Modern Analytical Methods for the Analysis of Pesticides in Grapes: A Review
Abstract
:1. Introduction
| Number | Pesticides | Class of Pesticides | Application | LAC (mg/kg) | References |
|---|---|---|---|---|---|
| 1 | Abamectin | Avermectins, Biological pesticides | Rape and grape | 0.01 | [13,14] |
| 2 | Ametrine | Other substances | Grapes | [14,15] | |
| 3 | Boscalid | Contact fungicide from the carboxamide class | Against diseases of grapes (grey rot), against diseases of grapes (oidium) | 5 | [16,17] |
| 4 | Captan | Phthalimides | Cotton, grapes, apple tree, rapeseed | 0.03 | [18] |
| 5 | Carbendazim | Benzimidazoles | Grapes | 0.3 | [14,19,20,21,22] |
| 6 | Chlorpyrifos | Organophosphates | Cotton, sugar beet, apple, peach, potato, hops, alfalfa. Areas filled with locusts. Melons, grapes, onions, rapeseed, corn, sunflower | 0.01 | [15,22,23] |
| 7 | Cypermethrin | Pyrethroids | Cotton, sugar beet, apple, peach, potato, hops, alfalfa. Areas filled with locusts. Melons, grapes, onions, rapeseed, corn, sunflower | 0.5 | [18] |
| 8 | Cypermethrin-alpha | Pyrethroids | Spring wheat, locust filling, rapeseed, grapes, apple tree, sugar beet, potatoes, cotton | 0.5 | [18] |
| 9 | Cyprodinil | Aminopyrimidine | Grapes | 3 | [17,24] |
| 10 | Dichlorobenzamide | Benzamides | Grapes, wine, and raisins | [16] | |
| 11 | Dimethomorph | Other substances | Grapes | 3 | [20,22,25] |
| 12 | Diniconazole | Triazoles | Grapes | 0.01 | [15,25,26,27] |
| 13 | Ethion | Organothiophosphate | Grapes | 0.01 | [10,18,25] |
| 14 | Fenitrothion | Organophosphorus | Grapes | 0.01 | [23,25] |
| 15 | Fenthion | Organophosphorus | Grapes | 0.01 | [14,23] |
| 16 | Fludioxonil | Benzodioxoles | Grapes | 5 | [19,24] |
| 17 | Fluopicolide | Other substances | Grape or soil sample | 2 | [14,16,28] |
| 18 | Folpet | Phthalimide | Meadow, vineyards, tomato, cucumbers | 6 | [18,24,29] |
| 19 | Hexaconazole | Triazole | Grapes | 0.01 | [10,19,22] |
| 20 | Lambda-cyhalothrin | Pyrethroids | Grapes | 0.08 | [18] |
| 21 | Metalaxyl | Other substances | Grapes | 2 | [16,20,24,29] |
| 22 | Methomyl | Carbamate | Appletree, apricot, grapes, tomatoes, onions, cabbage, cucumbers, cotton | 0.01 | [18] |
| 23 | Oxadiazon | Aromatic pesticide | Grape | 0.01 | [15,26] |
| 24 | Penconazole | Triazoles | Grapes | 0.5 | [15,24,26] |
| 25 | Phosalone | Organophosphorus | Grapes | 0.01 | [10,22] |
| 26 | Picoxystrobin | Strobilurines | Grapes, wine, and raisins | 0.01 | [16] |
| 27 | Prochloraz | Imidazoles | Cabbage, apple, kiwi, pear, grape | 0.03 | [18,19] |
| 28 | Procymidone | Other substances | Grapes | 0.01 | [18,24] |
| 29 | Propiconazole | Triazole | To combat diseases of grain, grapevine | 0.01 | [17,20,25] |
| 30 | Pyraclostrobin | Strobilurines | Grapes | 0.3 | [16,17,30,31] |
| 31 | Pyrimethanil | Aminopyrimidines | Lettuce garlic shoot, yam, celery, carrot, pepper, chives, cowpea, tomato, spinach, cabbage, apple, kiwi, pear, grape | 5 | [17,20] |
| 32 | Tebuconazole | Third generation Triazole | For the treatment of grain seeds in the fight against phytopathogens transmitted with seeds, grape. | 0.5 | [15,19,20,21,26] |
| 33 | Thiophanate-methyl | Thioureas | Table grape | 0.1 | [19,20,21] |
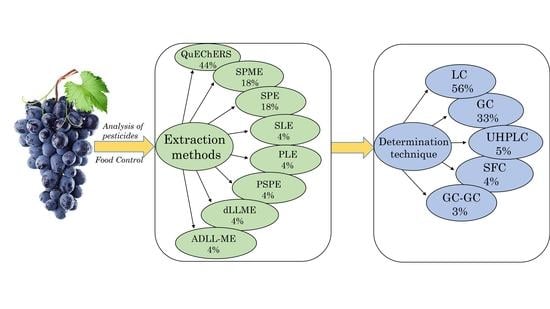
2. Sample Preparation Methods
2.1. Quick, Easy, Cheap, Effective, Rugged, and Safe Method (QuEChERS)
2.2. Solid-Phase Extraction (SPE)
2.3. Solid-Phase Micro-Extraction (SPME)
2.4. Other Sample Preparation Methods
3. Instrumental Detection Method
3.1. Gas Chromatography
3.2. Liquid Chromatography
| № | Detection Method | Number/Name of Analytes | LODs (mg/kg) | LOQs (mg/kg) | Reference |
|---|---|---|---|---|---|
| 1 | FI-MS/MS | 1 pesticide | [38] | ||
| 2 | GC/MS-MS | 8 pyrethroid pesticides | 0.02–0.5 | [25] | |
| 3 | GC-GC/TOF-MS | 5 organophosphorus pesticides | 0.001–0.01 | [34] | |
| 4 | GC-MS | 2 organophosphorus pesticides | 0.02–0.30 | 0.07–1.0 | [50] |
| 5 | GC-MSHPLC-MS-MS | 48 pesticides | 2.90–7.050.31–5.15 | [18] | |
| 6 | GC-MSGC-FID | 7 multiclass pesticides | 0.34–1.2 | 1.1–4.0 | [15] |
| 7 | GC-MS | 6 organophosphorus pesticides | 0.04–10 | 0.4–35 | [33] |
| 8 | GC–MSGC-FID | 9 multiclass pesticides | 0.34–1.2 | 1.1–4.0 | [26] |
| 9 | GC-MS/MS | 5 multiclass pesticides | [10] | ||
| 10 | GC-MS/MS | 6 multiclass pesticides | 3 | <10 | [23] |
| 11 | GC-MSD | 6 multiclass pesticides | [24] | ||
| 12 | GC–Q-TOF-MSLC–Q-TOF-MS | 733 pesticide multi-residues | 10 | [52] | |
| 13 | HPLC | 11 fungicides | [20] | ||
| 14 | HPLC | 6 triazole fungicides | 0.022–0.071 | [35] | |
| 15 | HPLC | 2 multiclass pesticides | 0.26–0.0039 | <0.001 | [31] |
| 16 | HPLC | 2 organophosphate pesticides | 1.2–4.2 | [32] | |
| 17 | HPLC | 5 multiclass pesticides | 0.02–0.0392 | 0.072–0.128 | [36] |
| 18 | HPLC-MS | Phoxim | [40] | ||
| 19 | HPLC-MS/MS | 7 multiclass pesticides | 0.0002–0.005 | 0.001–0.01 | [16] |
| 20 | HPLC-PDA | 5 pyrethroid pesticides | 0.02–0.039 | 0.072–0.128 | [36] |
| 21 | LC-MS | 14 fungicides | 0.002–0.01 | 0.01 | [19] |
| 22 | LC-MS | 7 multiclass pesticides | [37] | ||
| 23 | LC-MS/MS | 96 multiclass pesticides | 0.01–5.86 | [9] | |
| 24 | LC-MS/MS | 5 multiclass pesticides | 0.007–0.01 | [28] | |
| 25 | LC-MS/MS | 5 multiclass pesticides | [13] | ||
| 26 | LC-MS/MS | 3 multiclass pesticides | 2.1–8.7 | <0.1 | [21] |
| 27 | LC-MS/MS | 2 multiclass pesticides | [30] | ||
| 28 | LC-MS/MS | 49 fungicide and pesticides | 0.2–13 | [39] | |
| 29 | LC-MS-MS | 136 pesticides | 0.5–10 ng/g | [17] | |
| 30 | RP-HPLC | 3 multiclass pesticides | [29] | ||
| 31 | SFC-Q-TOF/MS | Diniconazole | 0.010–1.0 | 0.005 | [37] |
| 32 | UHPLC/TOF-MS | 60 multiclass pesticides | 0.3–3.8 | 0.8–11.8 | [22] |
| 33 | UHPLC-MS/MS | 250 pesticides | 0.6–6.0 | [14] | |
| 34 | UPLC-Q-TOF-MS | 134 pesticides | <10 | [55] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | enzyme acetylcholinesterase |
| ADLL-ME | assisted dispersive liquid-liquid microextraction method |
| dLLME | dispersive liquid-liquid microextraction |
| DSPE | dispersive solid-phase extraction |
| EC | European Commission |
| GC | gas chromatography |
| HPLC | high performance liquid chromatography |
| LAC | limits of acceptable concentrations |
| LC | liquid chromatography |
| MIP | molecular imprinted polymer |
| MSPD | matrix solid phase dispersion |
| OPPs | organophosphorus compounds |
| PDMS | polydimethylsiloxane |
| PLE | pressurized liquid extraction |
| PSA | primary secondary amine |
| PSPE | polymeric solid phase extraction |
| QuEChERS | Quick, Easy, Cheap, Effective, Rugged, and Safe |
| RSD | Relative Standard Deviation |
| SLE | solid–liquid extraction |
| SPE | solid-phase extraction |
| SPME | solid-phase microextraction |
| PDA | diode-array detection |
References
- International Organisation of Vine and Wine. World Vitiviniculture Situation. Available online: http://www.oiv.int/oiv/info/enpublicationsstatistiques (accessed on 15 April 2022).
- FAOSTAT. Food and Agriculture Data for All FAO Regional Groupings. Available online:https://www.fao.org/statistics/en (accessed on 17 April 2022).
- Flamini, R. Mass Spectrometry in Grape and Wine Chemistry. Part I: Polyphenols. Mass Spectrom. Rev. 2003, 22, 218–250. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.C. Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Bakirci, G.T.; Hişil, Y. Fast and Simple Extraction of Pesticide Residues in Selected Fruits and Vegetables Using Tetrafluoroethane and Toluene Followed by Ultrahigh-Performance Liquid Chromatography/Tandem Mass Spectrometry. Food Chem. 2012, 135, 1901–1913. [Google Scholar] [CrossRef]
- European Comission. Commission Regulation (EC) No 1881/2006: Commision Regulation (EC) 396/2005. 2006. Available online: https://www.legislation.gov.uk/eur/2006/1881 (accessed on 15 April 2022).
- EU Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/ (accessed on 20 April 2022).
- Grimalt, S.; Dehouck, P. Review of Analytical Methods for the Determination of Pesticide Residues in Grapes. J. Chromatogr. A 2016, 1433, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bouagga, A.; Chaabane, H.; Toumi, K.; Hamdane, A.M.; Nasraoui, B.; Joly, L. Pesticide Residues in Tunisian Table Grapes and Associated Risk for Consumer’s Health. Food Addit. Contam. Part B 2019, 12, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, A.; Nili-Ahmadabadi, A.; Rahimi, A.; Vahidinia, A.; Taheri, M. Dissipation Behavior and Risk Assessment of Fungicide and Insecticide Residues in Grape under Open-Field, Storage and Washing Conditions. J. Clean. Prod. 2020, 270, 122287. [Google Scholar] [CrossRef]
- Pu, C.H.; Lin, S.K.; Chuang, W.C.; Shyu, T.H. Modified QuEChERS Method for 24 Plant Growth Regulators in Grapes Using LC-MS/MS. J. Food Drug Anal. 2018, 26, 637–648. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services, Public Health Service Food and Drug Administration. Pesticide Analytical Manual, Volume I, 10/97 Revisions. Available online: https://www.fda.gov/media/74881/download (accessed on 15 April 2022).
- Zhou, Q.; Bian, Y.; Peng, Q.; Liu, F.; Wang, W.; Chen, F. The Effects and Mechanism of Using Ultrasonic Dishwasher to Remove Five Pesticides from Rape and Grape. Food Chem. 2019, 298, 125007. [Google Scholar] [CrossRef]
- Valera-Tarifa, N.M.; Santiago-Valverde, R.; Hernández-Torres, E.; Martínez-Vidal, J.L.; Garrido-Frenich, A. Development and Full Validation of a Multiresidue Method for the Analysis of a Wide Range of Pesticides in Processed Fruit by UHPLC-MS/MS. Food Chem. 2020, 315, 126304. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Dabbagh, M.S. Development of a Dispersive Solid Phase Extraction Method Based on in Situ Formation of Adsorbent Followed by Dispersive Liquid–Liquid Microextraction for Extraction of Some Pesticide Residues in Fruit Juice Samples. J. Chromatogr. A 2020, 1627, 461398. [Google Scholar] [CrossRef]
- Hou, X.; Xu, Z.; Zhao, Y.; Liu, D. Rapid Analysis and Residue Evaluation of Six Fungicides in Grape Wine-Making and Drying. J. Food Compos. Anal. 2020, 89, 103465. [Google Scholar] [CrossRef]
- Kasperkiewicz, A.; Pawliszyn, J. Multiresidue Pesticide Quantitation in Multiple Fruit Matrices via Automated Coated Blade Spray and Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry. Food Chem. 2021, 339, 127815. [Google Scholar] [CrossRef] [PubMed]
- İçli, N.; Kahyaoğlu, D.T. Investigation of Pesticide Residues in Fresh Sultani Grapes and Antioxidant Properties of Fresh/Sun-Dried/Oven-Dried Grapes. Turk. J. Agric. For. 2020, 44, 350–360. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhao, H.Y.; Shen, Q.; Qi, P.P.; Wang, X.Q.; Xu, H.; Di, S.S.; Wang, Z.W. High-Throughput Determination of Fungicides in Grapes Using Thin-Film Microextraction Coupled with Liquid Chromatography–Tandem Mass Spectrometry. J. Sep. Sci. 2020, 43, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Chen, Y.; He, F.; Yang, B.; Zou, K.; Shen, N.; Zuo, B.; Liu, R.; Zhang, W.; Li, Y. Risk Assessment of Fungicide Pesticide Residues in Vegetables and Fruits in the Mid-Western Region of China. J. Food Compos. Anal. 2021, 95, 103663. [Google Scholar] [CrossRef]
- Dong, B.; Yang, Y.; Pang, N.; Hu, J. Residue Dissipation and Risk Assessment of Tebuconazole, Thiophanate-Methyl and Its Metabolite in Table Grape by Liquid Chromatography-Tandem Mass Spectrometry. Food Chem. 2018, 260, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sivaperumal, P.; Anand, P.; Riddhi, L. Rapid Determination of Pesticide Residues in Fruits and Vegetables, Using Ultra-High-Performance Liquid Chromatography/Time-of-Flight Mass Spectrometry. Food Chem. 2015, 168, 356–365. [Google Scholar] [CrossRef]
- Venkatachalapathy, R.; Packirisamy, A.S.B.; Ramachandran, A.C.I.; Udhyasooriyan, L.P.; Peter, M.J.; Senthilnathan, K.; Basheer, V.A.; Muthusamy, S. Assessing the Effect of Chitosan on Pesticide Removal in Grape Juice during Clarification by Gas Chromatography with Tandem Mass Spectrometry. Process Biochem. 2020, 94, 305–312. [Google Scholar] [CrossRef]
- Bermúdez-Couso, A.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; López-Periago, E.; Soto-González, B.; Simal-Gándara, J. Seasonal Distributions of Fungicides in Soils and Sediments of a Small River Basin Partially Devoted to Vineyards. Water Res. 2007, 41, 4515–4525. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, W.; Chen, H.; Ding, Q.; Li, Q.; Zhang, L. In Situ Fabrication of Nitrogen Doped Graphitic Carbon Networks Coating for High-Performance Extraction of Pyrethroid Pesticides. Talanta 2021, 233, 122542. [Google Scholar] [CrossRef]
- Dabbagh, M.S.; Farajzadeh, M.A. Introduction of a New Procedure for the Synthesis of Polysulfone Magnetic Nanoparticles and Their Application in Magnetic Solid Phase Extraction for the Extraction of Some Pesticides from Fruit and Vegetable Juices. Microchem. J. 2020, 158, 105238. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Cui, X.; Wang, X.; Shen, H.; Chen, Z.; Huang, C.; Meruva, N.; Zhou, L.; Wang, F.; et al. Application and Enantiomeric Residue Determination of Diniconazole in Tea and Grape and Apple by Supercritical Fluid Chromatography Coupled with Quadrupole-Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2018, 1581-1582, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Feng, X.; Pan, L.; Jing, J.; Zhang, H. Residue and Risk Assessment of Fluopicolide and Cyazofamid in Grapes and Soil Using LC-MS/MS and Modified QuEChERS. RSC Adv. 2018, 8, 35485–35495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velkoska-Markovska, L.; Petanovska-Ilievska, B.; Jankulovska, M.S.; Ilievski, U. Development and Validation of High-Performance Liquid Chromatography Method for Determination of Some Pesticide Residues in Table Grape. Acta Chromatogr. 2018, 30, 250–254. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Yu, Y.; Chen, Y.; Zeng, S.; Lu, P.; Hu, D. Residues, Dissipation Kinetics, and Dietary Intake Risk Assessment of Two Fungicides in Grape and Soil. Regul. Toxicol. Pharmacol. 2018, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, S.; Gao, Y.; Ma, Y.; Hu, J.; Liu, X. Dissipation Behavior, Residue Distribution and Dietary Risk Assessment of Field-Incurred Boscalid and Pyraclostrobin in Grape and Grape Field Soil via MWCNTs-Based QuEChERS Using an RRLC-QqQ-MS/MS Technique. Food Chem. 2018, 274, 291–297. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Q.; Wang, Q.; Qiao, X.; Xu, Z. Study of a Molecularly Imprinted Solid-Phase Extraction Coupled with High-Performance Liquid Chromatography for Simultaneous Determination of Trace Trichlorfon and Monocrotophos Residues in Vegetables. J. Sci. Food Agric. 2013, 94, 1409–1415. [Google Scholar] [CrossRef]
- Moinfar, S.; Khodayari, A.; Sohrabnezhad, S.; Aghaei, A.; Jamil, L.A. MIL-53 (Al)/Fe2O3 Nanocomposite for Solid-Phase Microextraction of Organophosphorus Pesticides Followed by GC-MS Analysis. Microchim. Acta 2020, 187, 647. [Google Scholar] [CrossRef]
- Gionfriddo, E.; Souza-Silva, É.A.; Ho, T.D.; Anderson, J.L.; Pawliszyn, J. Exploiting the Tunable Selectivity Features of Polymeric Ionic Liquid-Based SPME Sorbents in Food Analysis. Talanta 2018, 188, 522–530. [Google Scholar] [CrossRef]
- Wang, Y.; He, M.; Chen, B.; Hu, B. Hydroxyl-Containing Porous Organic Framework Coated Stir Bar Sorption Extraction Combined with High Performance Liquid Chromatography-Diode Array Detector for Analysis of Triazole Fungicides in Grape and Cabbage Samples. J. Chromatogr. A 2020, 1633, 461628. [Google Scholar] [CrossRef]
- Yu, X.; Yang, H. Pyrethroid Residue Determination in Organic and Conventional Vegetables Using Liquid-Solid Extraction Coupled with Magnetic Solid Phase Extraction Based on Polystyrene-Coated Magnetic Nanoparticles. Food Chem. 2017, 217, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Oellig, C.; Schwack, W. Planar Solid Phase Extraction-A New Clean-up Concept in Multi-Residue Analysis of Pesticides by Liquid Chromatography-Mass Spectrometry. J. Chromatogr. A 2011, 1218, 6540–6547. [Google Scholar] [CrossRef] [PubMed]
- Ciasca, B.; Pecorelli, I.; Lepore, L.; Paoloni, A.; Catucci, L.; Pascale, M.; Lattanzio, V.M.T. Rapid and Reliable Detection of Glyphosate in Pome Fruits, Berries, Pulses and Cereals by Flow Injection—Mass Spectrometry. Food Chem. 2020, 310, 125813. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mayán, L.; Ramil, M.; Cela, R.; Rodríguez, I. Multiresidue Procedure to Assess the Occurrence and Dissipation of Fungicides and Insecticides in Vineyard Soils from Northwest Spain. Chemosphere 2020, 261, 127696. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, S.; Dang, J.; Wang, S.; Liu, Z.; Fang, J.; Han, P.; Zhang, J. Reduction of Phoxim Pesticide Residues from Grapes by Atmospheric Pressure Non-Thermal Air Plasma Activated Water. J. Hazard. Mater. 2019, 377, 98–105. [Google Scholar] [CrossRef]
- World Grape Production by Country. Available online: https://www.atlasbig.com/en-us/countries-grape-production (accessed on 21 April 2022).
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/about/en/ (accessed on 21 April 2022).
- Rong, L.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Pan, X.; Du, P.; Wei, D.; Zheng, Y. Simultaneous Determination of Three Pesticides and Their Metabolites in Unprocessed Foods Using Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry. Food Addit. Contam. Part A 2018, 35, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Collimore, W.A.; Bent, G.-A. A newly modified QuEChERS method for the analysis of organochlorine and organophosphate pesticide residues in fruits and vegetables. Environ. Monit. Assess. 2020, 192, 128. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24. [Google Scholar] [CrossRef] [Green Version]
- Narenderan, S.; Meyyanathan, S.; Karri, V.V.S.R.; Babu, B.; Chintamaneni, P. Multivariate response surface methodology assisted modified QuEChERS extraction method for the evaluation of organo-phosphate pesticides in fruits and vegetables cultivated in Nilgiris, South India. Food Chem. 2019, 300, 125188. [Google Scholar] [CrossRef]
- Xu, C.-H.; Chen, G.-S.; Xiong, Z.-H.; Fan, Y.-X.; Wang, X.-C.; Liu, Y. Applications of solid-phase microextraction in food analysis. TrAC Trends Anal. Chem. 2016, 80, 12–29. [Google Scholar] [CrossRef]
- Billiard, K.M.; Dershem, A.R.; Gionfriddo, E. Implementing Green Analytical Methodologies Using Solid-Phase Microextraction: A Review. Molecules 2020, 25, 5297. [Google Scholar] [CrossRef]
- Shamsipur, M.; Yazdanfar, N.; Ghambarian, M. Combination of Solid-Phase Extraction with Dispersive Liquid-Liquid Microextraction Followed by GC-MS for Determination of Pesticide Residues from Water, Milk, Honey and Fruit Juice. Food Chem. 2016, 204, 289–297. [Google Scholar] [CrossRef]
- Moinfar, S.; Jamil, L.A.; Sami, H.Z.; Ataei, S. An Innovative Continuous Sample Drop Flow Microextraction for GC–MS Determination of Pesticides in Grape Juice and Water Samples. J. Food Compos. Anal. 2021, 95, 103695. [Google Scholar] [CrossRef]
- Chawla, P.; Kaushik, R.; Swaraj, V.S.; Kumar, N. Organophosphorus Pesticides Residues in Food and Their Colorimetric Detection. Environ. Nanotechnol. Monit. Manag. 2018, 10, 292–307. [Google Scholar] [CrossRef]
- Pang, G.; Chang, Q.; Bai, R.; Fan, C.; Zhang, Z.; Yan, H.; Wu, X. Simultaneous Screening of 733 Pesticide Residues in Fruits and Vegetables by a GC/LC-Q-TOFMS Combination Technique. Engineering 2019, 6, 432–441. [Google Scholar] [CrossRef]
- Han, C.; Hu, B.; Liu, B.; Jin, J.; Ye, M.; Fu, C.; Shen, Y. Determination of Four Amide Fungicides in Grape Wine by Gas Chromatography Coupled with Tandem Mass Spectrometry. Food Anal. Methods 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Y.; Zheng, J.; Zhang, Y.; Yang, L.; Liao, C.; Su, L.; Zhou, Y.; Gong, D.; Chen, L.; et al. The Application of the QuEChERS Methodology in the Determination of Antibiotics in Food: A Review. TrAC-Trends Anal. Chem. 2019, 118, 517–537. [Google Scholar] [CrossRef]
- Meng, X.; Song, W.; Xiao, Y.; Zheng, P.; Cui, C.; Gao, W.; Hou, R. Rapid Determination of 134 Pesticides in Tea through Multi-Functional Filter Cleanup Followed by UPLC-QTOF-MS. Food Chem. 2022, 370, 130846. [Google Scholar] [CrossRef] [PubMed]

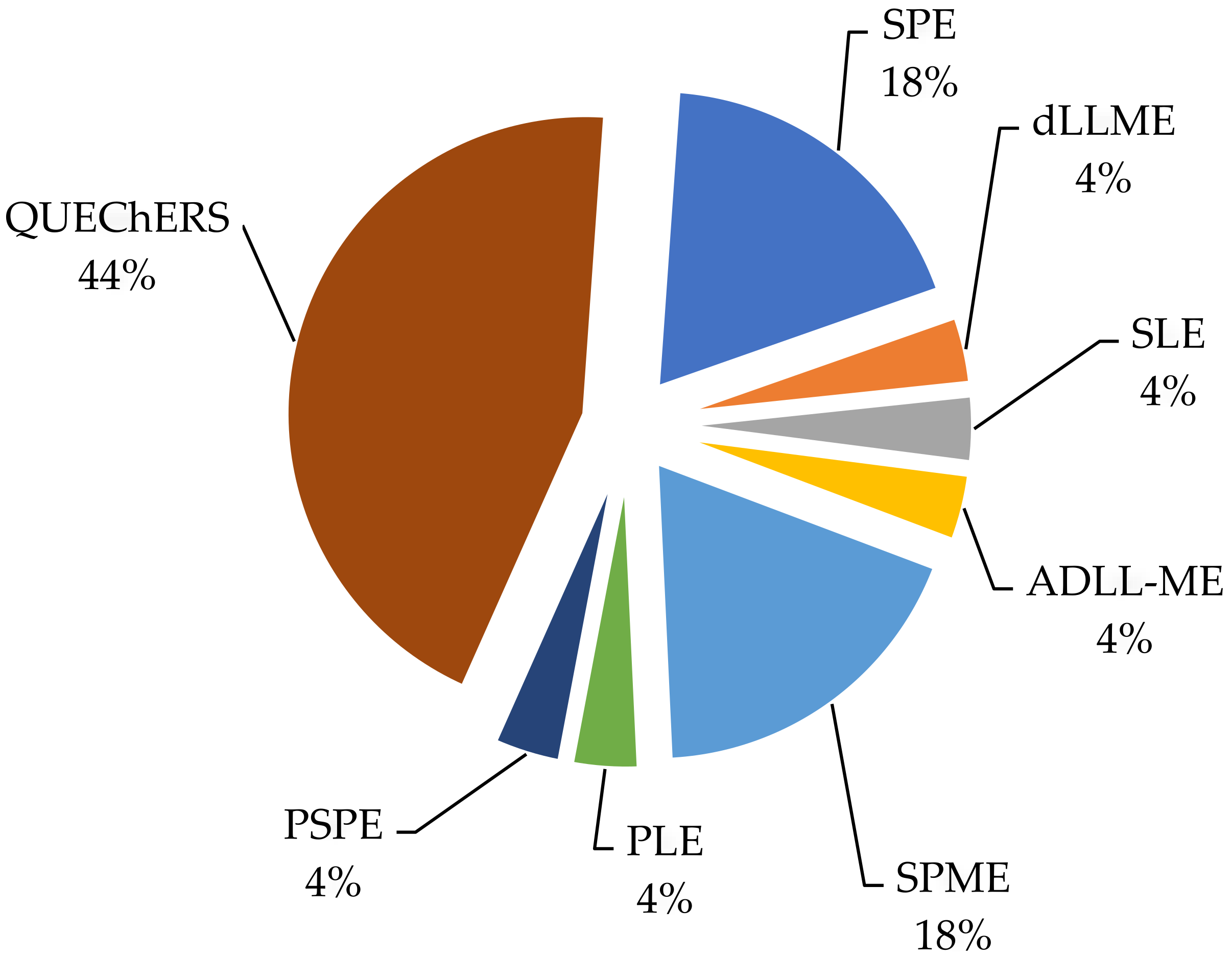
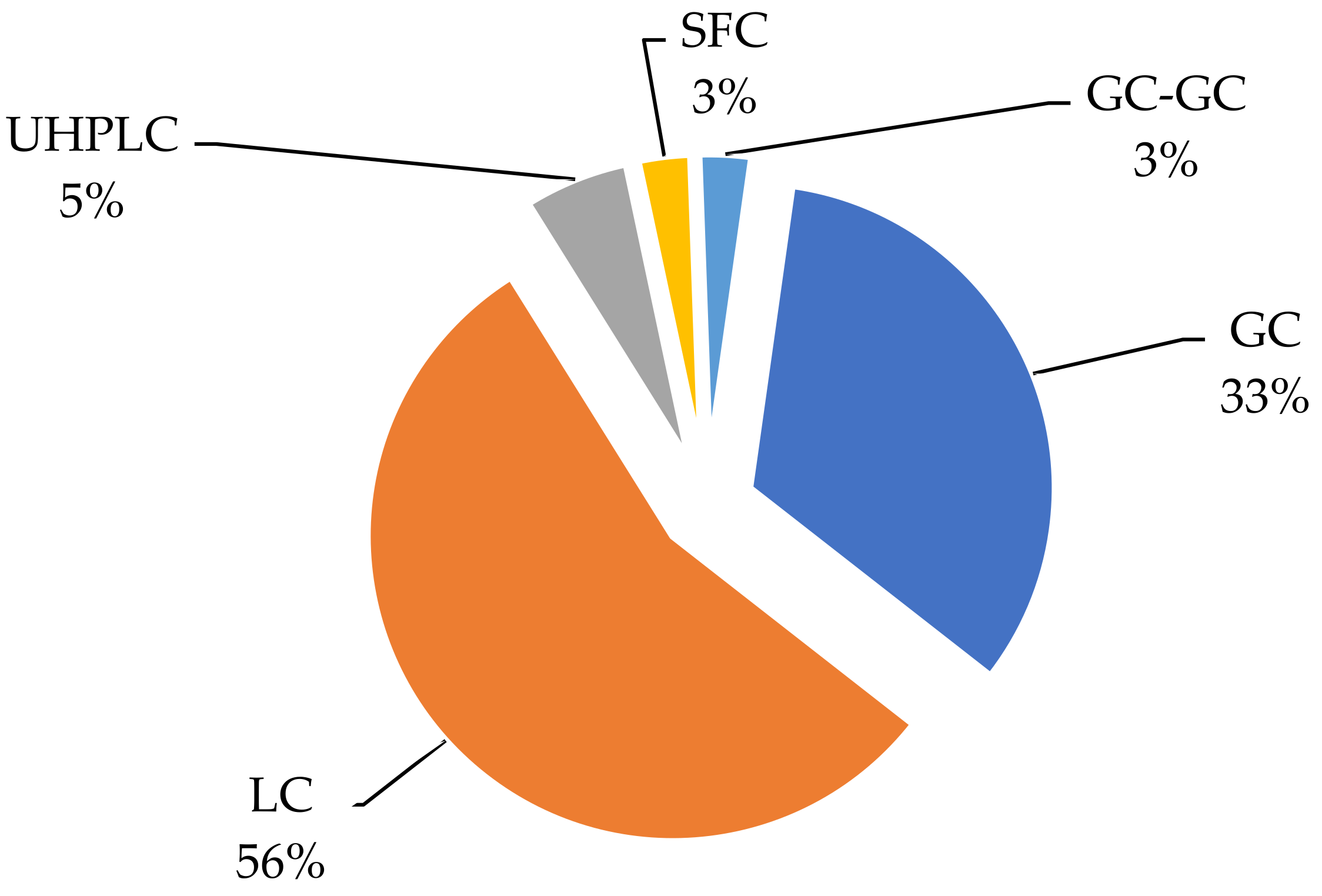

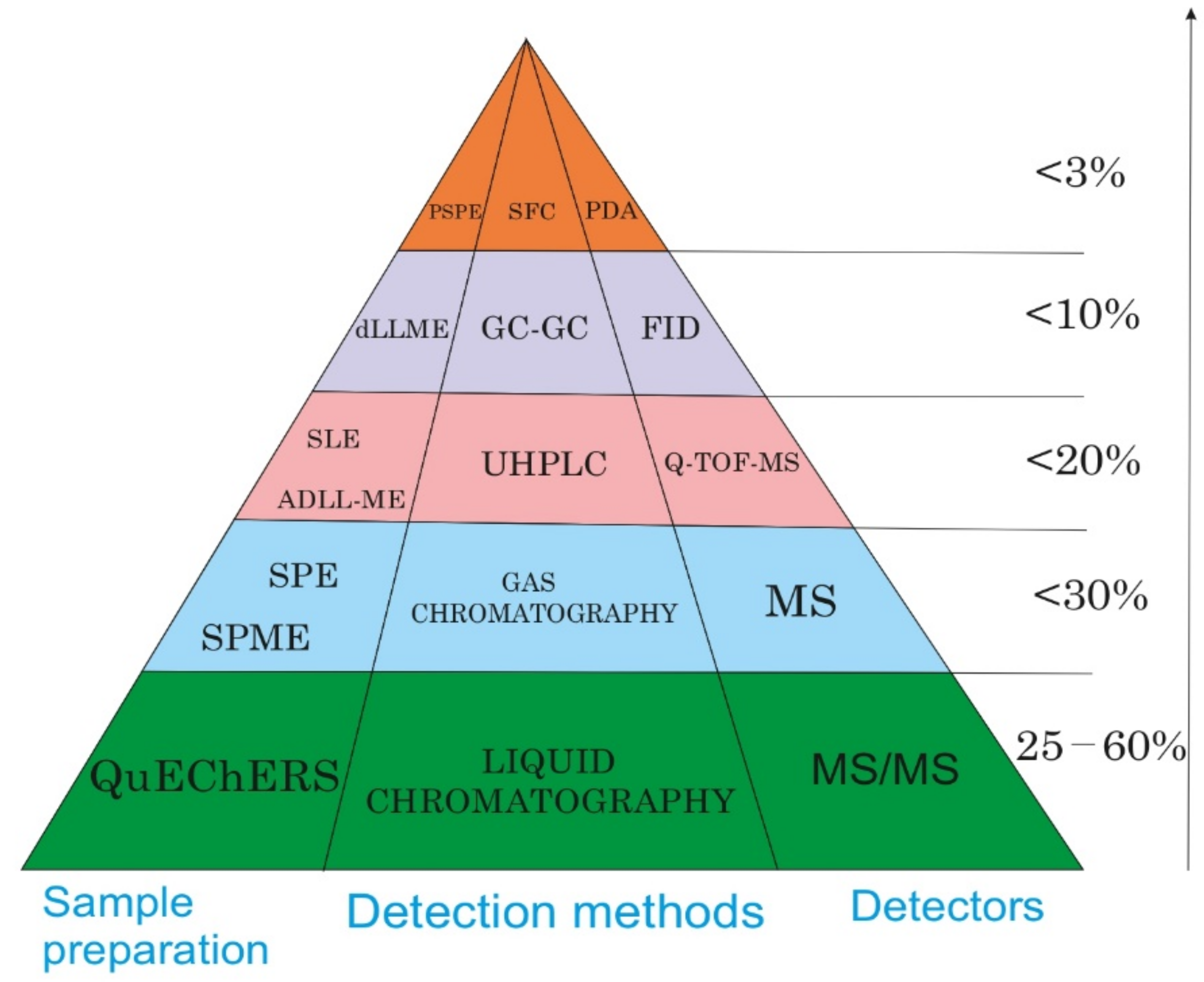
| № | Extraction Method | Matrix | Number/Name of Analytes | Recovery (%) | Study Region, Country | Reference |
|---|---|---|---|---|---|---|
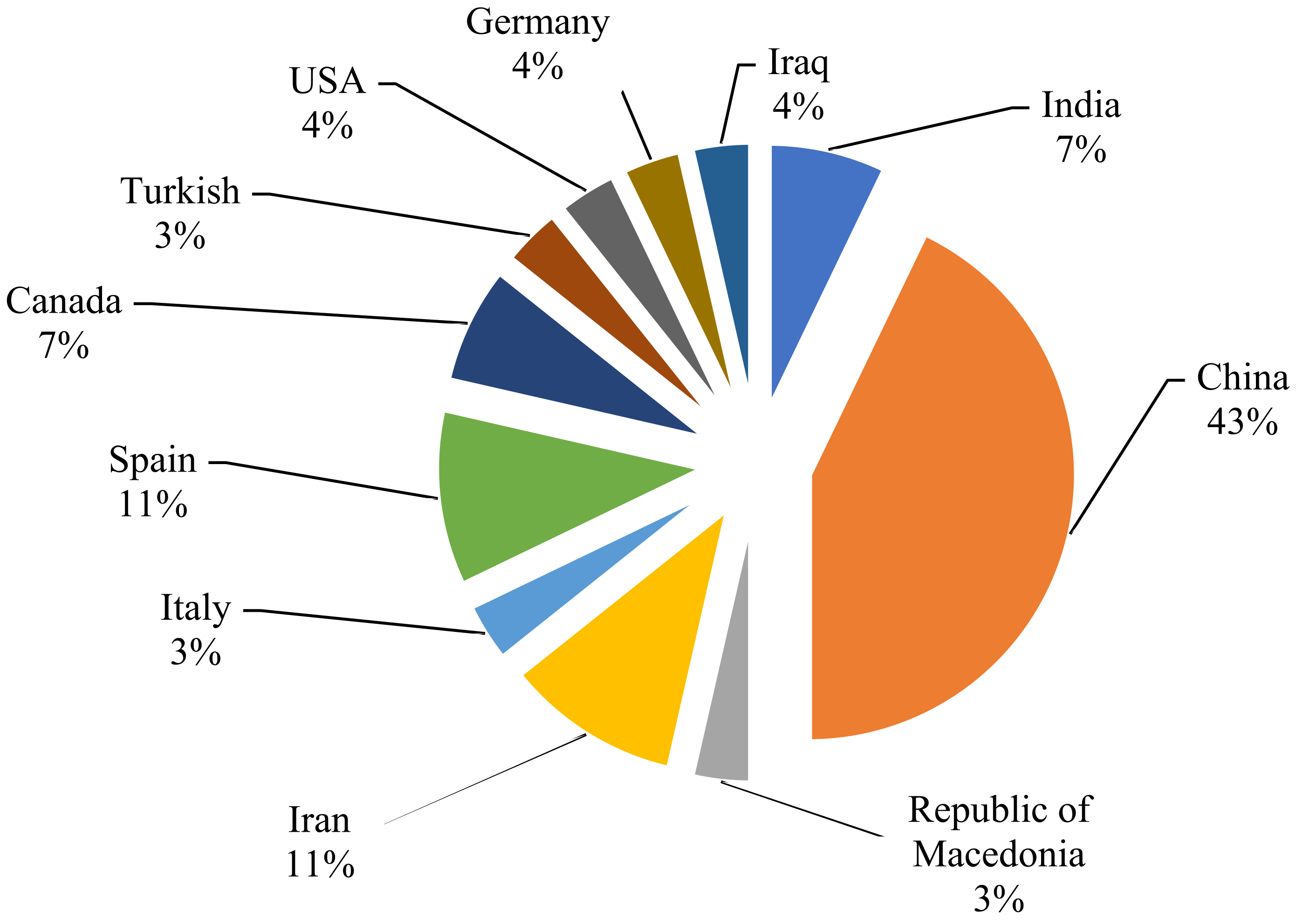
| 1 | Solid-phase extraction (SPE) | Grape, brinjal, cabbage, cauliflower, guava, okra, onion, potato, apple, banana, mango, orange, and pomegranate | 60 multiclass pesticides | 74–111 | India | [22] |
| 2 | Berry fruits, raspberry, strawberry, blueberry, and grape | 5 multiclass pesticides | 63–137 | China | [36] | |
| 3 | Grape, cauliflower, and leek | 2 pyrethroid pesticides | 88.5–94.2 | China | [32] | |
| 4 | Table grape | 3 multiclass pesticides | 90.55–105.40 | Republic of Macedonia | [29] | |
| 5 | Fruit juice (grape, sour cherry, peach, apple, orange, apricot, and mango) | 7 multiclass pesticides | 87–107 | Tabriz, Iran | [15] | |
| 6 | Grape | 7 multiclass pesticides | 90–104 | Germany | [37] | |
| 7 | Dispersive liquid-liquid microextraction (dLLME) | Mango, apricot, peach, apple, and grape | 9 multiclass pesticides | 46–95 | Karaj Iran | [26] |
| 8 | Solid–liquid extraction (SLE) | Chickpeas, apples, and grapes | Glyphosate | 60–111 | Italy | [38] |
| 9 | Assisted dispersive liquid-liquid microextraction method (ADLL-ME) | Vineyard soils, grapes | 6 multiclass pesticides | 75–100 | Spain | [24] |
| 10 | Solid-phase microextraction (SPME) | Vineyard soils, grapes | 49 multiclass fungicides and insecticides | 70–130 | Spain | |
| 11 | Apples, blueberries, strawberries, and grapes | 136 pesticides | - | Canada | [17] | |
| 12 | Grapes | 8 pyrethroid pesticides | 80.9–104.6 | China | [25] | |
| 13 | Grape | 6 organophosphorus pesticides | 87.5–112 | Iraq | [33] | |
| 14 | Grape | 5 organophosphorus pesticides | - | Canada | [34] | |
| 15 | Pressurized liquid extraction (PLE) | Grapes and grape juice | 12 fungicides | 70–130 | Spain | [39] |
| 16 | Polymeric solid phase extraction (PSPE) | Grape | 5 multiclass pesticides | - | Iran | [10] |
| 17 | Quick, easy, cheap, effective, rugged, and safe method (QuEChERS) | Grape | 2 multiclass pesticides | 31.7–54 | China | [31] |
| 18 | Grape | 2 multiclass pesticides | 76.88–97.05 | China | [30] | |
| 19 | Table grape | 3 multiclass pesticides | 83.2–105.4 | China | [21] | |
| 20 | 11 vegetable samples (lettuce garlic shoot, yam, celery, carrot, pepper, chives, cowpea, tomato, spinach, cabbage, apple, kiwi, pear, grape) | 11 multiclass pesticides | 71.3–116.7 | China | [20] | |
| 21 | Grape | 250 pesticides | 70–120 | Spain | [14] | |
| 22 | Rape and grape | 5 multiclass pesticides | 14.7–59.8 (Rape)72.1–100 (Grape) | China | [13] | |
| 23 | Grape or soil sample | 5 multiclass pesticides | 71.6–107.7 | China | [28] | |
| 24 | Grapes, wine, and raisins | 7 multiclass pesticides | 78.8–106.3 | China | [16] | |
| 25 | Grape and grape juice | 6 multiclass pesticides | 74–101 | India | [23] | |
| 26 | Table grape | 48 pesticides | 51–127 | Turkish | [18] | |
| 27 | Grape | Phoxim | 73.60 | China | [40] | |
| 28 | Grape | Diniconazole | 69.8–102.1 | China, USA | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syrgabek, Y.; Alimzhanova, M. Modern Analytical Methods for the Analysis of Pesticides in Grapes: A Review. Foods 2022, 11, 1623. https://doi.org/10.3390/foods11111623
Syrgabek Y, Alimzhanova M. Modern Analytical Methods for the Analysis of Pesticides in Grapes: A Review. Foods. 2022; 11(11):1623. https://doi.org/10.3390/foods11111623
Chicago/Turabian StyleSyrgabek, Yerkanat, and Mereke Alimzhanova. 2022. "Modern Analytical Methods for the Analysis of Pesticides in Grapes: A Review" Foods 11, no. 11: 1623. https://doi.org/10.3390/foods11111623
APA StyleSyrgabek, Y., & Alimzhanova, M. (2022). Modern Analytical Methods for the Analysis of Pesticides in Grapes: A Review. Foods, 11(11), 1623. https://doi.org/10.3390/foods11111623