A Single Catalytic Endolysin Domain Plychap001: Characterization and Application to Control Vibrio parahaemolyticus and Its Biofilm Directly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Cloning, Expression and Purification of Plychap001
2.3. Identification of Recombinant Plychap001
2.4. Anti-V. parahaemolyticus Activity Assay
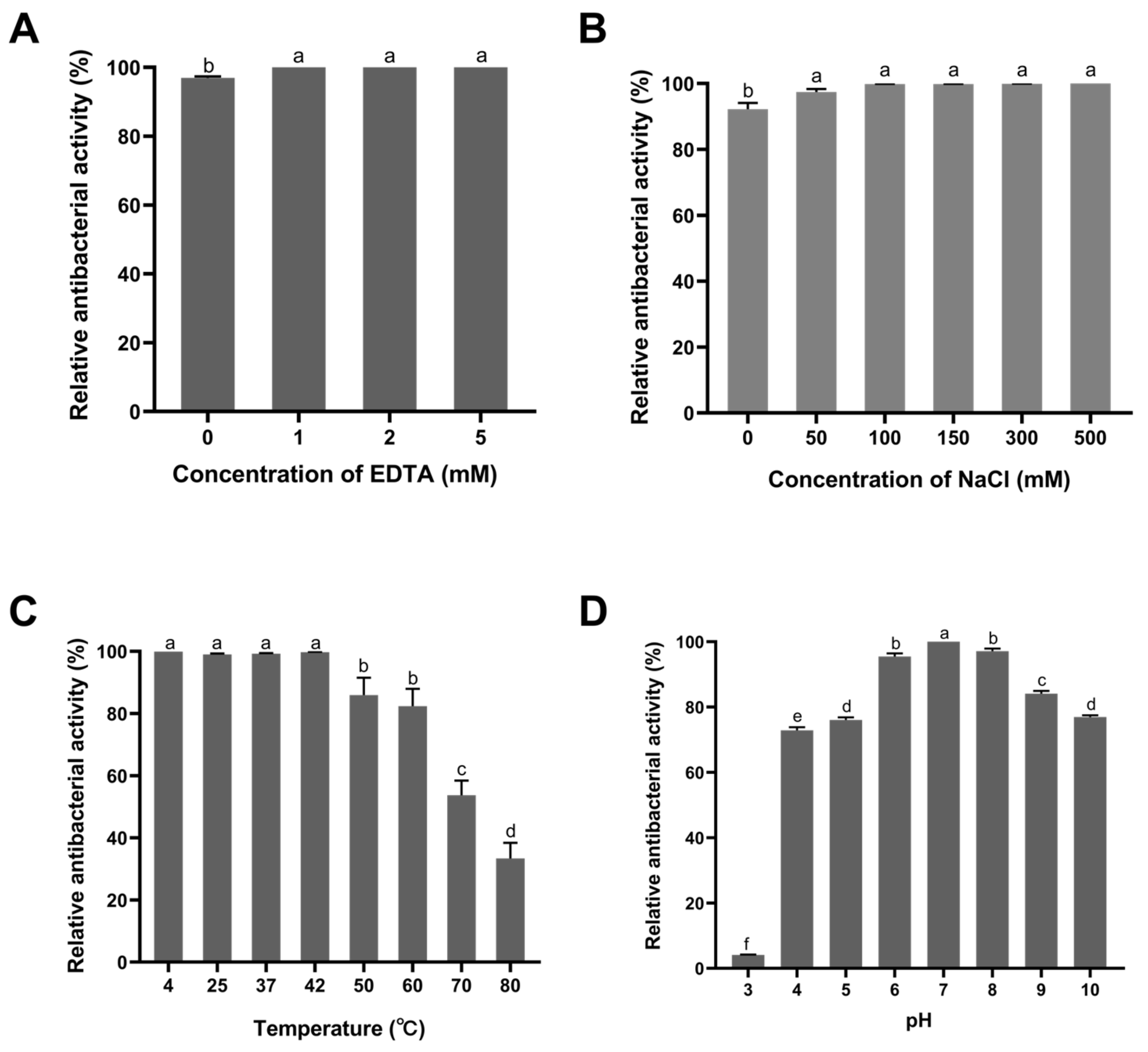
2.5. Stability of Recombinant Plychap001
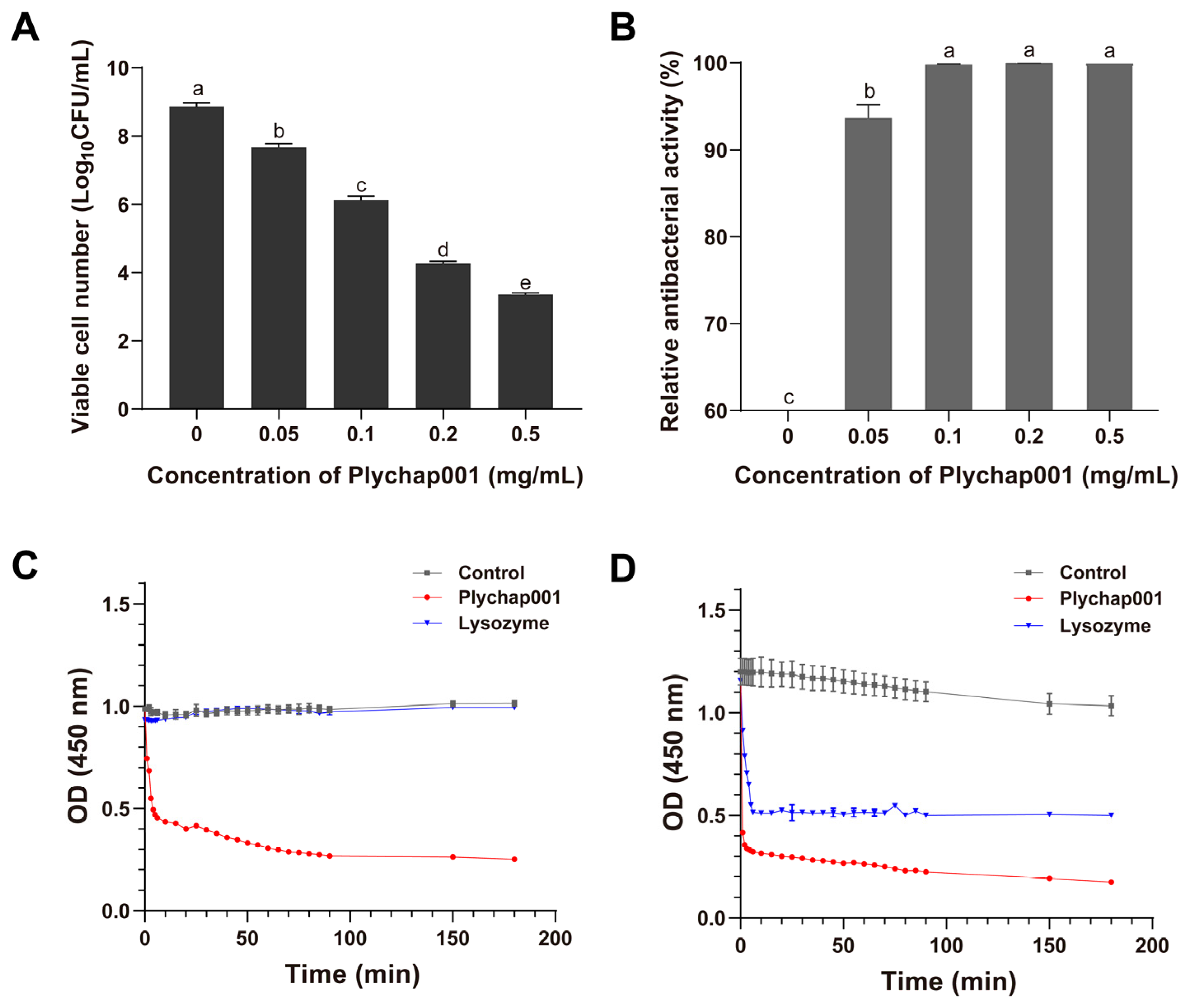
2.6. Turbidity Reduction Assay
2.7. Anti-Microbial Spectrum of Plychap001
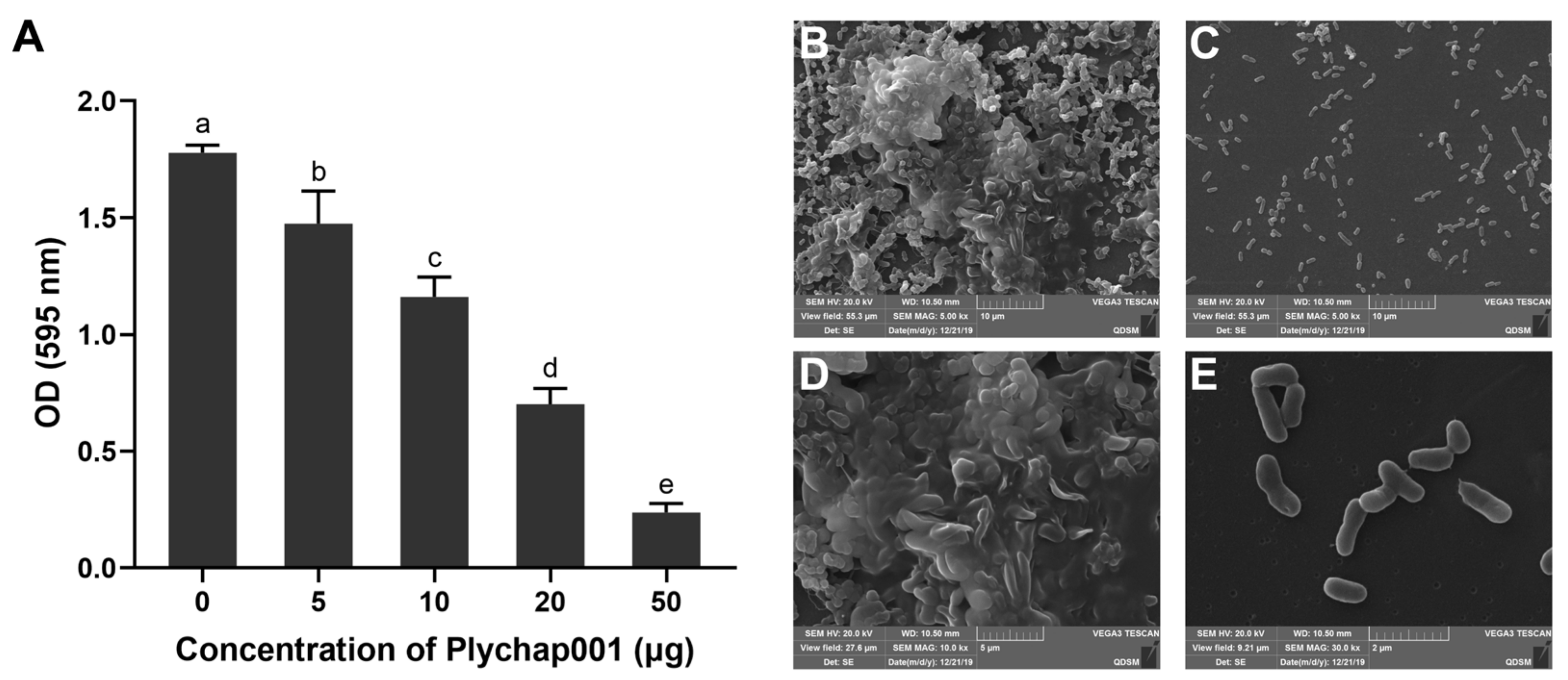
2.8. Analysis of the Bactericidal Effects of Plychap001 on V. parahaemolyticus Biofilm
2.9. Scanning Electron Microscope (SEM) Observation of Plychap001-Treated V. parahaemolyticus Biofilm
2.10. Statistical Analysis
3. Results
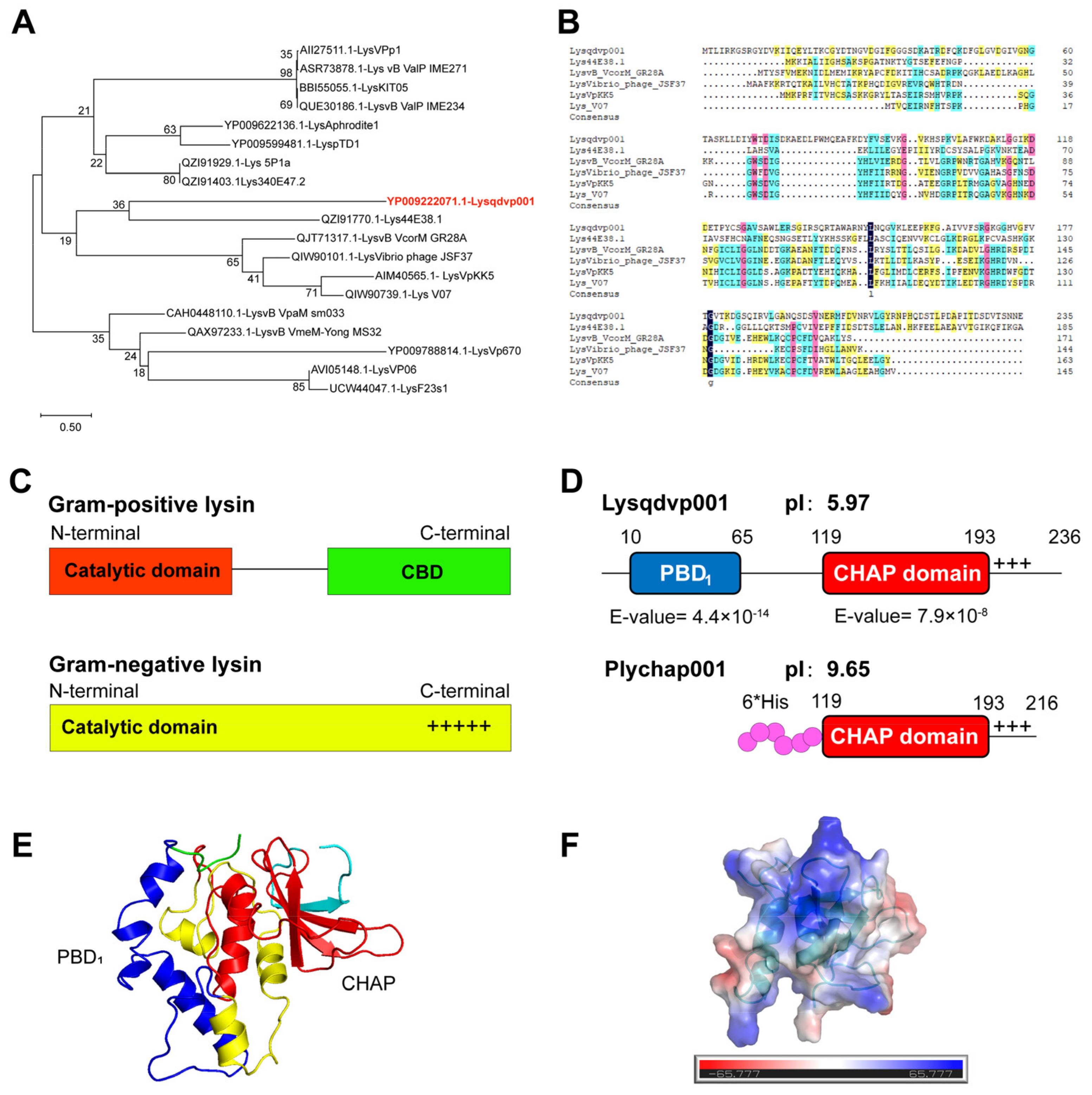
3.1. Modular Structure of Endolysin Lysqdvp001
3.2. Expression, Purification and Identification of Recombinant Plychap001
3.3. Antibacterial Activity of Recombinant Plychap001
3.4. Stability of Recombinant Plychap001
3.5. Antibacterial Spectrum of Plychap001
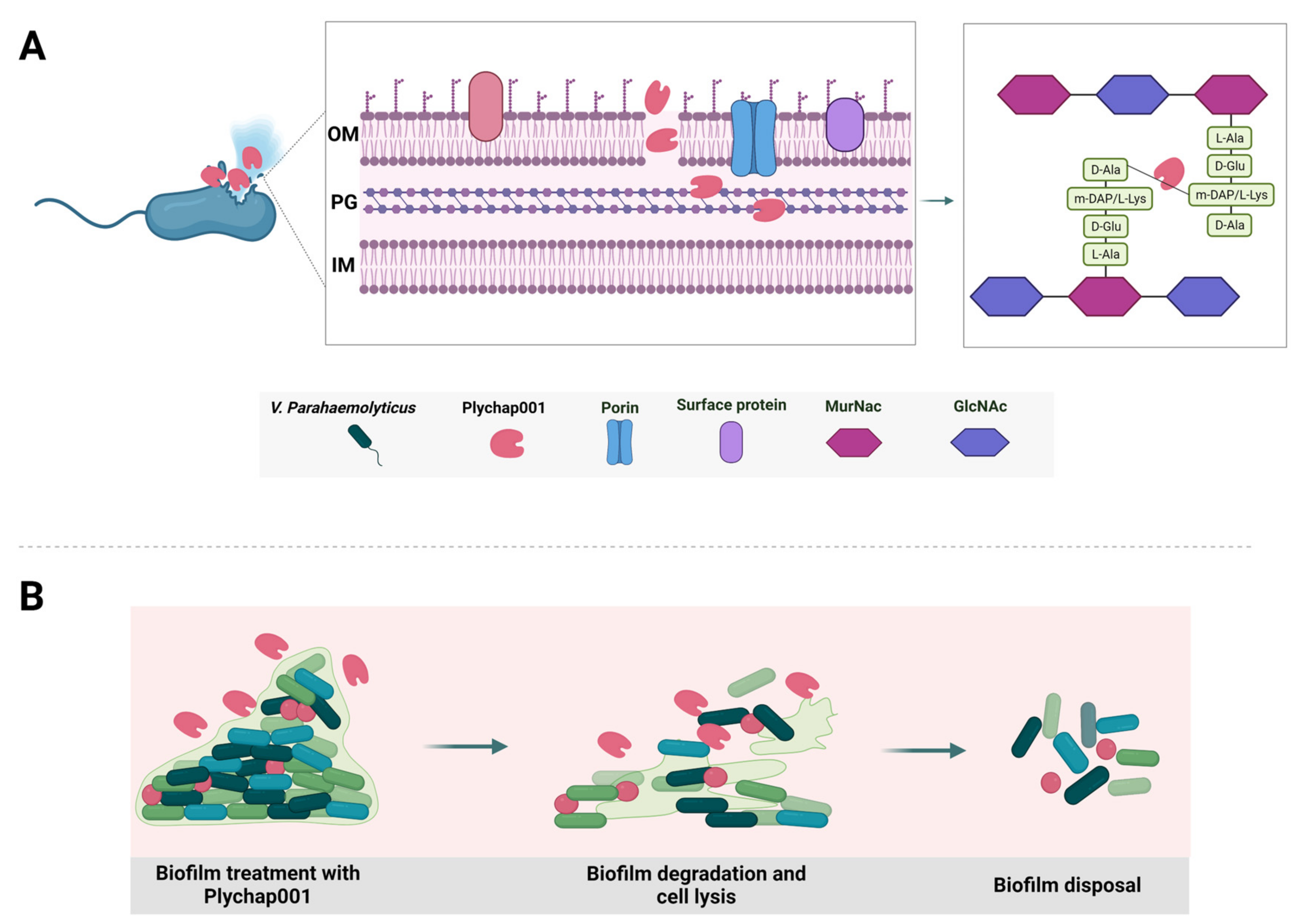
3.6. Plychap001 Can Effectively Degrade Mature V. parahaemolyticus Biofilm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banu, S.F.; Rubini, D.; Murugan, R.; Vadivei, V.; Gowrishankar, S.; Pandian, S.K.; Nithyanand, P. Exploring the antivirulent and sea food preservation efficacy of essential oil combined with DNase on Vibrio parahaemolyticus. LWT Food Sci. Technol. 2018, 95, 107–115. [Google Scholar] [CrossRef]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, T.; Jiang, F.; He, M.; Zhang, J.; Zeng, H.; Chen, M.; Pang, R.; Wu, S.; Wei, L.; Wang, J.; et al. Prevalence, virulence, antimicrobial resistance, and molecular characterization of fluoroquinolone resistance of Vibrio parahaemolyticus from different types of food samples in China. Int. J. Food Microbiol. 2020, 317, 108461. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, D.; Chen, K.; Wong, M.H.; Chen, S. First detection of AmpC beta-lactamase bla(CMY-2) on a conjugative IncA/C plasmid in a Vibrio parahaemolyticus isolate of food origin. Antimicrob. Agents Chemother. 2015, 59, 4106–4111. [Google Scholar] [CrossRef] [Green Version]
- Paterson, D.L.; Harris, P.N.A. Colistin resistance: A major breach in our last line of defence. Lancet Infect. Dis. 2016, 16, 132–133. [Google Scholar] [CrossRef]
- van Hal, S.J.; Willems, R.J.L.; Gouliouris, T.; Ballard, S.A.; Coque, T.M.; Hammerum, A.M.; Hegstad, K.; Westh, H.T.; Howden, B.P.; Malhotra-Kumar, S.; et al. The global dissemination of hospital clones of Enterococcus faecium. Genome Med. 2021, 13, 52. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, J.; Jiang, F.; He, M.; Zeng, H.; Chen, M.; Pang, R.; Wu, H.; Wu, S.; Wang, J.; et al. Characterization of class 1 integrons harboring blaVEB-1 in Vibrio parahaemolyticus isolated from ready-to-eat foods in China. Int. J. Food Microbiol. 2020, 318, 108473. [Google Scholar] [CrossRef]
- Shangguan, W.; Xie, T.; Zhang, R.; Lu, C.; Han, X.; Zhong, Q. Anti-biofilm potential of kefir-derived Lactobacillus paracasei L10 against Vibrio parahaemolyticus. Lett. Appl. Microbiol. 2021, 73, 750–758. [Google Scholar] [CrossRef]
- Rahman, M.U.; Wang, W.; Sun, Q.; Shah, J.A.; Li, C.; Sun, Y.; Li, Y.; Zhang, B.; Chen, W.; Wang, S. Endolysin, a Promising Solution against Antimicrobial Resistance. Antibiotics 2021, 10, 1277. [Google Scholar] [CrossRef]
- Shannon, R.; Radford, D.R.; Balamurugan, S. Impacts of food matrix on bacteriophage and endolysin antimicrobial efficacy and performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Trudil, D. Phage lytic enzymes: A history. Virol. Sin. 2015, 30, 26–32. [Google Scholar] [CrossRef]
- Fursov, M.V.; Abdrakhmanova, R.O.; Antonova, N.P.; Vasina, D.V.; Kolchanova, A.D.; Bashkina, O.A.; Rubalsky, O.V.; Samotrueva, M.A.; Potapov, V.D.; Makarov, V.V.; et al. Antibiofilm Activity of a Broad-Range Recombinant Endolysin LysECD7: In Vitro and In Vivo Study. Viruses 2020, 12, 545. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.-H.; Duc, H.M.; Masuda, Y.; Honjoh, K.-i.; Miyamoto, T. Application of endolysin LysSTG2 as a potential biocontrol agent against planktonic and biofilm cells of Pseudomonas on various food and food contact surfaces. Food Control 2022, 131, 108460. [Google Scholar] [CrossRef]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef]
- Gu, J.; Feng, Y.; Feng, X.; Sun, C.; Lei, L.; Ding, W.; Niu, F.; Jiao, L.; Yang, M.; Li, Y.; et al. Structural and biochemical characterization reveals LysGH15 as an unprecedented “EF-hand-like” calcium-binding phage lysin. PLoS Pathog. 2014, 10, e1004109. [Google Scholar] [CrossRef] [Green Version]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, H.H.; Duc, H.M.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T. Endolysin LysSTG2: Characterization and application to control Salmonella typhimurium biofilm alone and in combination with slightly acidic hypochlorous water. Food Microbiol. 2021, 98, 103791. [Google Scholar] [CrossRef]
- Zhou, B.; Zhen, X.; Zhou, H.; Zhao, F.; Fan, C.; Perculija, V.; Tong, Y.; Mi, Z.; Ouyang, S. Structural and functional insights into a novel two-component endolysin encoded by a single gene in Enterococcus faecalis phage. PLoS Pathog. 2020, 16, e1008394. [Google Scholar] [CrossRef]
- Liu, A.; Wang, Y.; Cai, X.; Jiang, S.; Cai, X.; Shen, L.; Liu, Y.; Han, G.; Chen, S.; Wang, J.; et al. Characterization of endolysins from bacteriophage LPST10 and evaluation of their potential for controlling Salmonella typhimurium on lettuce. LWT 2019, 114, 108372. [Google Scholar] [CrossRef]
- Oliveira, H.; Thiagarajan, V.; Walmagh, M.; Sillankorva, S.; Lavigne, R.; Neves-Petersen, M.T.; Kluskens, L.D.; Azeredo, J. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE 2014, 9, e108376. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, M.; Lin, H.; Wang, J.; Mao, X. The Vibrio parahaemolyticus-infecting bacteriophage qdvp001: Genome sequence and endolysin with a modular structure. Arch. Virol. 2016, 161, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Cong, Y.; Lin, H.; Wang, J. Development of cationic peptide chimeric lysins based on phage lysin Lysqdvp001 and their antibacterial effects against Vibrio parahaemolyticus: A preliminary study. Int. J. Food Microbiol. 2021, 358, 109396. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Meng, F.; Zhou, L.; Lu, F.; Bie, X.; Lu, Z.; Lu, Y. In Silico Development of Novel Chimeric Lysins with Highly Specific Inhibition against Salmonella by Computer-Aided Design. J. Agric. Food Chem. 2021, 69, 3751–3760. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A Novel Antimicrobial Endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Xu, L.; Huang, Y.; Lin, H.; Ahmed, I.; Li, Z. Identification and comparison of allergenicity of native and recombinant fish major allergen parvalbumins from Japanese flounder (Paralichthys olivaceus). Food Funct. 2019, 10, 6615–6623. [Google Scholar] [CrossRef]
- Yu, P.; Mathieu, J.; Yang, Y.; Alvarez, P.J.J. Suppression of Enteric Bacteria by Bacteriophages: Importance of Phage Polyvalence in the Presence of Soil Bacteria. Environ. Sci. Technol. 2017, 51, 5270–5278. [Google Scholar] [CrossRef]
- Lopatek, M.; Wieczorek, K.; Osek, J. Antimicrobial Resistance, Virulence Factors, and Genetic Profiles of Vibrio parahaemolyticus from Seafood. Appl. Environ. Microbiol. 2018, 84, e00537-18. [Google Scholar] [CrossRef] [Green Version]
- Matamp, N.; Bhat, S.G. Phage Endolysins as Potential Antimicrobials against Multidrug Resistant Vibrio alginolyticus and Vibrio parahaemolyticus: Current Status of Research and Challenges Ahead. Microorganisms 2019, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, H.; Vilas Boas, D.; Mesnage, S.; Kluskens, L.D.; Lavigne, R.; Sillankorva, S.; Secundo, F.; Azeredo, J. Structural and Enzymatic Characterization of ABgp46, a Novel Phage Endolysin with Broad Anti-Gram-Negative Bacterial Activity. Front. Microbiol. 2016, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Plotka, M.; Kapusta, M.; Dorawa, S.; Kaczorowska, A.K.; Kaczorowski, T. Ts2631 Endolysin from the Extremophilic Thermus scotoductus Bacteriophage vB_Tsc2631 as an Antimicrobial Agent against Gram-Negative Multidrug-Resistant Bacteria. Viruses 2019, 11, 657. [Google Scholar] [CrossRef] [Green Version]
- Plotka, M.; Sancho-Vaello, E.; Dorawa, S.; Kaczorowska, A.K.; Kozlowski, L.P.; Kaczorowski, T.; Zeth, K. Structure and function of the Ts2631 endolysin of Thermus scotoductus phage vB_Tsc2631 with unique N-terminal extension used for peptidoglycan binding. Sci. Rep. 2019, 9, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotka, M.; Szadkowska, M.; Hakansson, M.; Kovacic, R.; Al-Karadaghi, S.; Walse, B.; Werbowy, O.; Kaczorowska, A.K.; Kaczorowski, T. Molecular Characterization of a Novel Lytic Enzyme LysC from Clostridium intestinale URNW and Its Antibacterial Activity Mediated by Positively Charged N-Terminal Extension. Int. J. Mol. Sci. 2020, 21, 4894. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, D.; Wang, L.; Qu, M.; Li, F.; Tan, Z.; Yao, L. Characterization of a broad-spectrum endolysin LysSP1 encoded by a Salmonella bacteriophage. Appl. Microbiol. Biotechnol. 2021, 105, 5461–5470. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Wang, L.; Deng, B.; Jiu, S.; Ma, C.; Zhang, C.; Almeida, A.; Wang, D.; Xu, W.; Wang, S. Characterization of a Lytic Bacteriophage against Pseudomonas syringae pv. actinidiae and Its Endolysin. Viruses 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, V.; Chan, K.G.; Lee, L.H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014, 5, 705. [Google Scholar] [CrossRef] [Green Version]
- Cha, Y.; Son, B.; Ryu, S. Effective removal of staphylococcal biofilms on various food contact surfaces by Staphylococcus aureus phage endolysin LysCSA13. Food Microbiol. 2019, 84, 103245. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 2005, 187, 7161–7164. [Google Scholar] [CrossRef] [Green Version]
- Ning, H.Q.; Lin, H.; Wang, J.X. Synergistic effects of endolysin Lysqdvp001 and epsilon-poly-lysine in controlling Vibrio parahaemolyticus and its biofilms. Int. J. Food Microbiol. 2021, 343, 109112. [Google Scholar] [CrossRef]
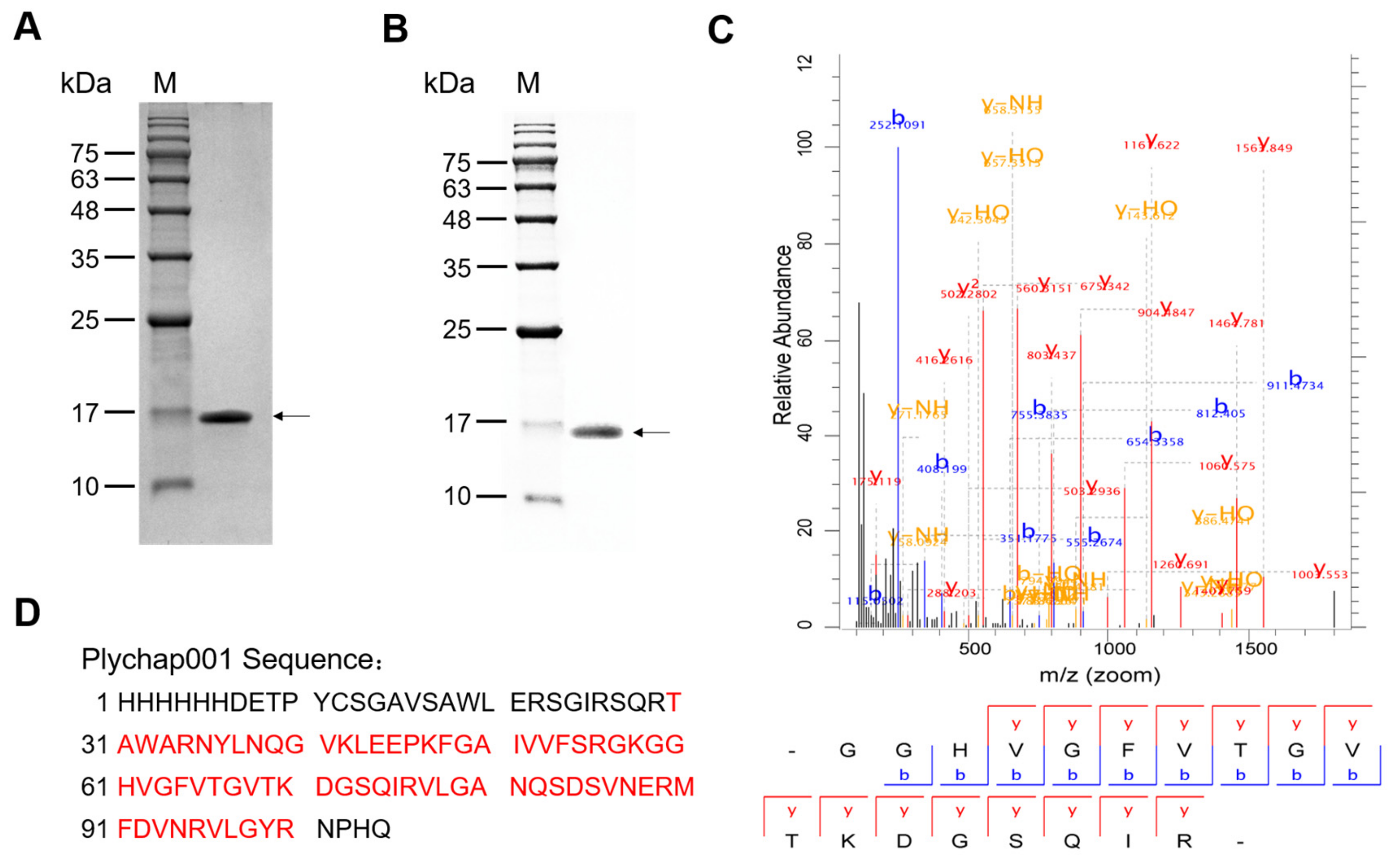
| Annotated Sequence | Protein | Mass | PSMs | Confidence | Score |
|---|---|---|---|---|---|
| GGHVGFVTGVTKDGSQIR | 1 | 1814.95 | 19 | High | 5.50 |
| GKGGHVGFVTGVTK | 1 | 1343.74 | 24 | High | 4.50 |
| DGSQIRVLGANQSDSVNERMFDVNR | 1 | 2807.35 | 1 | High | 4.47 |
| VLGANQSDSVNER | 1 | 1388.68 | 187 | High | 4.04 |
| GGHVGFVTGVTK | 1 | 1158.63 | 294 | High | 3.82 |
| NYLNQGVKLEEPK | 1 | 1531.81 | 7 | High | 3.58 |
| VLGANQSDSVNER | 1 | 1389.66 | 5 | High | 3.23 |
| FGAIVVFSR | 1 | 995.57 | 109 | High | 2.94 |
| NYLNQGVKLEEPK | 1 | 1532.80 | 9 | High | 2.68 |
| DGSQIRVLGANQSDSVNER | 1 | 2045.00 | 2 | High | 2.64 |
| VLGANQSDSVNERMFDVNRVLGYR | 1 | 2739.36 | 3 | High | 2.51 |
| VLGANQSDSVNERMFDVNR | 1 | 2151.02 | 1 | High | 2.42 |
| NYLNQGVK | 1 | 935.49 | 8 | High | 2.00 |
| TAWARNYLNQGVK | 1 | 1520.80 | 2 | High | 1.04 |
| Bacterial Isolate | Activity | Source b | |
|---|---|---|---|
| qdvp001 Phage a | Plychap001 a | ||
| VP 1.1 | − | + | isolated from sewage |
| VP 1.3 | − | + | isolated from sewage |
| VP 2.2 | − | + | isolated from sewage |
| VP 3.1 | − | + | isolated from sewage |
| VP 3.2 | − | + | isolated from sewage |
| VP 4.1 | − | + | isolated from sewage |
| VP 304 | + | + | stored in the lab |
| VP 461 | + | + | stored in the lab |
| VP 800 | − | + | stored in the lab |
| VP l4-90 | − | + | stored in the lab |
| V. parahaemolyticus 17802 | + | + | ATCC |
| Vibrio harveyi V1 | − | + | stored in the lab |
| Shewanella baltica S1 | − | + | stored in the lab |
| Acinetobacter baumannii A1 | − | + | stored in the lab |
| Staphylococcus aureus 43300 | − | − | ATCC |
| Pseudomonas fluorescens P1 | − | − | stored in the lab |
| Aeromonas hydrophila A1 | − | − | stored in the lab |
| Pseudomonas putrefaciens P1 | − | − | stored in the lab |
| Salmonella typhimurium 14028 | − | − | ATCC |
| Vibrio anguillarum V1 | − | − | stored in the lab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ju, X.; Cong, Y.; Lin, H.; Wang, J. A Single Catalytic Endolysin Domain Plychap001: Characterization and Application to Control Vibrio parahaemolyticus and Its Biofilm Directly. Foods 2022, 11, 1578. https://doi.org/10.3390/foods11111578
Wang L, Ju X, Cong Y, Lin H, Wang J. A Single Catalytic Endolysin Domain Plychap001: Characterization and Application to Control Vibrio parahaemolyticus and Its Biofilm Directly. Foods. 2022; 11(11):1578. https://doi.org/10.3390/foods11111578
Chicago/Turabian StyleWang, Luokai, Xiaochen Ju, Yu Cong, Hong Lin, and Jingxue Wang. 2022. "A Single Catalytic Endolysin Domain Plychap001: Characterization and Application to Control Vibrio parahaemolyticus and Its Biofilm Directly" Foods 11, no. 11: 1578. https://doi.org/10.3390/foods11111578
APA StyleWang, L., Ju, X., Cong, Y., Lin, H., & Wang, J. (2022). A Single Catalytic Endolysin Domain Plychap001: Characterization and Application to Control Vibrio parahaemolyticus and Its Biofilm Directly. Foods, 11(11), 1578. https://doi.org/10.3390/foods11111578





