Preliminary Characterization of a Spray-Dried Hydrocolloid from a High Andean Algae (Nostoc sphaericum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Determination of Fresh Nostoc Size
2.3. Proximal Analysis of Nostoc Samples
2.4. Nostoc Spray-Dried
2.5. Total Carbon Determination
2.6. Determination of Water Activity (aw)
2.7. Color Determination
- -
- If IC* −40 to −20, colors range from blue-violet to deep green.
- -
- If IC* −20 to −2, colors range from deep green to yellowish-green.
- -
- If IC* −2 to +2, represents greenish-yellow.
- -
- If IC* +2 to +20, colors range from pale yellow to deep orange.
- -
- If IC* +20 to +40, colors range from deep orange to deep red.
2.8. IR Analysis of Nostoc
2.9. Thermal Analysis
2.10. Determination of Particle Size and ζ Potential
2.11. Mineral Determination
2.12. Statistical Analysis
3. Results and Discussion
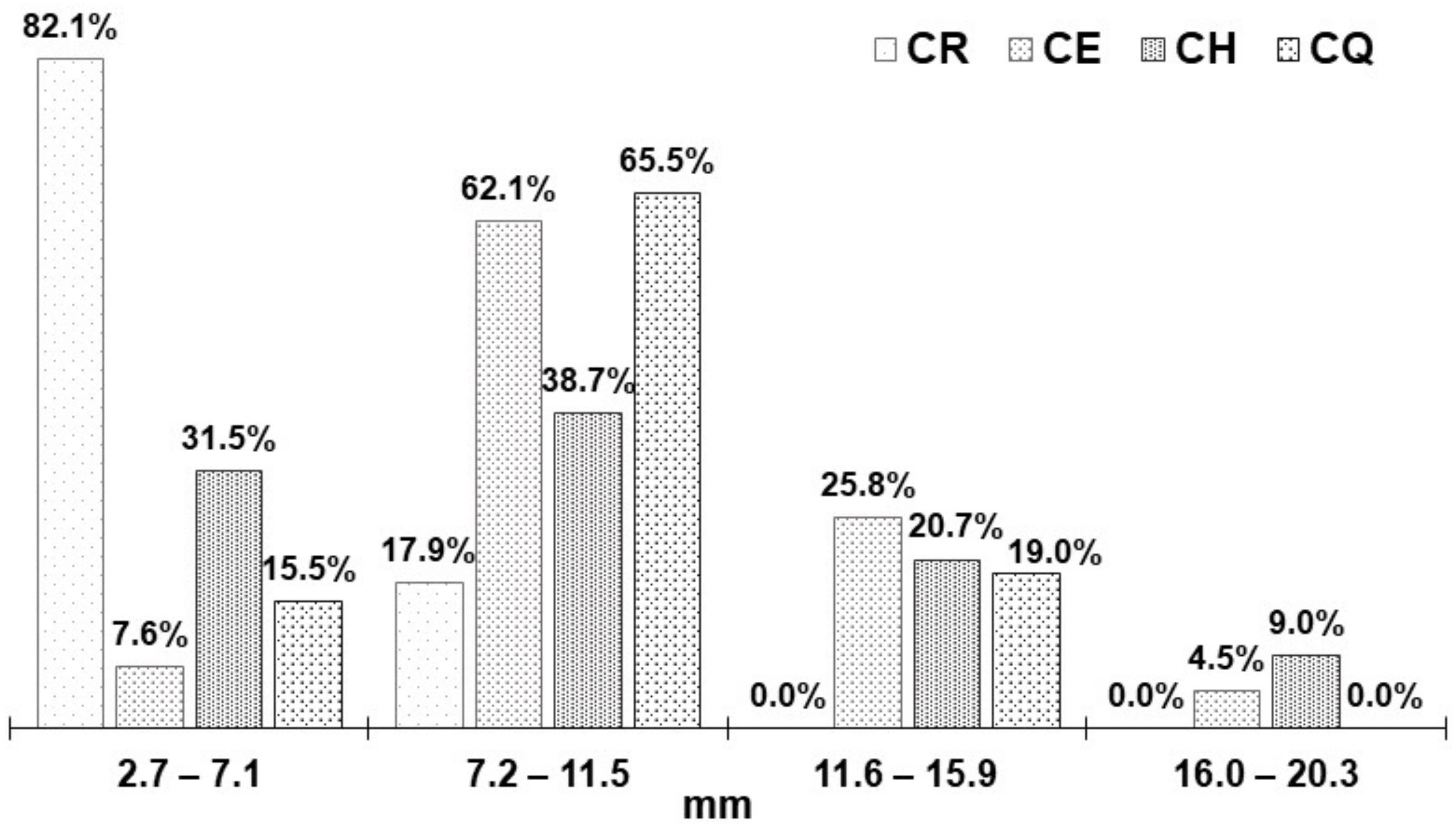
3.1. Fresh Nostoc Size
3.2. Nostoc Composition
3.3. Total Carbon in Dehydrated and Spray-Dried Nostoc
3.4. Water Activity
3.5. Color
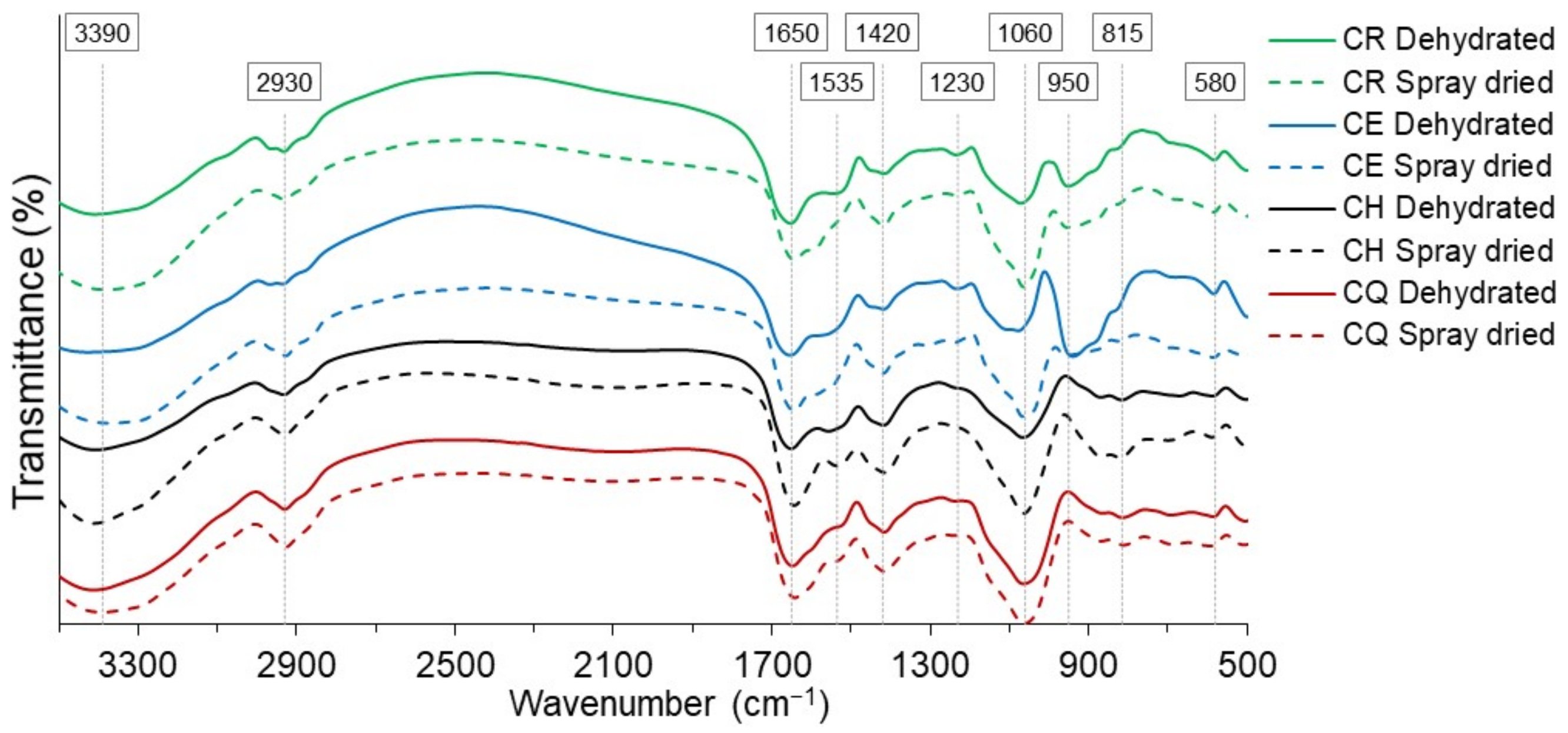
3.6. Infrared Analysis
| Wavelength (cm−1) | Functional Group | Vibration Type | Compound Type | Present in | |
|---|---|---|---|---|---|
| Range * | In Nostoc | ||||
| 3700–3100 | 3390 | -NH and -OH | Stretching | Water, secondary amide, and carboxylate | All dehydrated and spray-dried |
| 3000–2800 | 2930 | -CH- aliphatic | Stretching | Methyl groups | All dehydrated and spray-dried |
| 1870–1650 | 1650 | -OH, -COO- asymmetrical, and C=O carbonyl | Bending, stretching | carboxylate anion, ester carbonyl groups | All dehydrated and spray-dried |
| 1600–1500 | 1535 | -COO-, -C(=O)-NH-, C=O, -NH | Stretching | Carboxyl groups, amine | All dehydrated and spray-dried |
| 1500–1300 | 1420 | -CH2, -COO- symmetrical | Stretching | Methyl groups, carboxylate anion | All dehydrated and spray-dried |
| 1300–1200 | 1230 | C-O-C, C-OH, -C-H- | Stretching and bending | Carbohydrates | All dehydrated and spray-dried |
| 1100–920 | 1060 | C-O, C-O-C, C-OH | Stretching | absorption region of polysaccharides | All dehydrated and spray-dried |
| 950 | -OH out of the plane, C-O stretching | bending | Carboxylate anion | CR and CE | |
| 815 | -CH- glycosidic | bending | Methylene groups, Glucose | All dehydrated and spray-dried | |
| 580 | O=S=O, CHO | bending | Sulfate, Carbohydrates | All dehydrated and spray-dried | |
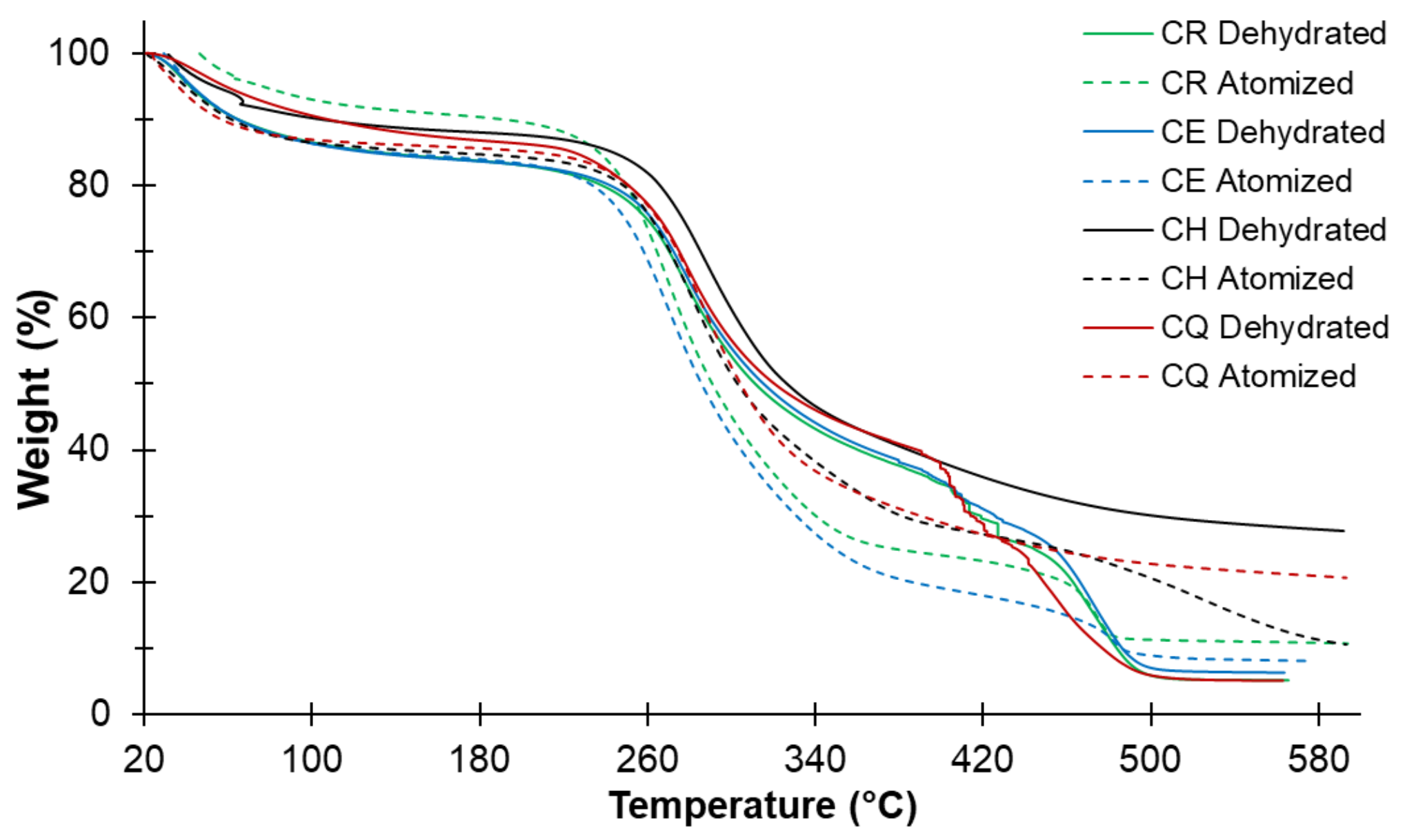
3.7. Thermal Analysis of Nostoc
3.8. Particle Size and ζ Potential
3.9. Mineral Content in Nostoc
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid gel particles: Formation, characterization, and application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; de Morais, M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Wang, W.; Li, Y. Advanced properties of gluten-free cookies, cakes, and crackers: A review. Trends Food Sci. Technol. 2020, 103, 200–213. [Google Scholar] [CrossRef]
- Culetu, A.; Duta, D.E.; Papageorgiou, M.; Varzakas, T. The Role of Hydrocolloids in Gluten-Free Bread and Pasta; Rheology, Characteristics, Staling and Glycemic Index. Foods 2021, 10, 3121. [Google Scholar] [CrossRef] [PubMed]
- Aftab, K.; Hameed, S.; Umbreen, H.; Ali, S.; Rizwan, M.; Alkahtani, S.; Abdel-Daim, M.M. Physicochemical and Functional Potential of Hydrocolloids Extracted from Some Solanaceae Plants. J. Chem. 2020, 2020, 3563945. [Google Scholar] [CrossRef]
- Martín-Esparza, M.E.; Raigón, M.D.; García-Martínez, M.D.; Albors, A. Role of Hydrocolloids in the Structure, Cooking, and Nutritional Properties of Fiber-Enriched, Fresh Egg Pasta Based on Tiger Nut Flour and Durum Wheat Semolina. Foods 2021, 10, 2510. [Google Scholar] [CrossRef]
- Jurado, B.; Fuertes, C.; Tomas, G.; Ramos, E.; Arroyo, J.; Cáceres, J.; Inocente, M.; Alvarado, B.; Rivera, B.; Ramírez, M.; et al. Estudio fisicoquímico, microbiológico y toxicológico de los polisacáridos del Nostoc commune y Nostoc sphaericum. Rev. Peru. Quím. E Ing. Quím. 2014, 17, 15–22. [Google Scholar]
- Perduca, M.J.; Spotti, M.J.; Santiago, L.G.; Judis, M.A.; Rubiolo, A.C.; Carrara, C.R. Rheological characterization of the hydrocolloid from Gleditsia amorphoides seeds. LWT-Food Sci. Technol. 2013, 51, 143–147. [Google Scholar] [CrossRef]
- Gomes, D.; Pereira, L.; Valadares, A. Cyanobacterial polyhydroxyalkanoates: A sustainable alternative in circular economy. Molecules 2020, 25, 4331. [Google Scholar] [CrossRef]
- Rodriguez, S.; Torres, F.G.; López, D. Preparation and characterization of polysaccharide films from the cyanobacteria Nostoc commune. Polym. Renew. Resour. 2017, 8, 133–150. [Google Scholar] [CrossRef]
- Jensen, S.; Petersen, B.O.; Omarsdottir, S.; Paulsen, B.S.; Duus, J.Ø.; Olafsdottir, E.S. Structural characterization of a complex heteroglycan from the cyanobacterium Nostoc commune. Carbohydr. Polym. 2013, 91, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Corpus-Gomez, A.; Alcantara-Callata, M.; Celis-Teodoro, H.; Echevarria-Alarcón, B.; Paredes-Julca, J.; Paucar-Menacho, L.M. Cushuro (Nostoc sphaericum): Hábitat, características fisicoquímicas, composición nutricional, formas de consumo y propiedades medicinales. Agroind. Sci. 2021, 11, 231–238. [Google Scholar] [CrossRef]
- Torres-Maza, A.; Yupanqui-Bacilio, C.; Castro, V.; Aguirre, E.; Villanueva, E.; Rodríguez, G. Comparison of the hydrocolloids Nostoc Commune and Nostoc sphaericum: Drying, spectroscopy, rheology and application in nectar. Sci. Agropecu. 2020, 11, 583–589. [Google Scholar] [CrossRef]
- Ponce, E. Nostoc: A different food and their presence in the precordillera of Arica. Idesia 2014, 32, 119–121. [Google Scholar] [CrossRef]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review. Mar. Drugs 2019, 17, 561. [Google Scholar] [CrossRef]
- Inocente, M.A.; Jurado, B.; Ramos, E.; Alvarado, B.; Fuentes, C.; Cárdenas, L.; Rivera, B. Actividad hipoglucemiante in vitro de los polisacáridos digeridos de Nostoc sphaericum Vaucher ex Bornet & Flahault (cushuro). Horiz. Med. 2019, 19, 26–31. [Google Scholar] [CrossRef]
- Rodriguez, S.; Gonzales, K.N.; Romero, E.G.; Troncoso, O.P.; Torres, F.G. Unusual reversible elastomeric gels from Nostoc commune. Int. J. Biol. Macromol. 2017, 97, 411–417. [Google Scholar] [CrossRef]
- Balaji, S.; Gopi, K.; Muthuvelan, B. A review on production of polyhydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res. 2013, 2, 278–285. [Google Scholar] [CrossRef]
- Singh, A.K.; Mallick, N. Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol. Lett 2017, 364, 20. [Google Scholar] [CrossRef]
- Rojas-Torres, S.A.; Quintana, S.E.; García-Zapateiro, L.A. Natural Yogurt Stabilized with Hydrocolloids from Butternut Squash (Cucurbita moschata) Seeds: Effect on Physicochemical, Rheological Properties and Sensory Perception. Fluids 2021, 6, 251. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, Y.S.; Chen, H.H.; Liu, S.; Li, M. Retardant effect of sodium alginate on the retrogradation properties of normal cornstarch and anti-retrogradation mechanism. Food Hydrocoll. 2017, 69, 1–9. [Google Scholar] [CrossRef]
- Gałkowska, D.; Długosz, M.; Juszczak, L. Effect of high methoxy pectin and sucrose on pasting, rheological, and textural properties of modified starch systems. Starch-Stärke 2013, 65, 499–508. [Google Scholar] [CrossRef]
- Cárdenas, A.; Alvites, H.; Valladares, G.; Obregón, J.; Vásquez-Villalobos, V. Optimization by Mixtures Design of Syneresis and Sensory Texture of Natural Smoothie Yogurt Using Three Types of Hydrocolloids. Agroind. Sci. 2013, 3, 35–40. [Google Scholar] [CrossRef][Green Version]
- Chandrika, K.S.V.; Singh, A.; Sarkar, D.J.; Rathore, A.; Kumar, A. pH-sensitive crosslinked guar gum-based superabsorbent hydrogels: Swelling response in simulated environments and water retention behavior in plant growth media. J. Appl. Polym. Sci. 2014, 131, 1–12. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Hussain, S.; Alamri, M.S.; Mohamed, A.A.; Ahmed, A.S.; Ibraheem, M.A.; Qasem, A.A.A. Use of hydrocolloid gums to modify the pasting, thermal, rheological, and textural properties of sweet potato starch. Int. J. Polym. Sci. 2019, 2019, 6308591. [Google Scholar] [CrossRef]
- Alamri, M.S.; Mohamed, A.A.; Hussain, S. Effects of alkaline-soluble okra gum on rheological and thermal properties of systems with wheat or corn starch. Food Hydrocoll. 2013, 30, 541–551. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.; Fellows, C.M. Effect of soluble fibre (guar gum and carboxymethylcellulose) addition on technological, sensory and structural properties of durum wheat spaghetti. Food Chem. 2012, 131, 893–900. [Google Scholar] [CrossRef]
- Woo, M.W.; Bhandari, B. Spray Drying for food powder Production. In Handbook of Food Science, Technology and Engineering; Hui, Y.H., Sherkat, F., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 29–56, (in press). [CrossRef]
- Elversson, J.; Millqvist-Fureby, A. Particle size and density in spray drying—Effects of carbohydrate properties. J. Pharm. Sci. 2005, 94, 2049–2060. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2016; ISBN 0935584870. [Google Scholar]
- Choque-Quispe, D.; Ramos-Pacheco, B.S.; Solano-Reynoso, A.M.; Ligarda-Samanez, C.A.; Choque-Quispe, Y.; Peralta-Guevara, D.E.; Quispe-Quispe, Y. Drying and color in punamuña leaves (Satureja boliviana). DYNA 2021, 88, 31–37. [Google Scholar] [CrossRef]
- Hadimani, L.; Mittal, N. Development of a computer vision system to estimate the colour indices of Kinnow mandarins. J. Food Sci. Technol. 2019, 56, 2305–2311. [Google Scholar] [CrossRef]
- Zimmerman, H.; Zimmerman, D.; Reuss, R.; Feilen, P.J.; Manz, B.; Katsen, A.; Weber, M.; Ihmig, F.R.; Ehrhart, F.; Gebner, P.; et al. Towards a medically approved technology for alginate-based microcapsules allowing long-term immunoisolated transplantation. J. Mater. Sci. Mater. Med. 2005, 16, 491–501. [Google Scholar] [CrossRef]
- Uhliariková, I.; Chválová, B.; Matulová, M.; Cepák, V.; Lukavský, J.; Capek, P. Extracellular biopolymers produced by freshwater cyanobacteria: A screening study. Chem. Pap. 2019, 73, 771–776. [Google Scholar] [CrossRef]
- Gao, Z.; Fang, Y.; Cao, Y.; Liao, H.; Nishinari, K.; Phillips, G.O. Hydrocolloid-food component interactions. Food Hydrocoll. 2017, 68, 149–156. [Google Scholar] [CrossRef]
- Ai, W.; Fang, Y.; Xiang, S.; Yao, X.; Nishinari, K.; Phillips, G. Protein/Polysaccharide Electrostatic Complexes and Their Applications in Stabilizing Oil-in-Water Emulsions. J. Nutr. Sci. Vitaminol. 2015, 61, S168–S169. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Smestad, B.; Klaveness, D. Studies on polysaccharides from three edible species of Nostoc (cyanobacteria) with different colony morphologies: Comparison of monosaccharide compositions and viscosities of polysaccharides from field colonies and suspension cultures. J. Phycol. 2002, 34, 962–968. [Google Scholar] [CrossRef]
- Gantar, M.; Svirčev, Z. Microalgae and Cyanobacteria: Food for Thought. J. Phycol. 2008, 44, 260–268. [Google Scholar] [CrossRef]
- Alves, A.; Caridade, S.G.; Mano, J.F.; Sousa, R.A.; Reis, R.L. Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydr. Res. 2010, 345, 2194–2200. [Google Scholar] [CrossRef]
- Parikh, A.; Madamwar, D. Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresour. Technol. 2006, 97, 1822–1827. [Google Scholar] [CrossRef]
- Helm, R.F.; Huang, Z.; Edwards, D.; Leeson, H.; Peery, W.; Potts, M. Structural Characterization of the Released Polysaccharide of Desiccation-Tolerant Nostoc commune DRH-1. J. Bacteriol. 2000, 182, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, S.; Ziaiifar, A.M.; Verkerk, R. Effect of thermal and non-thermal treatments on the color of citrus juice: A review. Food Rev. Int. 2022, 37, 1–23. [Google Scholar] [CrossRef]
- Spence, C.; Levitan, C.A.; Shankar, M.U.; Zampini, M. Does food color influence taste and flavor perception in humans? Chemosens. Percept. 2010, 3, 68–84. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Hernández, G.B.; Monaco, K.; Gómez, P.A.; Aguayo, E.; Artés, F.; Artés-Hernández, F. Preservation of bioactive compounds of a green vegetable smoothie using short time–high temperature mild thermal treatment. Food Sci. Technol. Int. 2016, 23, 46–60. [Google Scholar] [CrossRef]
- Aamir, M.; Ovissipour, M.; Rasco, B.; Tang, J.; Sablani, S. Seasonality of the thermal kinetics of color changes in whole spinach (Spinacia oleracea) leaves under pasteurization conditions. Int. J. Food Prop. 2014, 17, 2012–2024. [Google Scholar] [CrossRef]
- Putriani, N.; Perdana, J.; Meiliana; Nugrahedi, P.Y. Effect of thermal processing on key phytochemical compounds in green leafy vegetables: A review. Food Rev. Int. 2020, 36, 1–29. [Google Scholar] [CrossRef]
- Erge, H.S.; Karadeniz, F.; Koca, N.; Soyer, Y. Effect of heat treatment on chlorophyll degradation and color loss in green peas. Gida 2008, 33, 225–233. [Google Scholar]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Cozzolino, D. Uso de la espectroscopia de reflectancia en el infrarrojo cercano (NIRS) en el análisis de alimentos para animales. Agrociencia 2002, 6, 25–32. [Google Scholar]
- Hamrun, N.; Talib, B.; Ruslin, M.; Pangeran, H.; Hatta, M.; Marlina, E.; Yusuf, A.S.H.; Saito, T.; Ou, K.-L. A Promising Potential of Brown Algae Sargassum polycystum as Irreversible Hydrocolloid Impression Material. Mar. Drugs 2022, 20, 55. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.-M. Production of new cellulose nanomaterial from red algae marine biomass Gelidiu melegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; Elghoul, Y.; Alminderej, F.M. Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorptionof cationic and anionic dyes from water. Int. J. Biol. Macromol. 2019, 135, 152–162. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Ramakrishnan, Y.; Adzahan, N.M.; Muhammad, K. Functional and preliminary characterization of hydrocolloid from tamarillo (Solanum betaceum Cav.) puree. Molecules 2012, 17, 6869–6885. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Froehner, S.; Ligarda-Samanez, C.A.; Ramos-Pacheco, B.S.; Palomino-Rincón, H.; Choque-Quispe, Y.; Solano-Reynoso, A.M.; Taipe-Pardo, F.; Zamalloa-Puma, L.M.; Calla-Florez, M.; et al. Preparation and Chemical and Physical Characteristics of an Edible Film Based on Native Potato Starch and Nopal Mucilage. Polymers 2021, 13, 3719. [Google Scholar] [CrossRef]
- Bet, C.D.; de Oliveira, C.S.; Beninca, C.; Colman, T.A.D.; Lacerda, L.G.; Schnitzler, E. Influence of the addition of hydrocolloids on the thermal, pasting and structural properties of starch from common vetch seeds (Vicia sativa sp.). J. Therm. Anal. Calorim. 2018, 133, 549–557. [Google Scholar] [CrossRef]
- Adamovicz, J.A.L.; Cordoba, L.P.; Ribeiro, L.S.; Oliveira, C.S.; Schnitzler, E. Evaluation on thermal, rheological and structural properties on the mixture of potato starch and pectin. Carpath. J. Food Sci. Technol. 2015, 7, 45–52. [Google Scholar]
- Roos, Y.H.; Labuza, T.P.; Levine, H.; Mathlouthi, M.; Reid, D.; Shalev, E.; Slade, L. Melting and crystallization of sugars in high-solids system. J. Agric. Food Chem. 2013, 61, 3167–3178. [Google Scholar] [CrossRef]
- Vuillemin, M.E.; Michaux, F.; Adam, A.A.; Linder, M.; Muniglia, L.; Jasniewski, J. Physicochemical characterizations of gum Arabic modified with oxidation products of ferulic acid. Food Hydrocoll. 2020, 107, 105919. [Google Scholar] [CrossRef]
- Abu, M.H.E.; Mohamed, R.R.; Elhafeez, E.A.; Sabaa, M.W. Synthesis of novel biodegradable antibacterial grafted xanthan gum. Carbohyd. Polym. 2017, 173, 305–311. [Google Scholar] [CrossRef]
- Han, F.; Xiong, D.; Wang, Q.; Shao, B.; Chen, M. Thermal properties of carboxymethylcellulose and methyl methacrylate graft copolymers. J. Macromol. Sci. Phys. 2013, 52, 1242–1249. [Google Scholar] [CrossRef]
- Gliko-Kabir, I.; Penhasi, A.; Rubinstein, A. Characterization of crosslinked guar by thermal analysis. Carbohyd. Res. 1999, 316, 6–13. [Google Scholar] [CrossRef]
- Niu, X.; Ma, Q.; Li, S.; Wang, W.; Ma, Y.; Zhao, H.; Sun, J.; Wang, J. Preparation and Characterization of Biodegradable Composited Films Based on Potato Starch/Glycerol/Gelatin. J. Food Qual. 2021, 2021, 6633711. [Google Scholar] [CrossRef]
- Mikol, V.; Vincendon, P.; Eriani, G.; Hirsh, E.; Giegé, R. Diagnostic of protein crystallization by dynamic light scattering; an application to an aminoacyl-tRNA synthetase. J. Cryst. Growth 1991, 110, 195–200. [Google Scholar] [CrossRef]
- Cuadros-Moreno, A.; Pimentel, R.; Martínez, E.S.M.; Fernández, J.A. Dispersión de luz dinámica en la determinación de tamaño de nanopartículas poliméricas. Lat.-Am. J. Phys. Educ. 2014, 8, 14. [Google Scholar]
- Medina-López, S.V.; Zuluaga-Domínguez, C.M.; Fernández-Trujillo, J.P.; Hernández-Gómez, M.S. Nonconventional Hydrocolloids’ Technological and Functional Potential for Food Applications. Foods 2022, 11, 401. [Google Scholar] [CrossRef]
- Putro, J.N.; Lunardi, V.B.; Soetaredjo, F.E.; Yuliana, M.; Santoso, S.P.; Wenten, I.G.; Ismadji, S. A Review of Gum Hydrocolloid Polyelectrolyte Complexes (PEC) for Biomedical Applications: Their Properties and Drug Delivery Studies. Processes 2021, 9, 1796. [Google Scholar] [CrossRef]
- Lin, J.; Cai, X.; Tang, M.; Wang, S. Preparation and evaluation of the chelating nanocomposite fabricated with marine algae Schizochytrium sp. protein hydrolysate and calcium. J. Agric. Food Chem. 2015, 63, 9704–9714. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mazumder, B.; Nautiyal, V. Rheological characterization of Isabgol husk, gum katira hydrocolloids, and their blends. Int. J. Food Sci. 2014, 2014, 506591. [Google Scholar] [CrossRef]
- Malhotra, A.; Coupland, J.N. The effect of surfactants on the solubility, zeta potential, and viscosity of soy protein isolates. Food Hydrocoll. 2004, 18, 101–108. [Google Scholar] [CrossRef]
- Furusawa, K.; Uchiyama, K. Collaborative studies of zeta-potential measurements and electrophoretic measurements using reference sample. Colloids Surf. A Physicochem. Eng. Asp. 1998, 140, 217–226. [Google Scholar] [CrossRef]
- Dhawale, S.; Wadodkar, S.G.; Dorle, A. Behavior of suspending and wetting agents in aqueous environment. Asian J. Pharm 2009, 3, 9–12. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; Wiley-VCH Verlag GmbH y Co. KGaA: Weinheim, Germany, 2005; ISBN 9783527307432. [Google Scholar]
- Contreras-Lozano, K.P.; Ciro-Velásquez, H.J.; Arango-Tobón, J.C. Hidrocoloides como estabilizantes en bebidas de maíz dulce (Zea mays var. saccharata) y gel de aloe vera (Aloe barbadensis Miller). Rev. UDCA Actual. Divulg. Cien. 2019, 22, 2. [Google Scholar] [CrossRef]
- Singh, S.; Bothara, S.B. Physico-chemical and structural characterization of mucilage isolated from seeds of Diospyros melonoxylon Roxb. Braz. J. Pharm. Sci. 2014, 50, 713–725. [Google Scholar] [CrossRef]

| Ecotype Code | Source | Community | District | Region | Coordinates | Altitude (m) | |
|---|---|---|---|---|---|---|---|
| S | W | ||||||
| CR | Rayanniyoc lagoon | Rayanniyoc | Taray | Cusco | 13°28′36″ | 71°54′23″ | 3775 |
| CE | Concaja spring | Champimayu | Suycutambo—Espinar | Cusco | 15°04′13″ | 71°44′33″ | 4851 |
| CQ | Ccopi lagoon | Qquencco | Coya | Cusco | 13°22′24″ | 71°51′06″ | 4211 |
| CH | Pucccocha spring | Huamanilla | José María Arguedas | Apurímac | 13°47′52″ | 73°17′55″ | 4251 |
| Ecotype | pH | Humidity (%, d.b.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | * | C.V. (%) | ± | SD | * | C.V. (%) | |||
| CR | 7.57 | ± | 0.12 | a | 1.59 | 97.29 | ± | 0.08 | a | 0.08 |
| CE | 6.33 | ± | 0.07 | b | 1.17 | 98.23 | ± | 0.05 | b | 0.06 |
| CQ | 7.75 | ± | 0.00 | c | 0.03 | 97.94 | ± | 0.06 | c | 0.06 |
| CH | 6.91 | ± | 0.00 | d | 0.01 | 98.57 | ± | 0.07 | d | 0.08 |
| Composition (%) | CR | CE | CQ | CH | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | * | C.V. (%) | ± | SD | * | C.V. (%) | ± | SD | * | C.V. (%) | ± | s | * | C.V. (%) | |||||
| Humidity | 10.78 | ± | 0.15 | a | 1.40 | 9.44 | ± | 0.11 | b | 1.16 | 11.30 | ± | 0.11 | c | 0.97 | 10.57 | ± | 0.23 | a | 2.14 |
| Protein | 24.70 | ± | 0.05 | a | 0.19 | 24.60 | ± | 0.02 | a | 0.08 | 17.03 | ± | 0.25 | b | 1.45 | 24.01 | ± | 0.12 | c | 0.50 |
| Fat | 1.73 | ± | 0.04 | a | 2.08 | 1.79 | ± | 0.02 | a | 1.12 | 1.90 | ± | 0.02 | b | 1.09 | 1.88 | ± | 0.04 | b | 1.87 |
| Ash | 4.78 | ± | 0.07 | a | 1.37 | 5.52 | ± | 0.13 | b | 2.29 | 5.16 | ± | 0.11 | c | 2.16 | 6.19 | ± | 0.06 | d | 1.04 |
| Soluble fiber | 8.58 | ± | 0.08 | a | 0.97 | 8.67 | ± | 0.04 | a,b | 0.48 | 9.07 | ± | 0.13 | c | 1.47 | 8.84 | ± | 0.11 | b,c | 1.26 |
| Carbohydrates | 57.30 | ± | 0.50 | a | 0.87 | 58.52 | ± | 0.10 | b | 0.16 | 64.38 | ± | 0.28 | c | 0.44 | 57.32 | ± | 0.10 | a | 0.18 |
| Ecotype | Dehydrated | Spray-Dried | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | * | C.V. (%) | ± | SD | * | C.V. (%) | |||
| CR | 0.568 | ± | 0.006 | a | 1.00 | 0.628 | ± | 0.004 | a | 0.55 |
| CE | 0.542 | ± | 0.002 | b | 0.28 | 0.612 | ± | 0.002 | b | 0.25 |
| CQ | 0.458 | ± | 0.004 | c | 0.77 | 0.533 | ± | 0.001 | c | 0.22 |
| CH | 0.437 | ± | 0.004 | d | 1.00 | 0.530 | ± | 0.004 | c | 0.82 |
| Ecotype | L* | a* | b* | CI* | * | Color | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | SD | C.V. (%) | ± | SD | C.V. (%) | ± | SD | C.V. (%) | ± | SD | C.V. (%) | Referential | Fresh Nostoc | |||||||
| CR | Fresh | 11.4 | ± | 0.5 | 4.3 | −17.9 | ± | 1.3 | 7.4 | 44.9 | ± | 0.9 | 2.1 | −35.0 | ± | 4.9 | 13.9 | <0.05 |  | |
| Dehydrated | 40.8 | ± | 2.9 | 7.1 | 0.7 | ± | 0.1 | 10.1 | 48.2 | ± | 2.4 | 4.9 | 0.4 | ± | 0.0 | 13.5 | ||||
| Spray-dried | 45.3 | ± | 0.8 | 1.9 | −6.1 | ± | 0.6 | 9.7 | 38.3 | ± | 1.9 | 4.9 | −3.5 | ± | 0.4 | 12.6 | ||||
| CE | Fresh | 17.8 | ± | 0.7 | 4.1 | −20.6 | ± | 2 | 9.8 | 52.7 | ± | 0.9 | 1.7 | −22.1 | ± | 1.7 | 7.5 | <0.05 |  | |
| Dehydrated | 24.5 | ± | 0.9 | 3.5 | −9.3 | ± | 0.7 | 7.3 | 47.4 | ± | 1.4 | 3.0 | −8.0 | ± | 0.3 | 3.1 | ||||
| Spray-dried | 51.5 | ± | 2.7 | 5.3 | −7.5 | ± | 0.7 | 9.8 | 36.1 | ± | 2.4 | 6.5 | −4.1 | ± | 0.4 | 9.1 | ||||
| CQ | Fresh | 32.1 | ± | 1.8 | 5.7 | −9.8 | ± | 0.4 | 4.4 | 37.5 | ± | 1.4 | 3.8 | −8.1 | ± | 0.8 | 9.6 | <0.05 |  | |
| Dehydrated | 43.5 | ± | 3.4 | 7.9 | −4.3 | ± | 0.3 | 7.0 | 34.1 | ± | 1.8 | 5.3 | 0.3 | ± | 0.0 | 10.8 | ||||
| Spray-dried | 53.7 | ± | 0.9 | 1.6 | −7.1 | ± | 0.4 | 5.4 | 19.0 | ± | 0.3 | 1.5 | −7.0 | ± | 0.3 | 4.5 | ||||
| CH | Fresh | 30.1 | ± | 1.4 | 4.6 | −26.1 | ± | 1.1 | 4.4 | 36.6 | ± | 2.4 | 6.5 | −23.8 | ± | 2.7 | 11.4 | <0.06 |  | |
| Dehydrated | 35.6 | ± | 2.6 | 7.2 | −21.8 | ± | 2.4 | 10.9 | 59.7 | ± | 3.2 | 5.4 | −4.9 | ± | 0.2 | 4.7 | ||||
| Spray-dried | 55.3 | ± | 0.4 | 0.7 | −12.1 | ± | 0.4 | 3.5 | 32.4 | ± | 0.6 | 1.9 | −6.8 | ± | 0.1 | 1.5 | ||||
| Ecotype | First Stage | Second Stage | Third Stage | Residue (%) | Max. Weight Loss (%) | |||
|---|---|---|---|---|---|---|---|---|
| Weight Loss (%) | Temp. (°C) | Weight Loss (%) | Temp. (°C) | Weight Loss (%) | Temp. (°C) | |||
| CR Dehydrated | 13.54 | 101.45 | 36.53 | 311.56 | 39.25 | 482.09 | 10.69 | 89.32 |
| CR spray-dried | 7.44 | 107.96 | 64.63 | 349.54 | 10.50 | 468.81 | 17.43 | 82.57 |
| CE Dehydrated | 13.69 | 101.19 | 36.51 | 316.03 | 39.62 | 479.30 | 13.18 | 86.82 |
| CE spray-dried | 12.56 | 85.31 | 61.90 | 347.02 | 10.93 | 463.05 | 14.61 | 85.39 |
| CH Dehydrated | 10.18 | 108.65 | 45.39 | 352.22 | 14.57 | 492.14 | 29.86 | 70.14 |
| CH spray-dried | 12.19 | 78.67 | 48.71 | 318.24 | 27.06 | 527.09 | 12.05 | 87.95 |
| CQ Dehydrated | 13.47 | 91.63 | 53.31 | 370.25 | 18.31 | 451.96 | 14.91 | 85.09 |
| CQ spray-dried | 13.02 | 98.81 | 55.25 | 358.81 | 10.42 | 485.27 | 21.31 | 78.69 |
| Ecotype | NICOMP Distribution | Gaussian Distribution | |||||
|---|---|---|---|---|---|---|---|
| Peak | Size (nm) | % | ± | SD | C.V. (%) | ||
| CR | 1 | 11.1 | 0.9 | 379.1 | ± | 254.0 | 67.0 |
| 2 | 48.8 | 8.9 | |||||
| 3 | 217.3 | 90.2 | |||||
| CE | 1 | 16.5 | 1.3 | 496.1 | ± | 460.3 | 92.8 |
| 2 | 76.7 | 11 | |||||
| CH | 1 | 317.2 | 87.7 | 454.0 | ± | 269.2 | 59.3 |
| 2 | 43.4 | 4.2 | |||||
| 3 | 421.7 | 95.8 | |||||
| CQ | 1 | 22.1 | 1.3 | 413.8 | ± | 257.4 | 62.2 |
| 2 | 121.6 | 15.1 | |||||
| 3 | 648.7 | 83.6 | |||||
| Ecotype | ζ Potential (mV) | Viscosity Solution (cP) | Sample Temperature (°C) | pH Solution |
|---|---|---|---|---|
| CR | −30.88 | 0.933 | 23 | Neutral |
| CE | −27.17 | 0.933 | 23 | Neutral |
| CH | −27.14 | 0.933 | 23 | Neutral |
| CQ | −28.96 | 0.933 | 23 | Neutral |
| Metal | Wavelength (nm) | CR | CE | CH | CQ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dehydrated | |||||||||||||||||
| ± | SD | * | ± | SD | * | ± | SD | * | ± | SD | * | ||||||
| Al | 394.403 | 8.340 | ± | 0.010 | a | 5.163 | ± | 0.006 | b | 10.240 | ± | 0.010 | c | 15.730 | ± | 0.066 | d |
| As | 189.042 | 1.930 | ± | 0.022 | a | 2.377 | ± | 0.024 | b | 4.414 | ± | 0.072 | c | 3.793 | ± | 0.026 | d |
| Ba | 389.178 | 19.435 | ± | 0.233 | a | 13.235 | ± | 0.059 | b | 4.492 | ± | 0.023 | c | 65.107 | ± | 0.221 | d |
| Be | 313.042 | 0.001 | ± | 0.000 | a | 0.001 | ± | 0.000 | b | 0.001 | ± | 0.000 | c | 0.001 | ± | 0.000 | d |
| Ca | 317.933 | 186.559 | ± | 1.733 | a | 248.226 | ± | 0.577 | b | 221.790 | ± | 0.289 | c | 212.956 | ± | 0.362 | d |
| Cd | 214.438 | 0.140 | ± | 0.003 | a | 0.170 | ± | 0.001 | b | 0.320 | ± | 0.001 | c | 0.299 | ± | 0.001 | d |
| Cr | 267.716 | 0.086 | ± | 0.001 | a | 0.078 | ± | 0.001 | b | 0.149 | ± | 0.001 | c | 0.221 | ± | 0.003 | d |
| Cu | 324.754 | 0.055 | ± | 0.001 | c | 0.053 | ± | 0.001 | c | 0.184 | ± | 0.001 | a | 0.170 | ± | 0.005 | b |
| Fe | 239.562 | 8.841 | ± | 0.047 | a | 2.394 | ± | 0.006 | b | 8.354 | ± | 0.060 | c | 14.495 | ± | 0.066 | d |
| K | 766.490 | 4.420 | ± | 0.061 | a | 18.447 | ± | 0.100 | b | 60.847 | ± | 0.095 | c | 34.463 | ± | 0.015 | d |
| Mg | 285.213 | 25.996 | ± | 0.289 | a | 30.430 | ± | 0.153 | b | 48.996 | ± | 0.058 | c | 32.457 | ± | 0.001 | d |
| Mn | 257.610 | 1.040 | ± | 0.006 | a | 1.103 | ± | 0.001 | b | 1.723 | ± | 0.010 | c | 3.770 | ± | 0.011 | d |
| Na | 588.995 | 14.247 | ± | 0.176 | a | 6.213 | ± | 0.039 | b | 13.380 | ± | 0.055 | c | 16.650 | ± | 0.004 | d |
| Ni | 341.476 | 0.017 | ± | 0.003 | c | 0.010 | ± | 0.000 | c | 0.543 | ± | 0.006 | a | 0.367 | ± | 0.032 | b |
| Pb | 220.353 | 0.507 | ± | 0.015 | a | 0.577 | ± | 0.006 | b | 1.183 | ± | 0.006 | c | 1.083 | ± | 0.038 | d |
| Se | 203.985 | 1.723 | ± | 0.050 | a | 2.053 | ± | 0.032 | b | 4.137 | ± | 0.025 | c | 3.647 | ± | 0.055 | d |
| V | 311.071 | 0.027 | ± | 0.001 | b | 0.022 | ± | 0.001 | c | 0.087 | ± | 0.002 | a | 0.083 | ± | 0.002 | a |
| Zn | 213.856 | 0.197 | ± | 0.002 | a | 0.362 | ± | 0.002 | b | 0.268 | ± | 0.002 | c | 0.301 | ± | 0.002 | d |
| Spray-dried | |||||||||||||||||
| Al | 394.403 | 9.610 | ± | 0.000 | a | 6.027 | ± | 0.006 | b | 6.420 | ± | 0.020 | c | 11.677 | ± | 0.058 | d |
| As | 189.042 | 2.732 | ± | 0.018 | a | 2.597 | ± | 0.036 | b | 2.532 | ± | 0.050 | b,c | 2.452 | ± | 0.038 | c |
| Ba | 389.178 | 34.790 | ± | 0.057 | a | 20.528 | ± | 0.060 | b | 2.307 | ± | 0.006 | c | 49.723 | ± | 0.345 | d |
| Be | 313.042 | 0.001 | ± | 0.000 | a | 0.001 | ± | 0.000 | b | 0.002 | ± | 0.000 | c | 0.001 | ± | 0.000 | d |
| Ca | 317.933 | 283.248 | ± | 0.578 | a | 277.241 | ± | 0.576 | b | 306.582 | ± | 1.000 | c | 271.915 | ± | 2.310 | d |
| Cd | 214.438 | 0.216 | ± | 0.002 | a | 0.199 | ± | 0.002 | b | 0.181 | ± | 0.001 | c | 0.190 | ± | 0.002 | d |
| Cr | 267.716 | 0.139 | ± | 0.001 | a | 0.077 | ± | 0.001 | b | 2.436 | ± | 0.010 | c | 0.104 | ± | 0.001 | d |
| Cu | 324.754 | 0.122 | ± | 0.002 | b | 0.087 | ± | 0.001 | c | 0.141 | ± | 0.001 | a | 0.122 | ± | 0.001 | b |
| Fe | 239.562 | 12.555 | ± | 0.058 | a | 2.754 | ± | 0.011 | b | 20.621 | ± | 0.100 | c | 8.755 | ± | 0.035 | d |
| K | 766.490 | 8.423 | ± | 0.055 | c | 26.530 | ± | 0.154 | b | 27.890 | ± | 0.252 | a | 26.523 | ± | 0.251 | b |
| Mg | 285.213 | 43.240 | ± | 0.116 | a | 38.173 | ± | 0.100 | b | 23.407 | ± | 0.152 | c | 19.774 | ± | 0.201 | d |
| Mn | 257.610 | 1.361 | ± | 0.001 | a | 1.160 | ± | 0.000 | b | 1.474 | ± | 0.006 | c | 1.917 | ± | 0.011 | d |
| Na | 588.995 | 28.430 | ± | 0.102 | a | 8.863 | ± | 0.039 | b | 6.130 | ± | 0.064 | c | 10.796 | ± | 0.122 | d |
| Ni | 341.476 | 0.280 | ± | 0.000 | a | 0.093 | ± | 0.006 | b | 2.183 | ± | 0.015 | c | 0.257 | ± | 0.006 | d |
| Pb | 220.353 | 0.679 | ± | 0.011 | b | 0.701 | ± | 0.010 | b | 0.609 | ± | 0.008 | c | 0.725 | ± | 0.003 | a |
| Se | 203.985 | 2.650 | ± | 0.036 | b | 2.347 | ± | 0.042 | c | 2.860 | ± | 0.040 | a | 2.277 | ± | 0.029 | c |
| V | 311.071 | 0.050 | ± | 0.001 | a | 0.032 | ± | 0.001 | b | 0.022 | ± | 0.001 | c | 0.045 | ± | 0.001 | d |
| Zn | 213.856 | 0.763 | ± | 0.002 | a | 0.176 | ± | 0.001 | b | 0.289 | ± | 0.002 | c | 0.345 | ± | 0.002 | d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choque-Quispe, D.; Mojo-Quisani, A.; Ligarda-Samanez, C.A.; Calla-Florez, M.; Ramos-Pacheco, B.S.; Zamalloa-Puma, L.M.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Zamalloa-Puma, A.; et al. Preliminary Characterization of a Spray-Dried Hydrocolloid from a High Andean Algae (Nostoc sphaericum). Foods 2022, 11, 1640. https://doi.org/10.3390/foods11111640
Choque-Quispe D, Mojo-Quisani A, Ligarda-Samanez CA, Calla-Florez M, Ramos-Pacheco BS, Zamalloa-Puma LM, Peralta-Guevara DE, Solano-Reynoso AM, Choque-Quispe Y, Zamalloa-Puma A, et al. Preliminary Characterization of a Spray-Dried Hydrocolloid from a High Andean Algae (Nostoc sphaericum). Foods. 2022; 11(11):1640. https://doi.org/10.3390/foods11111640
Chicago/Turabian StyleChoque-Quispe, David, Antonieta Mojo-Quisani, Carlos A. Ligarda-Samanez, Miriam Calla-Florez, Betsy S. Ramos-Pacheco, Lourdes Magaly Zamalloa-Puma, Diego E. Peralta-Guevara, Aydeé M. Solano-Reynoso, Yudith Choque-Quispe, Alan Zamalloa-Puma, and et al. 2022. "Preliminary Characterization of a Spray-Dried Hydrocolloid from a High Andean Algae (Nostoc sphaericum)" Foods 11, no. 11: 1640. https://doi.org/10.3390/foods11111640
APA StyleChoque-Quispe, D., Mojo-Quisani, A., Ligarda-Samanez, C. A., Calla-Florez, M., Ramos-Pacheco, B. S., Zamalloa-Puma, L. M., Peralta-Guevara, D. E., Solano-Reynoso, A. M., Choque-Quispe, Y., Zamalloa-Puma, A., Palomino-Malpartida, Y. G., Medina-Quiquin, L. D., & Kari-Ferro, A. (2022). Preliminary Characterization of a Spray-Dried Hydrocolloid from a High Andean Algae (Nostoc sphaericum). Foods, 11(11), 1640. https://doi.org/10.3390/foods11111640











