Metabolomic Characterization of Pigmented and Non-Pigmented Potato Cultivars Using a Joint and Individual Variation Explained (JIVE)
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Potato Materials
2.3. Steam Cooking Method
2.4. Antioxidants, Total Polyphenolic Content, Polyphenols, and Sugars Extraction Method
2.5. Anthocyanin Extraction Method
2.6. Spectrophotometric Assays
2.6.1. Total Polyphenolic Content (TPC)
2.6.2. Antioxidant Activity, ABTS Assay
2.6.3. Antioxidant Activity, FRAP Assay
2.6.4. Total Monomeric Content and Total Anthocyanins Content (Anto Mono and Anto Tot)
2.7. Analysis of Polyphenolic Compounds
2.8. Analysis of Sugar Compounds
2.9. Analysis of Anthocyanins
2.10. Datasets
2.11. JIVE, a Data Fusion Approach
3. Results and Discussion
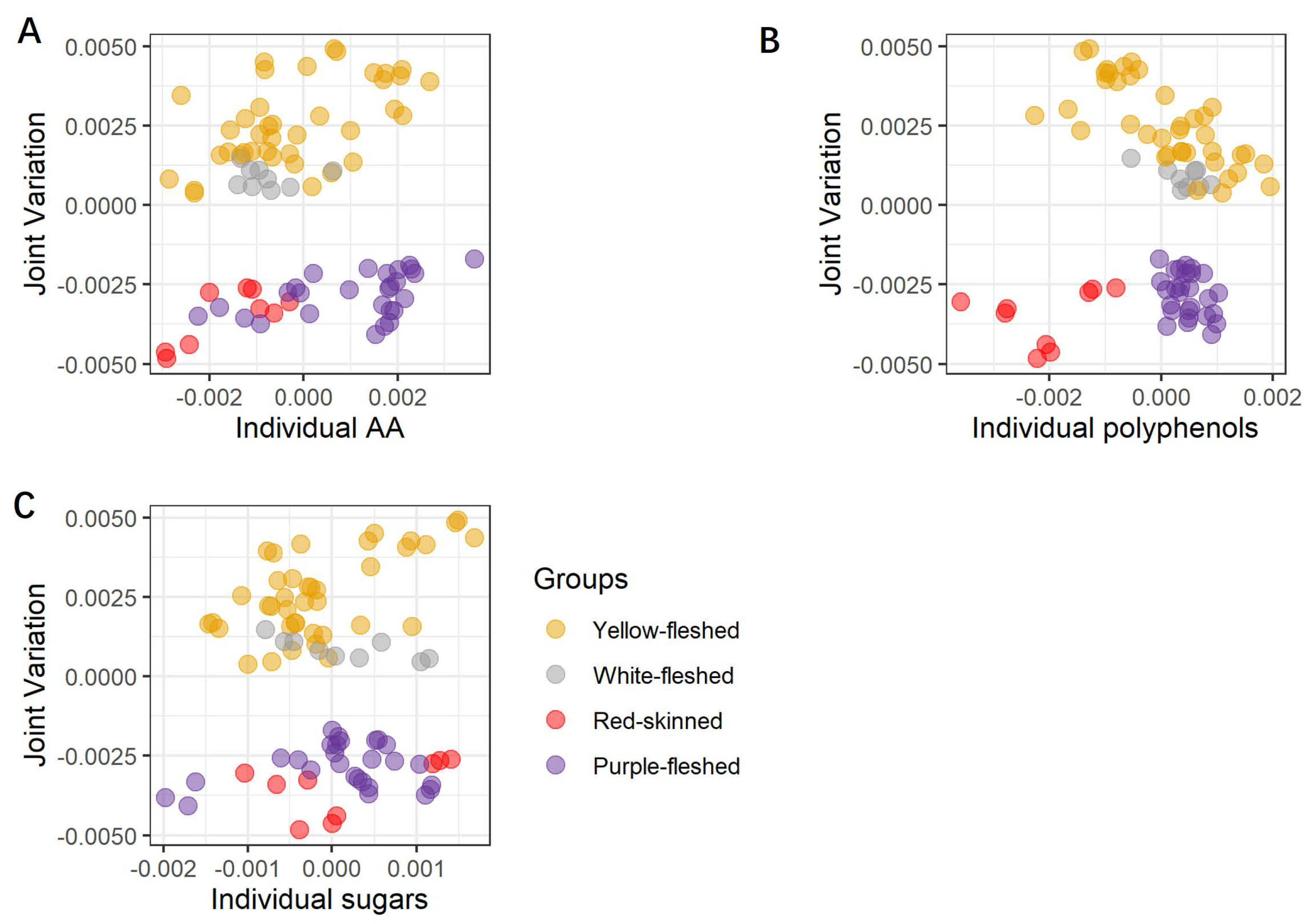
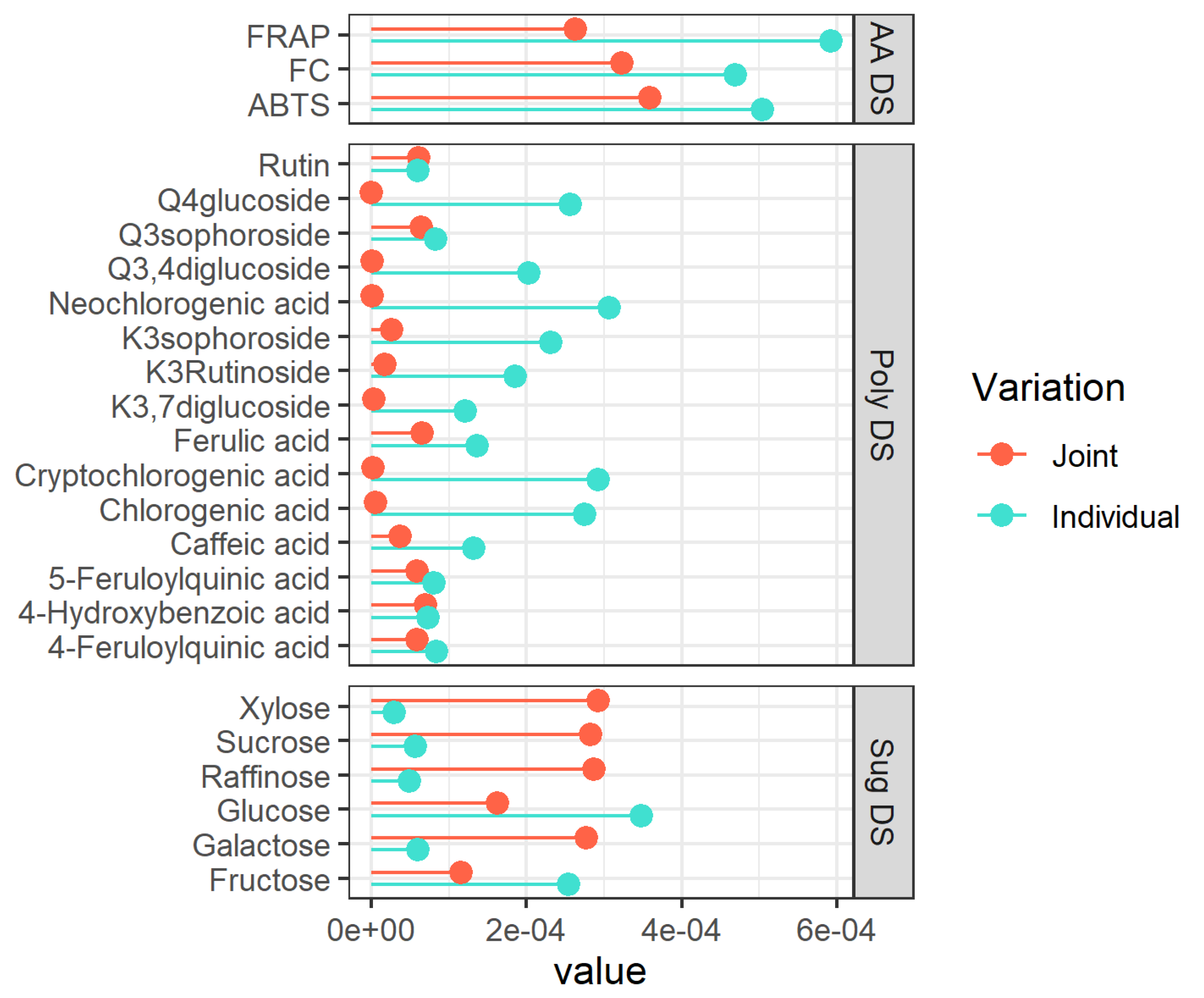
3.1. Interpretation of JIVE Model
3.2. Individual Metabolites
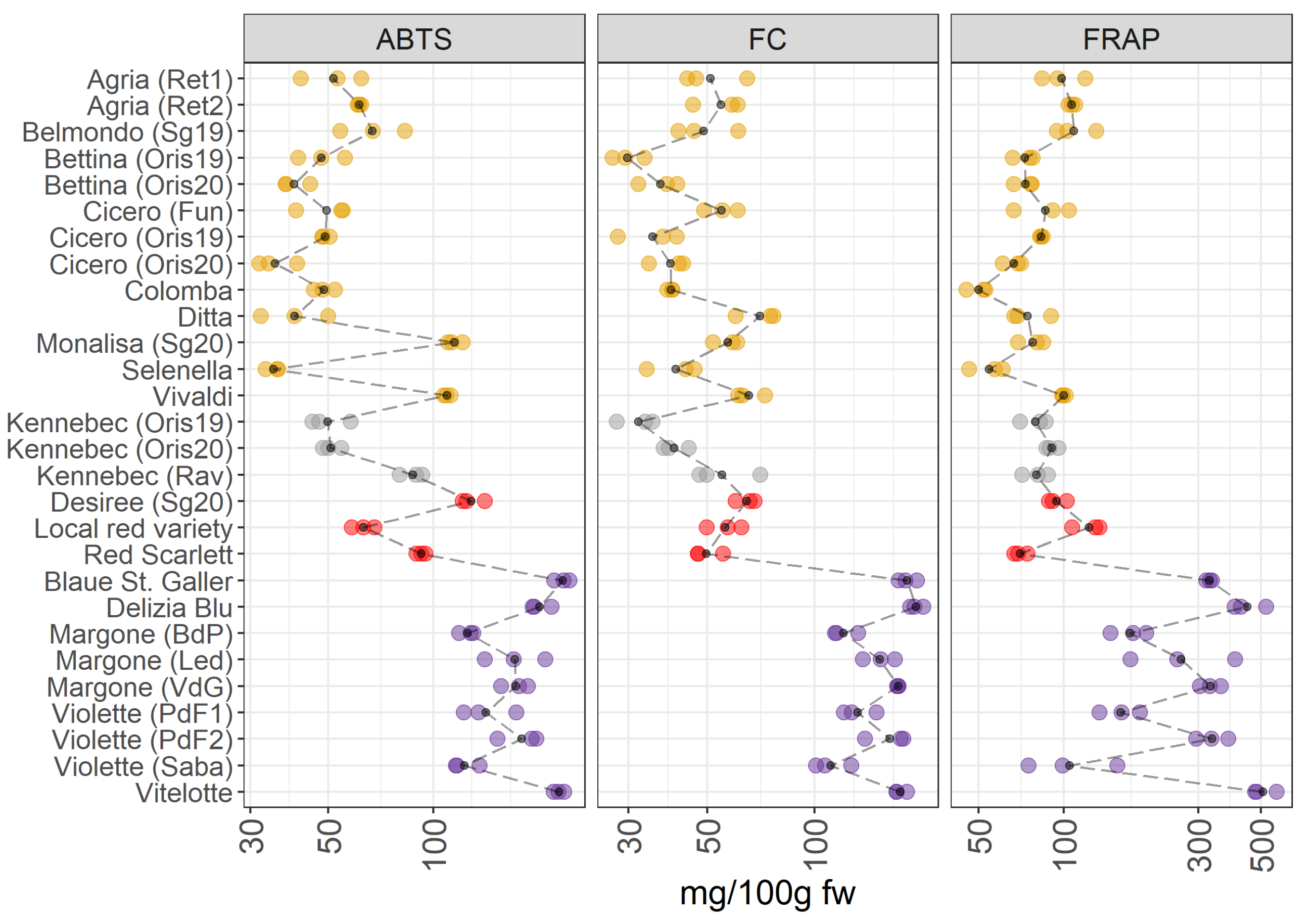
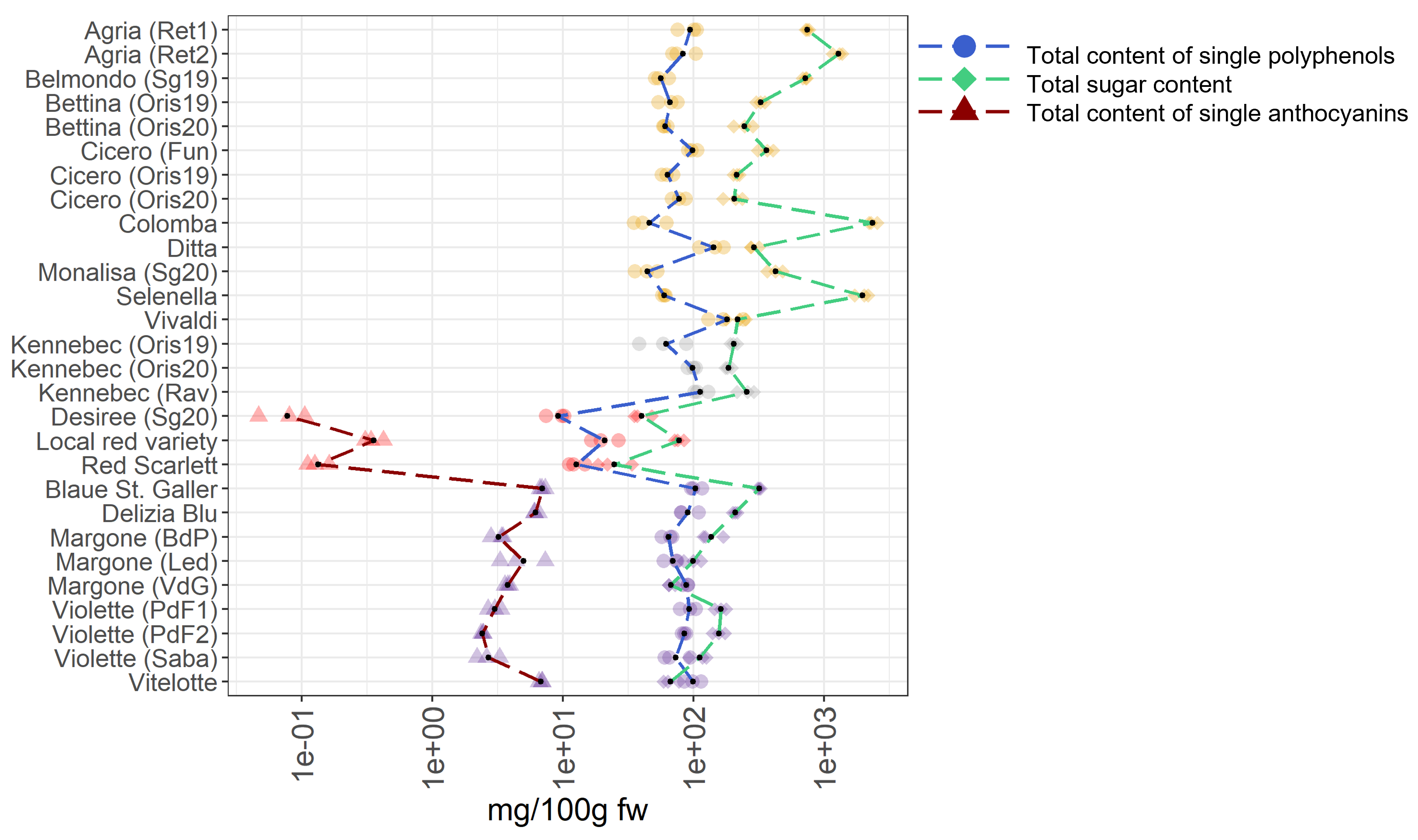
3.2.1. Antioxidant Activities and Total Polyphenolic Content
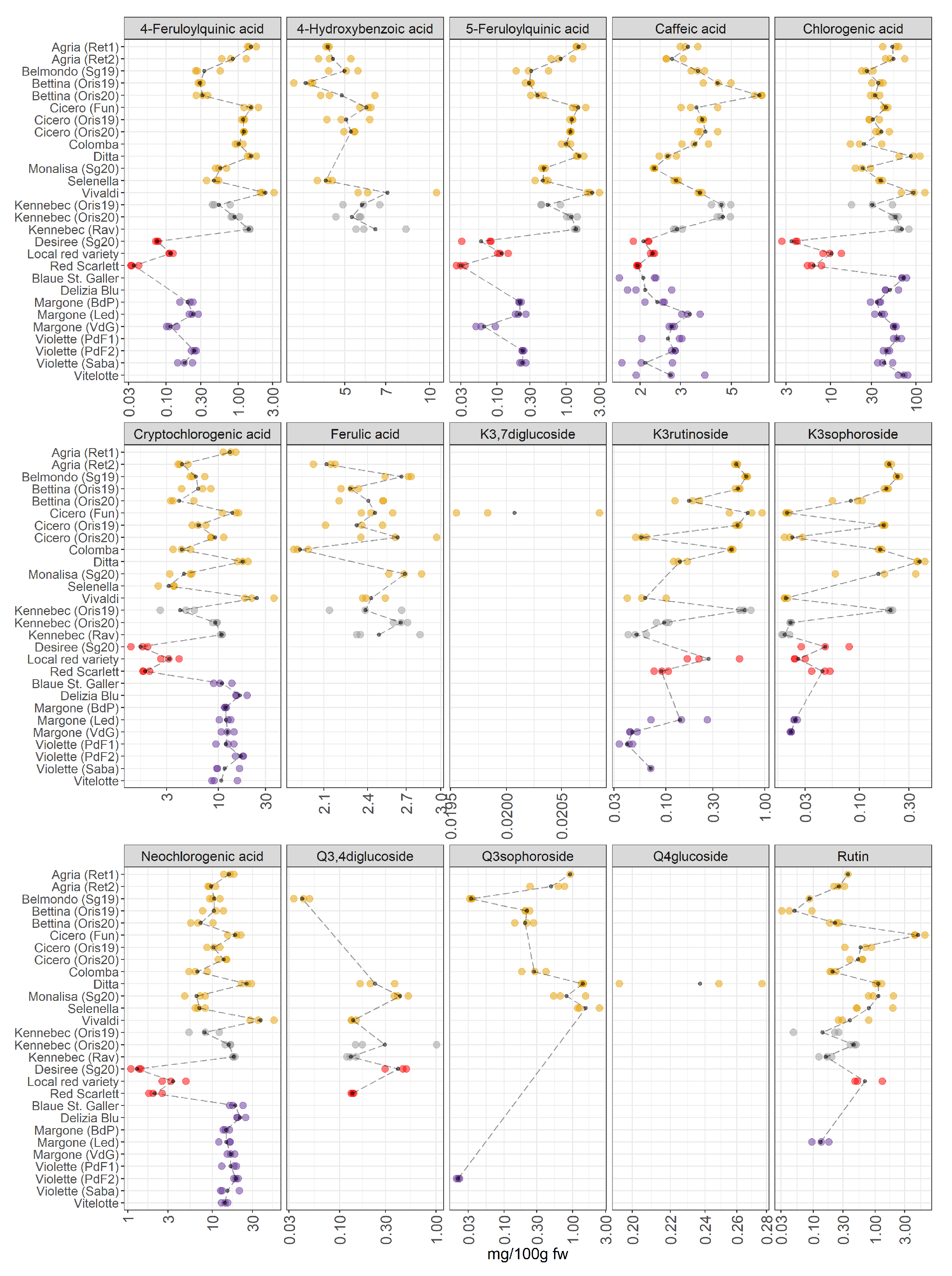
3.2.2. Polyphenolic Compounds
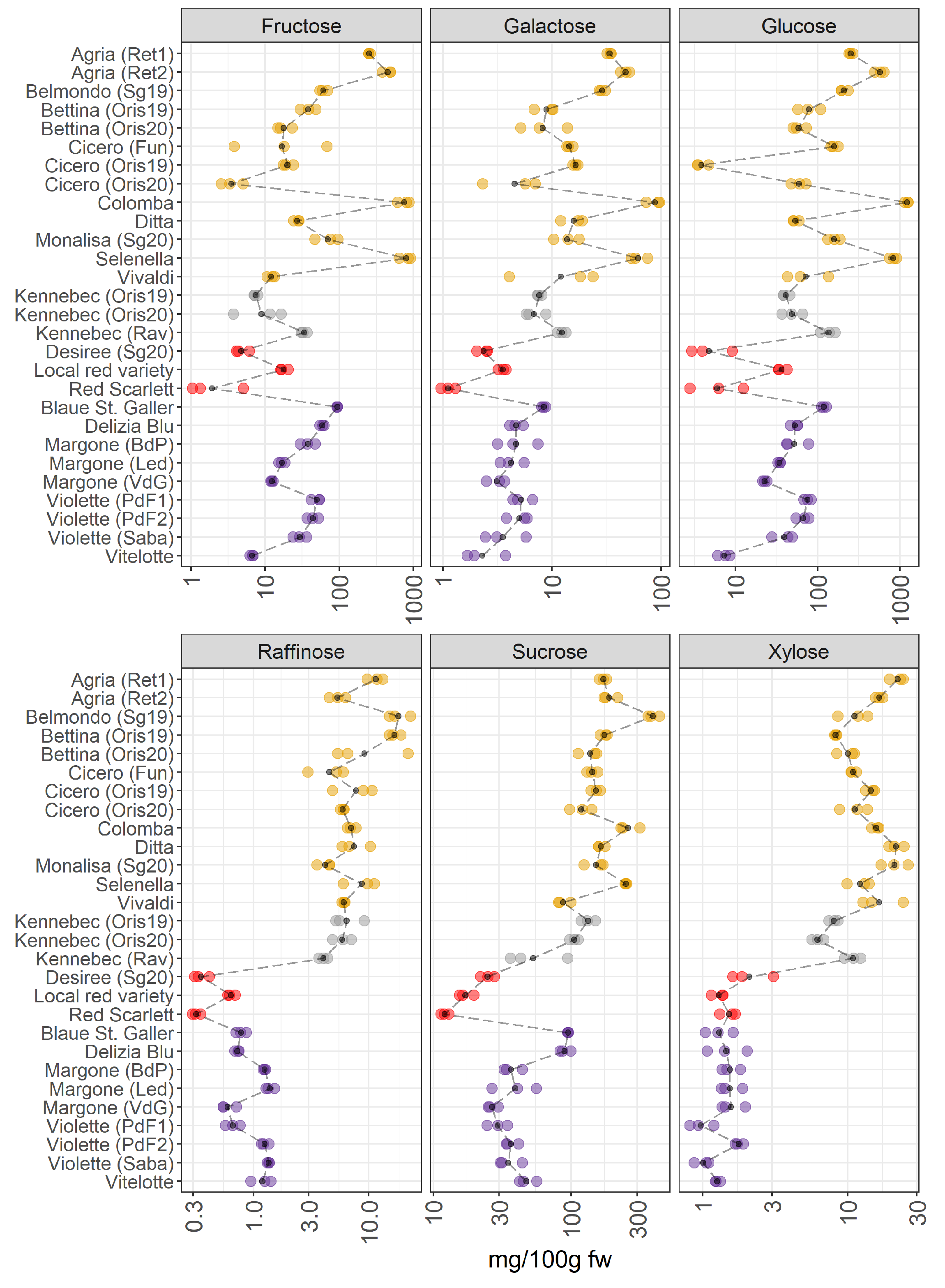
3.2.3. Sugars and Polyols
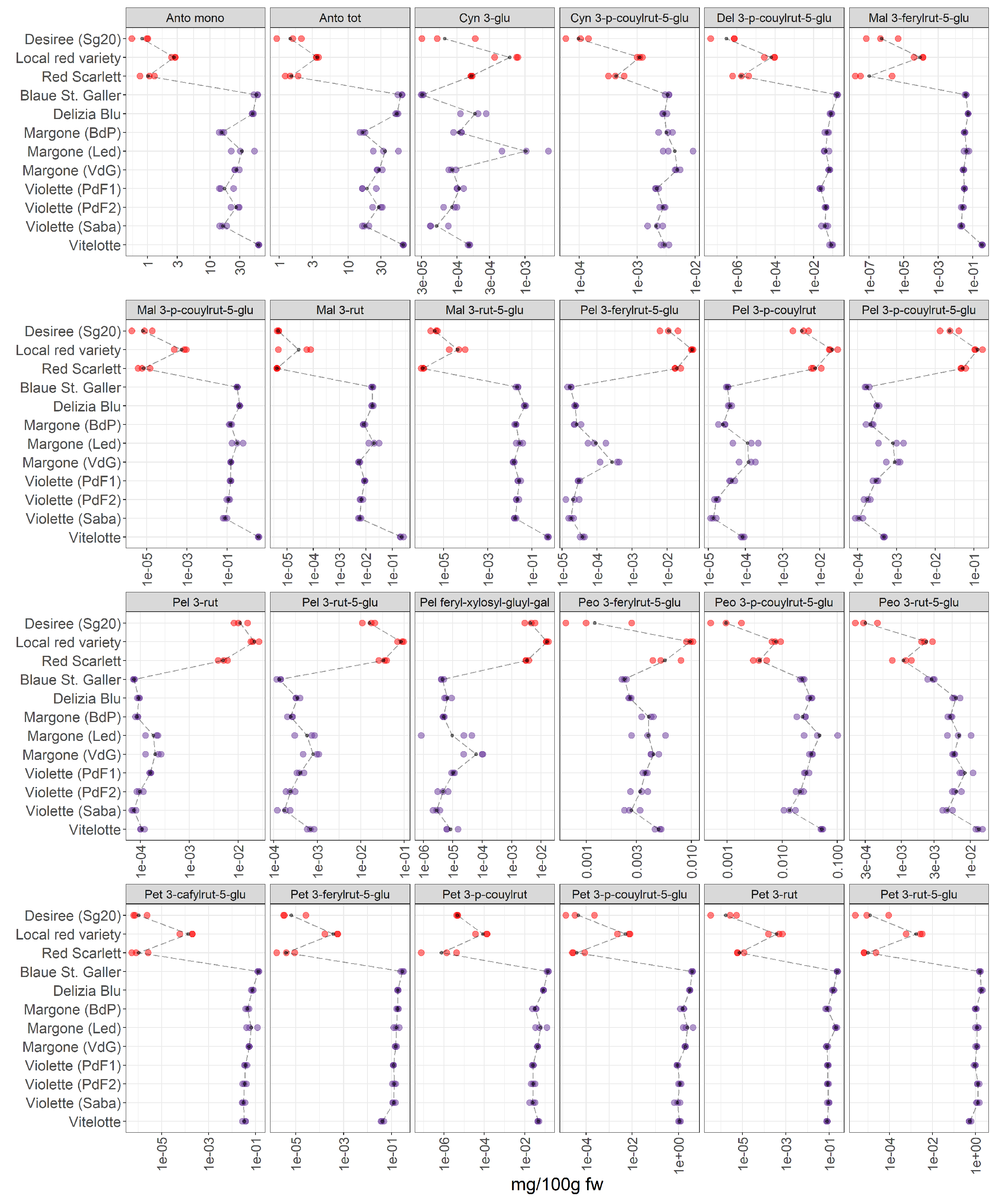
3.2.4. Anthocyanins
3.3. Identification of the Most Valuable Potato Varieties for Human Diets by Comparing the Different Metabolomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Thomas, G.; Sansonetti, G. (Eds.) New Light on a Hidden Treasure: International Year of the Potato 2008, an End-of-Year Review, 1st ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Velangi, A.; Wolever, T.M. Glycemic index of potatoes commonly consumed in North America. J. Am. Diet. Assoc. 2005, 105, 557–562. [Google Scholar] [CrossRef] [PubMed]
- ADA. Myths about Diabetes. Available online: https://www.diabetes.org/diabetes-risk/prediabetes/myths-about-diabetes (accessed on 31 January 2022).
- Bellumori, M.; Innocenti, M.; Michelozzi, M.; Cerretani, L.; Mulinacci, N. Coloured-fleshed potatoes after boiling: Promising sources of known antioxidant compounds. J. Food Compos. Anal. 2017, 59, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ramdath, D.D.; Padhi, E.; Hawke, A.; Sivaramalingam, T.; Tsao, R. The glycemic index of pigmented potatoes is related to their polyphenol content. Food Funct. 2014, 5, 909–915. [Google Scholar] [CrossRef]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Spano, M.; Sobolev, A.P.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L. Characterization of Local Products for Their Industrial Use: The Case of Italian Potato Cultivars Analyzed by Untargeted and Targeted Methodologies. Foods 2020, 9, 1216. [Google Scholar] [CrossRef]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.P. Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef]
- Mulinacci, N.; Ieri, F.; Giaccherini, C.; Innocenti, M.; Andrenelli, L.; Canova, G.; Saracchi, M.; Casiraghi, M.C. Effect of cooking on the anthocyanins, phenolic acids, glycoalkaloids, and resistant starch content in two pigmented cultivars of Solanum tuberosum L. J. Agric. Food Chem. 2008, 56, 11830–11837. [Google Scholar] [CrossRef]
- Zhou, X.; Ulaszewska, M.M.; Cuparencu, C.; Gobba, C.D.; Vázquez-Manjarrez, N.; Gürdeniz, G.; Chen, J.; Mattivi, F.; Dragsted, L.O. Urine Metabolome Profiling Reveals Imprints of Food Heating Processes after Dietary Intervention with Differently Cooked Potatoes. J. Agric. Food Chem. 2020, 68, 6122–6131. [Google Scholar] [CrossRef]
- Lock, E.F.; Hoadley, K.A.; Marron, J.S.; Nobel, A.B. Joint and individual variation explained (JIVE) for integrated analysis of multiple data types. Ann. Appl. Stat. 2013, 7, 523. [Google Scholar] [CrossRef]
- Rommens, C.M.; Richael, C.M.; Yan, H.; Navarre, D.A.; Ye, J.; Krucker, M.; Swords, K. Engineered native pathways for high kaempferol and caffeoylquinate production in potato. Plant Biotechnol. J. 2008, 6, 870–886. [Google Scholar] [CrossRef] [PubMed]
- Perla, V.; Holm, D.G.; Jayanty, S.S. Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT-Food Sci. Technol. 2012, 45, 161–171. [Google Scholar] [CrossRef]
- Tierno, R.; Hornero-Méndez, D.; Gallardo-Guerrero, L.; López-Pardo, R.; de Galarreta, J.I.R. Effect of boiling on the total phenolic, anthocyanin and carotenoid concentrations of potato tubers from selected cultivars and introgressed breeding lines from native potato species. J. Food Compos. Anal. 2015, 41, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Valls, J.; Agnolet, S.; Haas, F.; Struffi, I.; Ciesa, F.; Robatscher, P.; Oberhuber, M. Valorization of Lagrein grape pomace as a source of phenolic compounds: Analysis of the contents of anthocyanins, flavanols and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 2211–2224. [Google Scholar] [CrossRef]
- Ieri, F.; Innocenti, M.; Andrenelli, L.; Vecchio, V.; Mulinacci, N. Rapid HPLC/DAD/MS method to determine phenolic acids, glycoalkaloids and anthocyanins in pigmented potatoes (Solanum tuberosum L.) and correlations with variety and geographical origin. Food Chem. 2011, 125, 750–759. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef]
- Eisenstecken, D.; Panarese, A.; Robatscher, P.; Huck, C.W.; Zanella, A.; Oberhuber, M. A Near Infrared Spectroscopy (NIRS) and Chemometric Approach to Improve Apple Fruit Quality Management: A Case Study on the Cultivars “Cripps Pink” and “Braeburn”. Molecules 2015, 20, 13603–13619. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. References—Scientific Research Publishing, 2020. Available online: https://scirp.org/reference/referencespapers.aspx?referenceid=3067217 (accessed on 31 January 2022).
- Kita, A.; Bakowska-Barczak, A.; Hamouz, K.; Kułakowska, K.; Lisińska, G. The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2013, 32, 169–175. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Dvořák, P. The influence of flesh colour and growing locality on polyphenolic content and antioxidant activity in potatoes. Sci. Hortic. 2008, 117, 109–114. [Google Scholar] [CrossRef]
- Silveira, A.C.; Orena, S.; Medel-Maraboli, M.; Escalona, V.H. Determination of some functional and sensory attributes and suitability of colored-and noncolored-flesh potatoes for different cooking methods. Food Sci. Technol. 2020, 40, 395–404. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Hejtmánková, K.; Pazderů, K.; Dvořák, P.; Čepl, J. Impact of selected factors—Cultivar, storage, cooking and baking on the content of anthocyanins in coloured-flesh potatoes. Food Chem. 2012, 133, 1107–1116. [Google Scholar] [CrossRef]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Hamouz, K.; Lachman, J.; Pazderů, K.; Tomášek, J.; Hejtmánková, K.; Pivec, V. Differences in anthocyanin content and antioxidant activity of potato tubers with different flesh colour. Plant Soil Environ. 2011, 57, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Hellmann, H.; Goyer, A.; Navarre, D.A. Antioxidants in Potatoes: A Functional View on One of the Major Food Crops Worldwide. Molecules 2021, 26, 2446. [Google Scholar] [CrossRef]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Li, W.; Lu, Z.; Beta, T.; Hydamaka, A.W. Phenolic content, composition, antioxidant activity, and their changes during domestic cooking of potatoes. J. Agric. Food. Chem. 2009, 57, 10231–10236. [Google Scholar] [CrossRef]
- Wang, S.; Pan, D.; Lv, X.; Song, X.; Qiu, Z.; Huang, C.; Huang, R.; Chen, W. Proteomic approach reveals that starch degradation contributes to anthocyanin accumulation in tuberous root of purple sweet potato. J. Proteom. 2016, 143, 298–305. [Google Scholar] [CrossRef]
- Reddivari, L.; Hale, A.L.; Miller, J.C. Genotype, Location, and Year Influence Antioxidant Activity, Carotenoid Content, Phenolic Content, and Composition in Specialty Potatoes. J. Agric. Food Chem. 2007, 55, 8073–8079. [Google Scholar] [CrossRef]
- Payyavula, R.S.; Navarre, D.A.; Kuhl, J.C.; Pantoja, A.; Pillai, S.S. Differential effects of environment on potato phenylpropanoid and carotenoid expression. BMC Plant Biol. 2012, 12, 1–17. [Google Scholar] [CrossRef] [Green Version]
- André, C.M.; Oufir, M.; Hoffmann, L.; Hausman, J.F.; Rogez, H.; Larondelle, Y.; Evers, D. Influence of environment and genotype on polyphenol compounds and in vitro antioxidant capacity of native Andean potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2009, 22, 517–524. [Google Scholar] [CrossRef]
- Bassoli, B.K.; Cassolla, P.; Borba-Murad, G.R.; Constantin, J.; Salgueiro-Pagadigorria, C.L.; Bazotte, R.B.; Silva, R.S.D.S.F.D.; Souza, H.M.D. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: Effects on hepatic glucose release and glycaemia. Cell Biochem. Funct. 2008, 26, 320–328. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowokinos, J.R. Relationship of harvest sucrose content to processing maturity and storage life of potatoes. Am. Potato J. 1978, 55, 333–344. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.P.; Kumar, P. An overview of the factors affecting sugar content of potatoes. Ann. Appl. Biol. 2004, 145, 247–256. [Google Scholar] [CrossRef]
- Bontempo, P.; Carafa, V.; Grassi, R.; Basile, A.; Tenore, G.C.; Formisano, C.; Rigano, D.; Altucci, L. Antioxidant, antimicrobial and anti-proliferative activities of Solanum tuberosum L. var. Vitelotte. Food Chem. Toxicol. 2013, 55, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Musilová, J.; Hejtmánková, K.; Kotíková, Z.; Pazderů, K.; Domkářová, J.; Pivec, V.; Cimr, J. Effect of peeling and three cooking methods on the content of selected phytochemicals in potato tubers with various colour of flesh. Food Chem. 2013, 138, 1189–1197. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Ye, X.; Chen, S. Health benefits of the potato affected by domestic cooking: A review. Food Chem. 2016, 202, 165–175. [Google Scholar] [CrossRef]
- Fossen, T.; Cabrita, L.; Andersen, O.M. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chem. 1998, 63, 435–440. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.; Aragon, I.; Furrer, A.; Klinken, J.W.V.; Kaczmarczyk, M.; Lee, B.H.; George, J.; Hamaker, B.R.; Mattes, R.; Ferruzzi, M.G. Potato phenolics impact starch digestion and glucose transport in model systems but translation to phenolic rich potato chips results in only modest modification of glycemic response in humans. Nutr. Res. 2018, 52, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Keppler, K.; Humpf, H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
| Sample Number | Variety | Origin | Year | Sample ID | Group | Pigmentation | Skin Color | Pulp Color | Maturity | Shape |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Belmondo | San Genesio (BZ) | 2019 | Belmondo (Sg19) | Yellow | Non-pigmented | Yellowish | Yellow | Early | Round-oval |
| 2 | Monalisa | San Genesio (BZ) | 2020 | Monalisa (Sg20) | Yellow | Non-pigmented | Golden-yellow | Golden-yellow | Medium early | Oval |
| 3 4 | Bettina | Oris (BZ) | 2019 2020 | Bettina (Sg19) | Yellow | Non-pigmented | Yellow | Light yellow | Medium early | Oval |
| 5 6 7 | Cicero | Oris (BZ) Oris (BZ) Funes (BZ) | 2019 2020 2020 | Cicero (Oris19) Cicero (Oris20) Cicero (Fun) | Yellow | Non-pigmented | Yellow | Pale Yellow | Medium early | Oval |
| 8 9 | Agria (Patata di Montagna) | Ret1 Ret2 | 2019 | Agria (Ret1) Agria (Ret2) | Yellow | Non-pigmented | Yellow | Yellow | Early | Long oval |
| 10 | Selenella | Ret3 | 2019 | Selenella | Yellow | Non-pigmented | Yellow | Yellow | Early | Oval |
| 11 | Colomba | Ret4 | 2019 | Colomba | Yellow | Non-pigmented | Yellow | Yellow | Early | Round oval |
| 12 | Ditta | Merano | 2020 | Ditta | Yellow | Non-pigmented | Yellow | Light yellow | Early | Long oval |
| 13 | Vivaldi | Calceranica al lago (TN) | 2020 | Vivaldi | Yellow | Non-pigmented | Yellow | Light yellow | Medium early | Oval |
| 14 15 16 | Kennebec | Oris (BZ) Oris (BZ) Ravina (TN) | 2019 2020 2020 | Kennebec (Oris19) Kennebec (Oris20) Kennebec (Rav) | White | Non-pigmented | Yellow | White | Early | Oval |
| 14 | Red Scarlett | Calceranica al lago(TN) | 2020 | Red Scarlett | Red | Pigmented | Red | Light yellow | Medium early | Long oval |
| 18 | Desire | San Genesio (BZ) | 2020 | Desire (Sg20) | Red | Pigmented | Red | Light yellow | Medium late | Oval |
| 19 | Local red variety | Val di Ledro (TN) | 2020 | Local red variety | Red | Pigmented | Red | Light yellow | Medium late | Round oval |
| 20 21 22 | Margone | Val di Ledro (TN) Val di Gresta (TN) Baselga di Pinè (TN) | 2020 | Margone (Led) Margone (VdG) Margone (BdP) | Purple | Pigmented | Dark violet | Dark violet | Very late | Round oval |
| 23 24 25 | Violette | Sabaudia (LT) Piana del Fucino1 (AG) Piana del Fucino2 (AG) | 2020 | Violette (Saba) Violette (PdF1) Violette (Pdf2) | Purple | Pigmented | Dark violet | Dark violet | Very late | Long irregular |
| 26 | Vitellotte | Grotte di Castro (VT) | 2020 | Vitellotte | Purple | Pigmented | Dark violet | Dark violet | Very late | Long irregular |
| 27 | Delizia Blu | Grotte di Castro (VT) | 2020 | Delizia Blu | Purple | Pigmented | Dark violet | Dark violet | Very late | Round oval |
| 28 | Blaue St. Galler | Val Pusteria (BZ) | 2020 | Blaue St. Galler | Purple | Pigmented | Dark violet | Dark violet | Very late | Long irregular |
| Dataset | Dataset ID | N. of Analytes | Analytical Methods | JIVE Model |
|---|---|---|---|---|
| Antioxidants Total polyphenolic content | AA | 3 | Spectrophotometric assays (FRAP, ABTS, FC) | yes |
| Polyphenols | Poly | 15 | UHPLC-QqQ-MS/MS | yes |
| Sugar | Sug | 7 | IC-HPAE-PAD | yes |
| Anthocyanins | Anthocyanins | 22 | UHPLC-QqQ-MS/MS | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceci, A.T.; Franceschi, P.; Serni, E.; Perenzoni, D.; Oberhuber, M.; Robatscher, P.; Mattivi, F. Metabolomic Characterization of Pigmented and Non-Pigmented Potato Cultivars Using a Joint and Individual Variation Explained (JIVE). Foods 2022, 11, 1708. https://doi.org/10.3390/foods11121708
Ceci AT, Franceschi P, Serni E, Perenzoni D, Oberhuber M, Robatscher P, Mattivi F. Metabolomic Characterization of Pigmented and Non-Pigmented Potato Cultivars Using a Joint and Individual Variation Explained (JIVE). Foods. 2022; 11(12):1708. https://doi.org/10.3390/foods11121708
Chicago/Turabian StyleCeci, Adriana Teresa, Pietro Franceschi, Enrico Serni, Daniele Perenzoni, Michael Oberhuber, Peter Robatscher, and Fulvio Mattivi. 2022. "Metabolomic Characterization of Pigmented and Non-Pigmented Potato Cultivars Using a Joint and Individual Variation Explained (JIVE)" Foods 11, no. 12: 1708. https://doi.org/10.3390/foods11121708
APA StyleCeci, A. T., Franceschi, P., Serni, E., Perenzoni, D., Oberhuber, M., Robatscher, P., & Mattivi, F. (2022). Metabolomic Characterization of Pigmented and Non-Pigmented Potato Cultivars Using a Joint and Individual Variation Explained (JIVE). Foods, 11(12), 1708. https://doi.org/10.3390/foods11121708








