b | 24 ± 0.35 b | 6.9 ± 0.18 b | 19 ± 0.33 b | 13 ± 0.44 b | 41 ± 0.42 b | 4.1 ± 0.38 b | 21 ± 0.59 b |
| CM-ADF | 4.6 ± 0.26 a | 30 ± 0.46 a | 7.6 ± 0.43 a | 25 ± 0.13 a | 13 ± 0.37 a | 44 ± 0.22 a | 4.7 ± 0.24 a | 27 ± 0.12 a |
| Group | AST (U/L) | ALT (U/L) | Creatinine (μmol/L) | Urea (mmol/L) |
|---|---|---|---|---|
| BG | 42.6 ± 2.11 a | 114.4 ± 5.37 a | 37.2 ± 3.42 a | 5.3 ± 0.25 a |
| CG | 122 ± 5.84 e | 290 ± 9.20 e | 92.4 ± 4.93 e | 12 ± 0.52 e |
| ADF | 105 ± 2.71 d | 263 ± 6.79 d | 79.0 ± 4.04 d | 10 ± 0.47 d |
| HPH-ADF | 84.0 ± 2.27 c | 220 ± 10.8 c | 66.6 ± 5.22 c | 8.2 ± 0.39 c |
| CM-ADF | 73.0 ± 6.89 b | 152 ± 7.83 b | 54.43 ± 4.16 b | 6.5 ± 0.29 b |
| Group | Red Blood Cells (1012/L) | White Blood Cells (109/L) | Neutrophil Proportion (%) | Hemoglobin Count (g/L) |
|---|---|---|---|---|
| BG | 9.9 ± 0.95 a | 2.28 ± 1.02 a | 20.3 ± 7.22 a | 159 ± 8.84 b |
| CG | 7.94 ± 1.77 ab | 4.36 ± 1.79 ab | 29.2 ± 8.54 ab | 110 ± 3.53 a |
| ADF | 8.9 ± 0.77 ab | 3.99 ± 1.65 ab | 28.0 ± 6.36 ab | 113 ± 9.39 c |
| HPH-ADF | 8.8 ± 0.83 ab | 2.6 ± 0.93 bc | 27.0 ± 1.81 b | 119 ± 6.37 ab |
| CM-ADF | 8.79 ± 1.64 b | 2.6 ± 0.63 c | 25.6 ± 3.29 b | 152 ± 8.73 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Xu, T.; He, B.; Wang, Y.; Zhang, L.; Huang, L. Modification of Artichoke Dietary Fiber by Superfine Grinding and High-Pressure Homogenization and Its Protection against Cadmium Poisoning in Rats. Foods 2022, 11, 1716. https://doi.org/10.3390/foods11121716
Zhu R, Xu T, He B, Wang Y, Zhang L, Huang L. Modification of Artichoke Dietary Fiber by Superfine Grinding and High-Pressure Homogenization and Its Protection against Cadmium Poisoning in Rats. Foods. 2022; 11(12):1716. https://doi.org/10.3390/foods11121716
Chicago/Turabian StyleZhu, Renwei, Tianhui Xu, Bian He, Yayi Wang, Linwei Zhang, and Liang Huang. 2022. "Modification of Artichoke Dietary Fiber by Superfine Grinding and High-Pressure Homogenization and Its Protection against Cadmium Poisoning in Rats" Foods 11, no. 12: 1716. https://doi.org/10.3390/foods11121716
APA StyleZhu, R., Xu, T., He, B., Wang, Y., Zhang, L., & Huang, L. (2022). Modification of Artichoke Dietary Fiber by Superfine Grinding and High-Pressure Homogenization and Its Protection against Cadmium Poisoning in Rats. Foods, 11(12), 1716. https://doi.org/10.3390/foods11121716






