Recent Advances for the Developing of Instant Flavor Peanut Powder: Generation and Challenges
Abstract
:1. Introduction
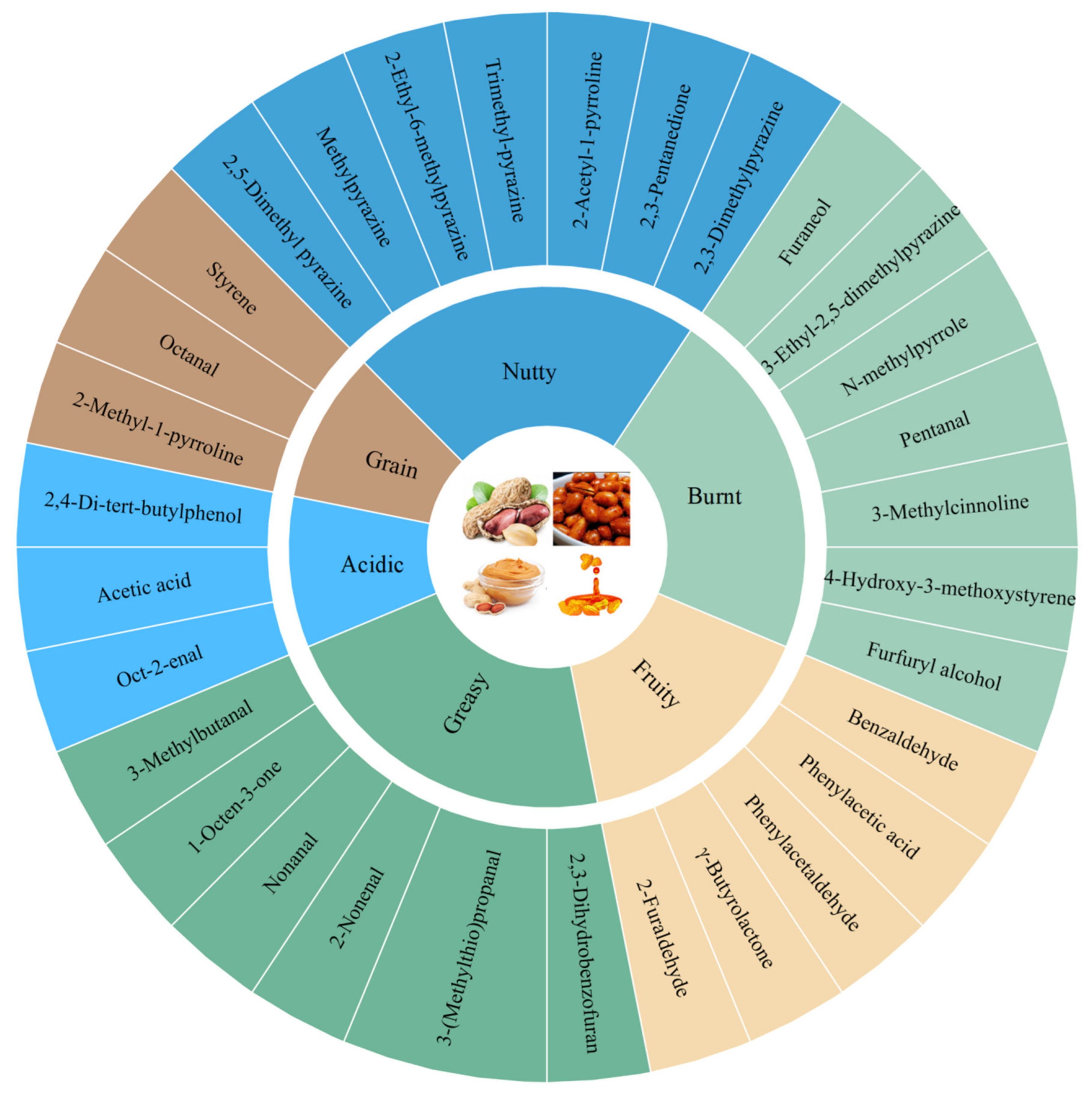
2. Characterization of Characteristic Flavor Substances in Raw Peanuts and Heat-Treatment Peanut Products
| No. | Compounds | CAS No. | Flavor Description | Reported Flavor of Peanut Product | Reference |
|---|---|---|---|---|---|
| 1 | 2-isobutyl-3-methoxypyrazine | 24683-00-9 | Bell pepper-like, earthy | Raw peanuts | [28] |
| 2 | Trans-4,5-epoxy-(E)-2-decenal | metallic | Raw peanuts | [28] | |
| 3 | 3-Isopropyl-2-methoxy-pyrazine | 25773-40-4 | Chocolate, nutty | Raw peanuts | [36] |
| 4 | Acetic acid | 64-19-7 | Sharp pungent | Raw peanuts | [36] |
| 5 | 2-isopropyl-3-methoxypyrazine | 93905-03-4 | Earthy, pea-like | Raw peanuts, roasted peanut | [28] |
| 6 | Nonanal | 124-19-6 | Beany aroma | Raw peanuts, roasted peanuts | [36] |
| 7 | 2-Acetylpyrroline | 85213-22-5 | Popcorn scent | Roasted peanuts, peanut oil | [36] |
| 8 | 1-Octen-3-one | 4312-99-6 | Mushroom aroma | Roasted peanuts, raw peanut | [36,37] |
| 9 | Trans-4,5-Epoxy-(E)-2-decanal | Metallic aroma | Roasted peanuts | [23,36] | |
| 10 | 2-Methyl-1-pyrroline | 872-32-2 | Rice aroma | Roasted peanuts | [37] |
| 11 | 2-Nonenal | 2463-53-8 | Greasy | Roasted peanuts | [17] |
| 12 | Phenylacetaldehyde | 122-78-1 | Fruity | Roasted peanuts | [38] |
| 13 | Phenylacetic acid | 103-82-2 | Honey, floral scent | Roasted peanuts | [36,37] |
| 14 | Methanethiol | 74-93-1 | Decomposed aroma | Roasted peanuts | [22] |
| 15 | 2,3-pentanedione | 600-14-6 | Nutty | Roasted peanuts | [22,36] |
| 16 | 3-(methylthio) propanal | 3268-49-3 | Musty potato, tomato | Roasted peanuts, peanut oil, raw peanut | [23,36,38] |
| 17 | 3-methylbutanal | 590-86-3 | Fatty | Roasted peanuts | [37] |
| 18 | 2-methylbutanal | 96-17-3 | Musty, cocoa, nutty | Roasted Peanuts, raw peanut | [36] |
| 19 | Trans-2,4-nonadienal-trans | 6750-03-4 | Greasy, fatty | Roasted peanuts | [38] |
| 20 | Octanal | 124-13-0 | Beany | Roasted peanuts, raw peanut | [16,17] |
| 21 | Pentanal | 110-62-3 | Fruity, nutty, berry | Roasted peanuts, raw peanut | [39] |
| 22 | N-methylpyrrole | 96-54-8 | Sweet, woody odor | Roasted peanuts | [38] |
| 23 | 4,5-dimethyloxazole | 20662-83-3 | Green, sweet, vegetable | Roasted peanuts | [38] |
| 24 | Hexanal | 66-25-1 | Grassy, refreshing, beany | Roasted peanuts, peanut oil, raw peanut | [23,37] |
| 25 | 2,3-dimethylpyrazine | 5910-89-4 | Nutty | Roasted peanuts, peanut oil | [23,38] |
| 26 | Oct-2-enal | 2363-89-5 | Fatty | Peanut oil | [38] |
| 27 | Benzaldehyde | 100-52-7 | Fruity | Roasted peanuts, peanut oil | [23,38] |
| 28 | 2-Acetyl-3-methylpyrazine | 23787-80-6 | Rose aroma | Peanut oil | [38] |
| 29 | Phenethyl alcohol | 60-12-8 | Bitter medicinal | Peanut oil | [38] |
| 30 | Pyrrole-2-carboxaldehyde | 1003-29-8 | Sweet fragrance | Peanut oil | [38] |
| 31 | Gamma butyrolactone | 96-48-0 | Alcohol odor | Peanut oil | [38] |
| 32 | 2-furaldehyde | 98-01-1 | Green, sweet, and vegetable | Roasted peanuts, peanut oil, peanut butter | [23,39,40] |
| 33 | Methylpyrazine | 109-08-0 | Nutty | Roasted peanuts, peanut oil, Peanut butter | [23,39,40] |
| 34 | 2,5-dimethyl pyrazine | 123-32-0 | Nutty | Roasted peanuts, peanut oil, peanut butter | [40] |
| 35 | Trimethyl-pyrazine | 14667-55-1 | Nutty | Roasted peanuts, peanut oil, peanut butter | [40] |
| 36 | Furaneol | 3658-77-3 | Caramel aroma | Roasted peanuts, peanut oil, peanut butter | [36,37] |
| 37 | 3-Ethyl-2,5-diMethylpyrazine | 13360-65-1 | Burnt aroma | Peanut oil, peanut butter | [23] |
| 38 | 4-Hydroxy-3-methoxystyrene | 7786-61-0 | Burnt aroma | Peanut butter | [40] |
2.1. Characterization of Raw Peanut Flavor Components
2.2. Characterization of Heat-Treatment Peanuts Products
2.2.1. Roasted Peanut Flavor Components
2.2.2. Characterization of Roasted Peanut Oil Flavor Components
2.2.3. Characterization of Peanut Butter Flavor Components
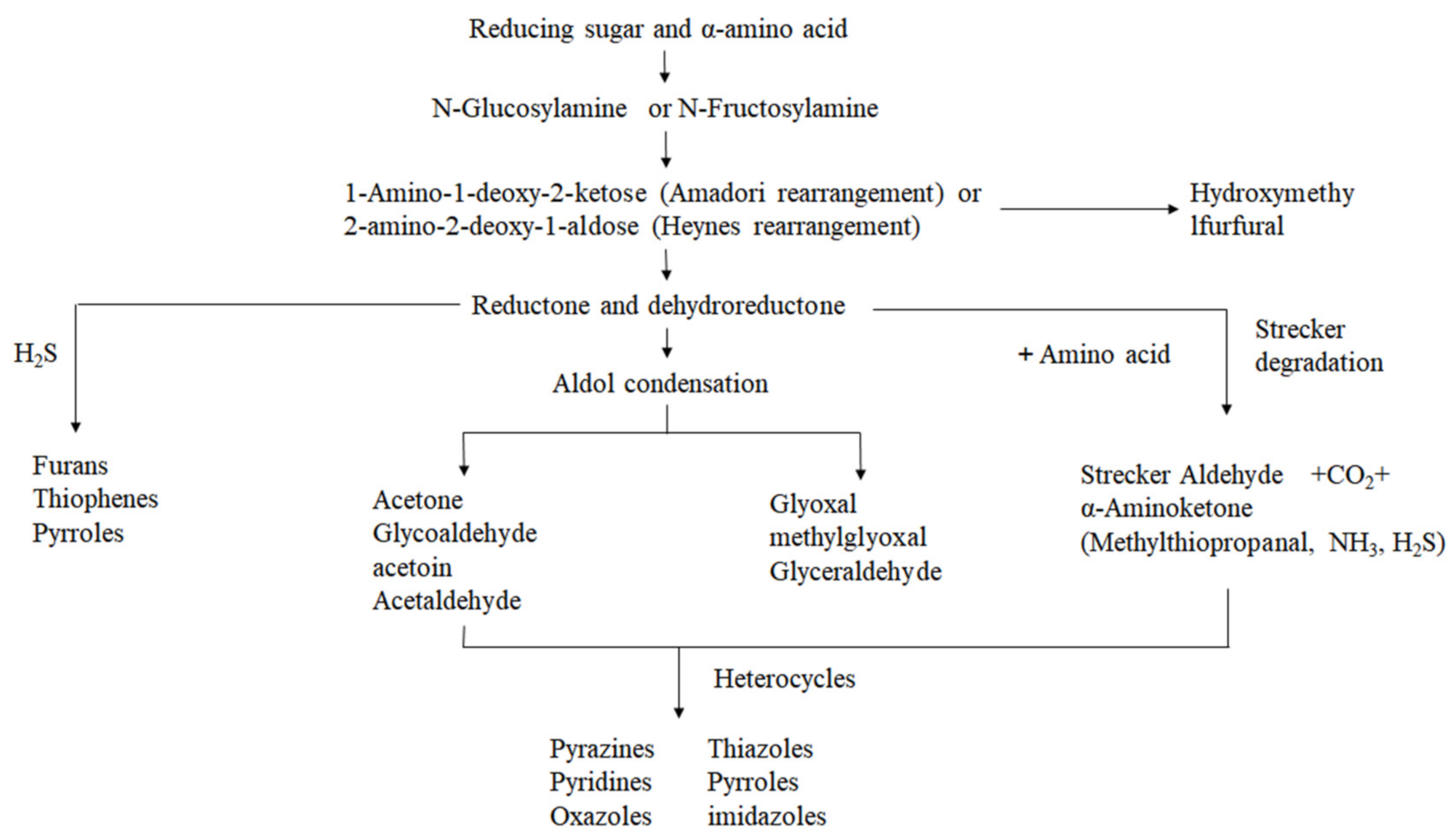
3. Formation of Characteristic Flavor Substances of Heat-Processed Peanuts and the Mechanism of Aroma Presentation
3.1. Maillard Reaction
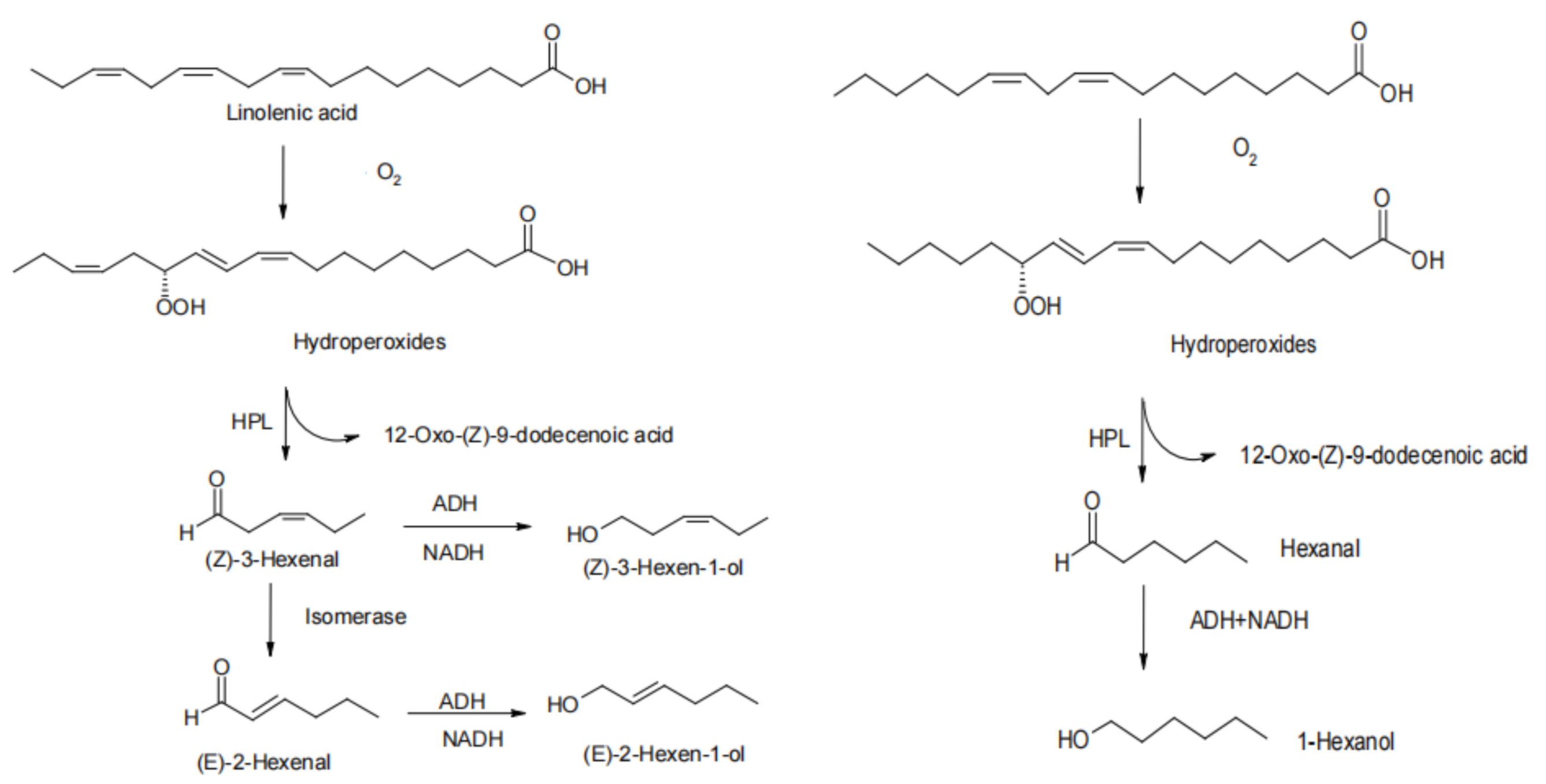
3.2. Lipid Oxidation Reaction
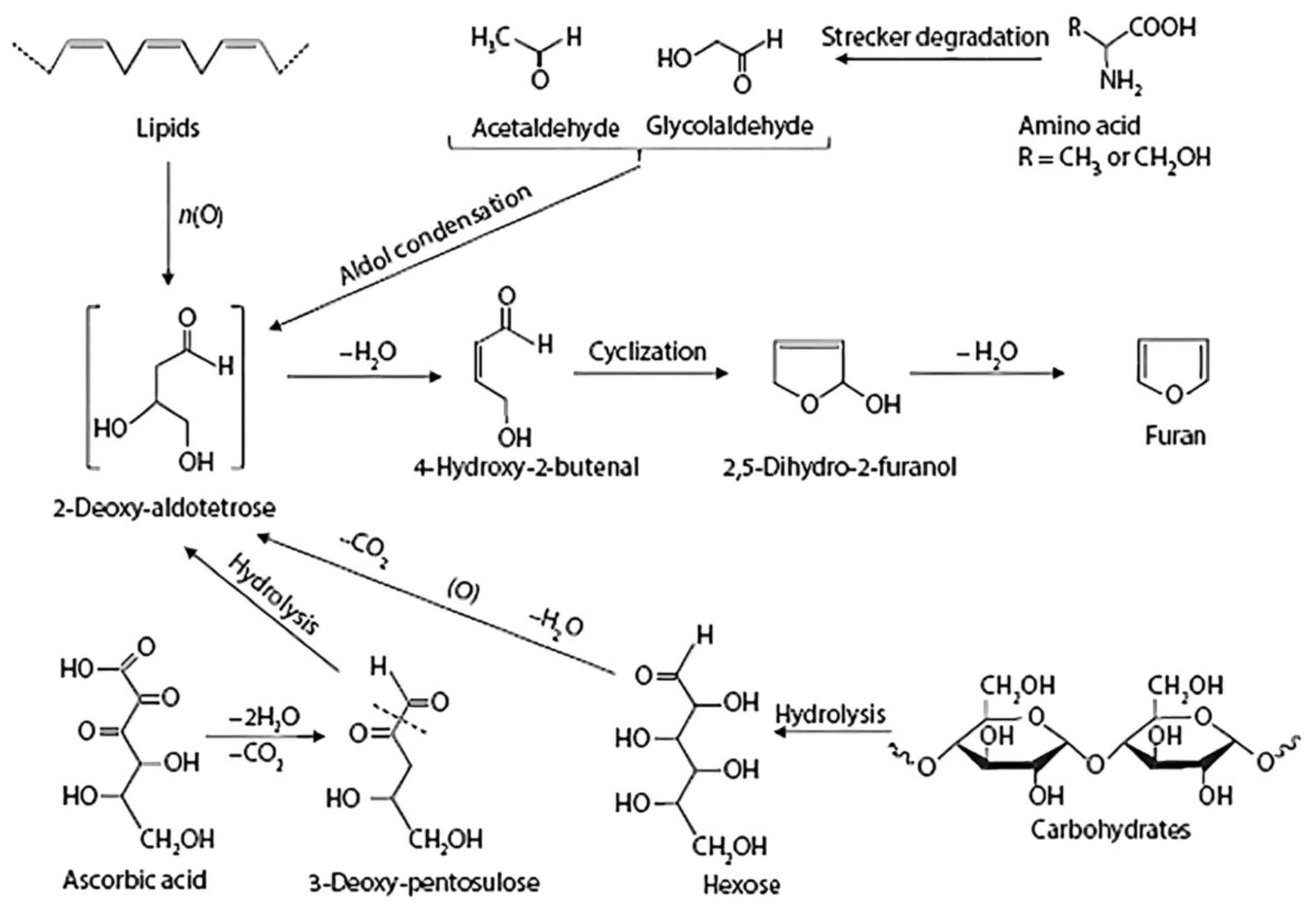
3.3. Caramelization Reaction
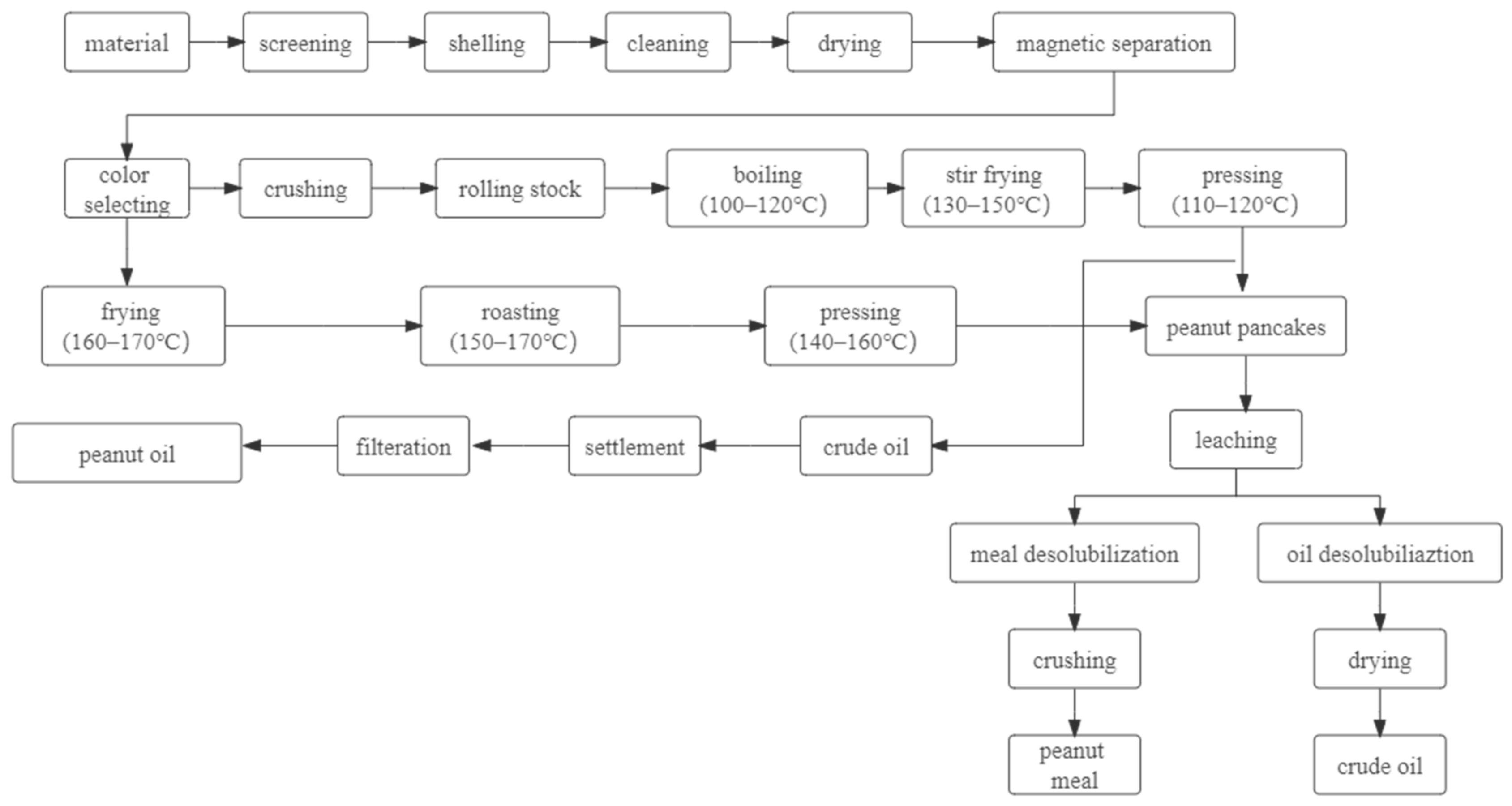
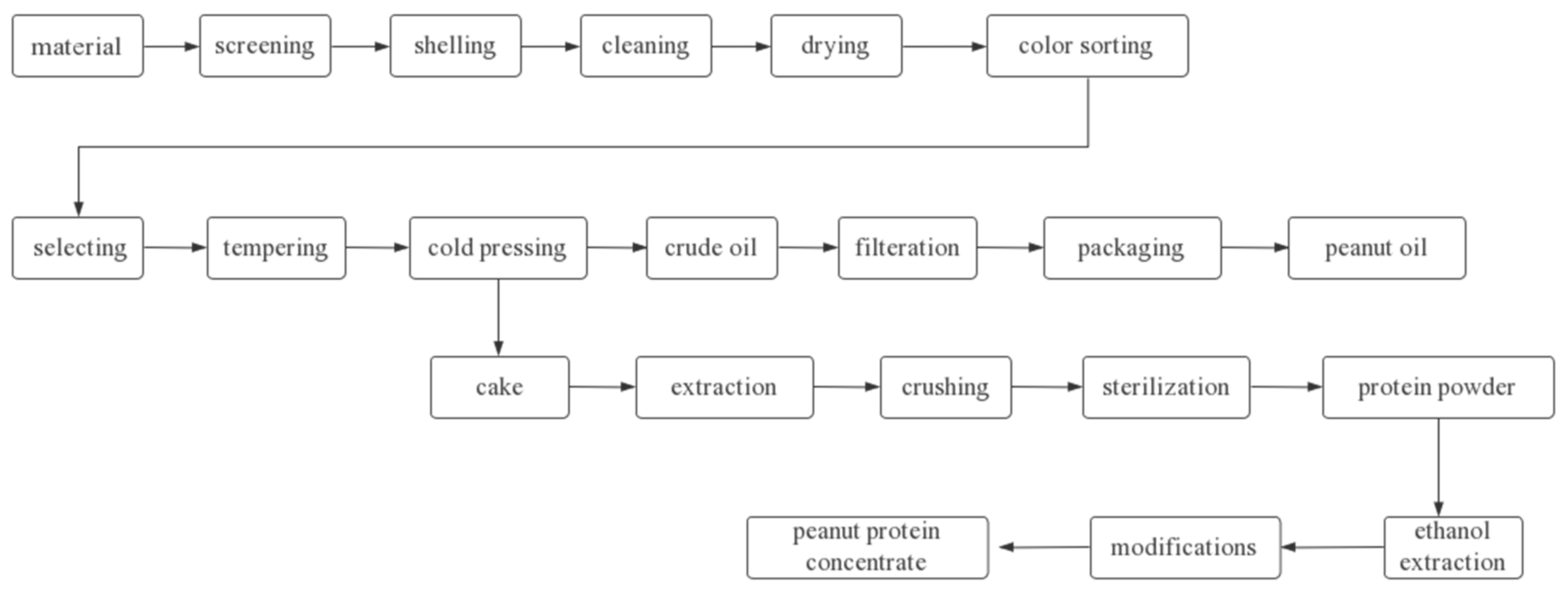
4. Effect of Heat Treatment on the Quality Characteristics of Peanut Protein
4.1. Thermal Degradation of Proteins
4.2. Effect of Heat Treatment on the Functional Properties of Peanut Proteins
5. Research Progress on Solubilization Modification of Peanut Protein
| No. | Authors | Sample | Modification Method | Modification Mechanism | Modified Results | Reference |
|---|---|---|---|---|---|---|
| 1 | Dong | Pea protein | Cold Plasma Technology | New oxygen- or nitrogen-containing hydrophilic groups are formed on the surface of the protein. | Significant improvement of zein solubility in both neutral and acidic solutions could be observed after treatment with max solubility at 75 V. | [106] |
| 2 | Zhao | Peanut protein | Baking | Part of the globulin aggregates or decomposes, improving the solubility of the isolated protein under alkaline conditions. | The solubility at pH 7.0 increased gradually from 76% to 95%. | [107] |
| 3 | Zhang | Peanut protein | Microwave | Using microwave effect to change protein aggregation degree and spatial structure. | Under the conditions of microwave power 480 W, modification time 60 s, and pH value 9, the NSI of modified peanut protein concentrate was 53.26%. | [108] |
| 4 | Tu | Peanut protein | Dynamic high-pressure microfluidization | As the content of UV-absorbing groups in arachidon increased, the degree of molecular unfolding became larger; as the content of sulfhydryl groups decreased, the three-dimensional structure of arachisin changed locally. | The solubility of arachidrin increased significantly; foaming and foaming stability increased with the increase of homogenization pressure and reached the maximum when the treatment pressure was 120 V. | [109] |
| 5 | Li | Egg white protein | Microwave-assisted phosphorylation | The microwave technique can significantly shorten reaction times and accelerate phosphorylation process. | The 3 conditions for optimal phosphorylation modification of egg white are the concentration of sodium tripolyphosphate of 33.84 g/L, microwave power of 419.38 W, and microwave time 90 s for maximum functional properties (solubility, foaming ability, and foaming stability). | [110] |
| 6 | Miedzianka | Potato protein | Sodium trimetaphosphate (STMP) | By binding phosphate groups to the active groups of protein side chains, the electronegativity of protein molecules can be changed to increase the electrostatic repulsion between protein molecules and lower their isoelectric points. | The solubility of potato protein increases to 26% at pH 5.2. | [111] |
| 7 | Lu | Peanut protein | Sulfonated styrene cation exchange resin | The isoelectric point of the acylate peanut protein shifted, the main protein components are broken into subunits, and the amide group selectively deamidate the protein. | The solubility of modified peanut protein was improved, and the isoelectric point pH was reduced to 0.5–1; the emulsification, emulsion stability, and foaming properties were increased by 215%, 122%, and 538%. | [112] |
| 8 | Liu | Peanut protein | Dextran glycosylation | Glycosylation forms protein-polysaccharide complexes by covalent binding of proteins to polysaccharides and the introduction of sugar chains into protein polypeptide chains. Cross-linking of proteins with polysaccharides with hydrophilic hydroxyl groups increases the hydrophilicity of proteins. | Peanut protein nitrogen solubility index increased by 75%. | [113] |
| 9 | Qi | Soy protein | Pepsin and phytase complex enzymes | Enzymes modify the amino acid side chain groups of protein molecules by modifying the amino acid side-chain groups of protein molecules to partially degrade or cross-link the protein molecules to polymerize solubility and other functional properties of the protein | The nitrogen solubility index increased from 10.0% to 80.0% at pH 4.0 compared to the unmodified soybean isolate. | [114] |
| 10 | Ma | Peanut protein | Limited enzymatic hydrolysis and high-pressure homogenization; compound modification | High-pressure homogenization exposes internal groups of proteins and affects their secondary bonds, increases free sulfhydryl groups in solution, and destroys disulfide bonds, exposing more enzyme cleavage sites, making it easier for enzymes to act on peptide bonds and peptide bonds to break; accelerates protein breakdown. | The nitrogen solubility index of peanut protein concentrate increased to 96.57%. | [34] |
| 11 | Zang | Wheat protein | Ultrasonic and Glycosylation Compound Modification | Appropriate ultrasonic treatment is beneficial to the glycosylation modification of wheat gluten, and the surface hydrophobicity of the ultrasonically treated wheat gluten is reduced after grafting with glucose | The solubility of the modified wheat gluten protein is improved in the pH range of 4–7, and the solubility at the isoelectric point is 82.15%. | [115] |
5.1. Physical Modification
5.2. Chemically Modified
5.3. Enzymatic Modification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDA. Available online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade/ (accessed on 9 November 2021).
- Wang, Q.; Liu, L.; Wang, L.; Guo, Y.; Wang, J. Introduction. In Peanuts: Processing Technology and Product Development, 1st ed.; Wang, Q., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–22. [Google Scholar]
- Liu, X.Q.; Yi, H.; Yao, L.; Ma, H.W.; Zhang, J.Y.; Wang, Z.M. Advances in plants of Polygonatum and discussion of its development prospects. J. Chin. Pharm. 2017, 52, 530–534. [Google Scholar]
- Matthäus, B.; Musazcan Özcan, M. Oil Content, Fatty Acid Composition and Distributions of Vitamin-E-Active Compounds of Some Fruit Seed Oils. Antioxidants 2015, 4, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Suchoszek-Łukaniuk, K.; Jaromin, A.; Korycińska, M.; Kozubek, A. Chapter 103: Health Benefits of Peanut (Arachis hypogaea L.) Seeds and Peanut Oil Consumption. Nuts Seeds Health Dis. Prev. 2011, 873–880. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Shi, A.; Liu, L.; Fauconnier, M.L.; Wang, Q. The Effect of Microwave Pretreatment on Micronutrient Contents, Oxidative Stability and Flavor Quality of Peanut Oil. Molecules 2018, 24, 62. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.; Ribotta, P.D.; León, A.E. Differential scanning calorimetry (DSC)studies on the thermal properties of peanut proteins. J. Agric. Food Chem. 2010, 58, 4434–4439. [Google Scholar] [CrossRef]
- Molino Artiz, S.E.; Wagner, J.R. Hydrolysates of native and modified soy protein isolates: Structural characteristics, solubility and foaming properties. Food Res. Int. 2002, 35, 511–518. [Google Scholar] [CrossRef]
- Dong, X.H.; Zhao, M.M.; Jiang, Y.M. Research progress of peanut protein modification. Chin. J. Cereals Oils 2011, 26, 109–117. [Google Scholar]
- Cherry, J.P. Peanut protein and product functionality. J. Am. Oil Chem. Soc. 1990, 67, 5473–5481. [Google Scholar] [CrossRef]
- Dong, X.H.; Zhao, M.M.; Yang, B.; Jiang, Y.M. Effect of high-pressure homogenization on the functional property of peanut protein. J. Food Process Eng. 2010, 34, 2191–2204. [Google Scholar] [CrossRef]
- Feng, X.L.; Liu, H.Z.; Shi, A.M.; Liu, L.; Wang, Q.; Adhikari, B. Effects of transglutaminase catalyzed crosslinking on physicochemical characteristics of arachin and conarachin-rich peanut protein fractions. Food Res. Int. 2014, 62, 84–90. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, C.; Yao, F.; Chen, F. Aqueous enzymatic extraction of peanut oil body and protein and evaluation of its physicochemical and functional properties. Int. J. Food Eng. 2021, 17, 897–908. [Google Scholar] [CrossRef]
- Boukid, F. Peanut protein—An underutilised by-product with great potential: A review. Int. J. Food Sci. Technol. 2021, 111438. [Google Scholar] [CrossRef]
- Gong, K.J.; Shi, A.M.; Liu, H.Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016, 170, 33–40. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, A.; Wang, Q.; Zhou, J. High oleic acid peanut oil and extra virgin olive oil supplementation attenuate metabolic syndrome in rats by modulating the gut microbiota. Nutrients 2019, 11, 3005. [Google Scholar] [CrossRef] [Green Version]
- Leunissen, M.; Davidson, V.J.; Kakuda, Y. Analysis of volatileflavor components in roasted peanuts using supercriticalfluid extraction and gas chromatography mass spectrometry. Agric. Food Chem. 1996, 44, 2694–2699. [Google Scholar] [CrossRef]
- Mu, J.; Yu, X. Determination of the composition of peanut protein powder and its properties. Molecules 2018, 24, 163–168. [Google Scholar]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q. Introduction to Peanut Bioactive Substances; China Agricultural University Press: Beijing, China, 2012. [Google Scholar]
- Chetschik, I.; Granvogl, M.; Schieberle, P. Comparison of the Key Aroma Compounds in Organically Grown, Raw West-African Peanuts (Arachis hypogaea) and in Ground, Pan-Roasted Meal Produced Thereof. J. Agric. Food Chem. 2008, 56, 10237. [Google Scholar] [CrossRef]
- Qian, D.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of hot and cold-pressed processes on volatile compounds of peanut oil and corresponding analysis of characteristic flavor components. LWT 2019, 112, 107648. [Google Scholar]
- Walradt, J.P.; Pittet, A.O.; Kinlin, T.E.; Muralidhara, R.; Sanderson, A. Volatile components of roasted peanuts. J. Agric. Food Chem. 1971, 19, 972–979. [Google Scholar] [CrossRef]
- Brown, D.F.; Stanley, J.B.; Senn, V.J.; Dollear, F.G. Comparison of carbonyl-compounds in raw and roasted runner peanuts. I. Major qualitative and some quantitative differences. J. Agric. Food Chem. 1972, 20, 700–706. [Google Scholar] [CrossRef]
- Balagiannis, D.P.; Parker, J.K.; Pyle, D.L.; Desforges, N.; Mottram, D.S. Modelling the generation of flavour in a real food system. In Expression of Multidisciplinary Flavour Science: Proceedings of the 12th Weurman Symposium; Blank, I., Wüst, M., Yeretzian, C., Eds.; Zürcher Hochschule für Angewandte Wissenschaften: Wädenswil, Switzerland, 2010; pp. 284–287. [Google Scholar]
- Ajandouz, E.H.; Tchiakpe, L.S.; Ore, F.D.; Benajiba, A.; Puigserver, A. Effects of pH on caramelization and Maillard reaction kinetics in fructose—Lysine model systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Shi, A.; Wang, Q.; Liu, H.; Wang, L.; Zhang, J.; Du, Y.; Chen, X. Peanut Processing Quality Evaluation Technology. In Peanuts: Processing Technology and Product Development; Academic Press: Cambridge, MA, USA, 2016; pp. 23–61. [Google Scholar] [CrossRef]
- Li, J.; Shi, A.; Liu, H. Research progress on the modification method and application of vegetable protein for solubility enhancement under acidic conditions. China Fats Oils 2019, 44, 59–65. [Google Scholar]
- Moure, A.; Sineiro, J.; Dominguez, H.; Parajó, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Zhang, S. Study on the Production Technology of Low Denatured Peanut Protein Powder. J. Wuhan Univ. Light Ind. 2003, 22, 10–11. [Google Scholar]
- Qin, M. Research on the Production of Low Denatured Peanut Protein Powder by Low Temperature Pressing and Low Temperature Leaching Process; Qingdao Liangquan Group Co., Ltd.: Qingdao, China, 2006. [Google Scholar]
- Sun, Q. Preparation and Modification of Low Denatured Peanut Protein; Qingdao Dongsheng Group Co., Ltd.: Qingdao, China, 2010. [Google Scholar]
- Wang, Q. Research on Key Technologies for the Preparation of Functional Short Peptides from Peanut; Institute of Agricultural Products Processing, Chinese Academy of Agricultural Sciences: Beijing, China, 2007. [Google Scholar]
- Ma, T.; Zhu, H.; Wang, J.; Wang, Q.; Yu, L.; Sun, B. Influence of extraction and solubilizing treatments on the molecular structure and functional properties of peanut protein. LWT Food Sci. Technol. 2017, 79, 197–204. [Google Scholar] [CrossRef]
- Dkv, A.; As, B.; Akn, B.; Mt, C.; Ns, D.; Ss, E. Recent trends in microbial flavour compounds: A review on chemistry, synthesis mechanism and their application in food. Saudi J. Biol. Sci. 2022, 29, 1565–1576. [Google Scholar]
- Chetschik, I.; Granvogl, M.; Schieberle, P. Quantitation of key peanut aroma compounds in raw peanuts and pan-Roasted peanut meal. Aroma Reconstitution and Comparison with Commercial Peanut Products. J. Agric. Food Chem. 2010, 58, 11018–11026. [Google Scholar] [CrossRef]
- Yun, L.; Hu, H.; Liu, H.; Wang, Q. Flavor analysis of peanut cake meal and peanut shell baking. J. Food Sci. 2017, 38, 146–153. [Google Scholar]
- Liu, X.; Jin, Q.; Liu, Y.; Liu, Y.; Huang, J.; Wang, X.; Mao, W.; Wang, S. Changes in volatile compounds of peanut oil during the roasting process for pduction of aromatic roasted peanut oil. Food Sci. 2011, 76, C404–C412. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Lee, M.H.; Chang, S.S. Isolation and identification of volatile compounds from roasted peanuts. Food Sci. 1981, 47, 127–133. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Erasmus, S.W.; Zhao, S.; Wang, Q.; van Ruth, S.M. An explorative study on the relationships between the quality traits of peanut varieties and their peanut butters. LWT 2021, 151, 112068. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, D.; Feng, Y.; Su, G.; Zhao, M.; Lin, L. The umami intensity enhancement of peanut protein isolate hydrolysate, and its derived factions and peptides by Maillard reaction and the analysis of peptide (EP) Maillard products. Food Res. Int. 2019, 120, 895–903. [Google Scholar] [CrossRef]
- Shen, J.; Liu, L.; Qian, J. Effects of Polygonatum polysaccharide on immunological activity of immunosuppressive mouse model. Drug Eval. Res. 2012, 35, 328–331. [Google Scholar]
- Nepote, V.; Olmedo, R.H.; Mest Ra Llet, M.G.; Grosso, N.R. A Study of the Relationships among Consumer Acceptance, Oxidation Chemical Indicators, and Sensory Attributes in High-Oleic and Normal Peanuts. Food Sci. 2009, 74, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.L.; Lee, R.S.; Young, C.T.; Chiou, R.Y.Y. Roasted peanutflavor and related compositional characteristics of peanut kernels of spring and fall crops grown in Taiwan. J. Agric. Food Chem. 1998, 46, 3220–3224. [Google Scholar] [CrossRef]
- Settaluri, V.S.; Kandala, C.; Puppala, N.; Sundaram, J. Peanuts and their nutritional aspects—A review. Food Nutr. Sci. 2015, 3, 1644–1650. [Google Scholar] [CrossRef] [Green Version]
- Maga, J.A. Phytate: Its chemistry, occurrence, food interactions, nutritional significance, and methods of analysis. J. Agric. Food Chem. 1982, 30, 1–9. [Google Scholar] [CrossRef]
- Hu, H.; Shi, A.; Liu, H.; Liu, L.; Fauconnier, M.L.; Wang, Q. Study on Key Aroma Compounds and Its Precursors of Peanut Oil Prepared with Normal- and High-Oleic Peanuts. Foods 2021, 10, 3036. [Google Scholar] [CrossRef]
- Mason, M.E.; Johnson, B.; Hamming, M. Flavor components of roasted peanuts. Some low molecular weight pyrazines and a pyrrole. Agric. Food Chem. 1966, 14, 454–460. [Google Scholar] [CrossRef]
- Baker, G.L.; Cornell, J.A.; Gorbet, D.W.; O’Keefe, S.F.; Sims, C.A.; Talcott, S.T. Determination of pyrazine and flavor variations in peanut genotypes during roasting. Food Sci. 2003, 68, 394–400. [Google Scholar] [CrossRef]
- Johnson, B.R.; Waller, G.R.; Foltz, R.L. Volatile components of roasted peanuts: Neutral fraction. Agric. Food Chem. 1971, 19, 1025–1027. [Google Scholar]
- Buckholz, L.L.; Withcombe, D.A.; Daun, H. Application and characteristics of a polymer adsorption method used to analyzeflavor volatiles from peanuts. J. Agric. Food Chem. 1980, 28, 760–765. [Google Scholar] [CrossRef]
- Schirack, A.V.; Drake, M.A.; Sanders, T.H.; Sandeep, K.P. Characterization of aroma-active compounds in microwave blanched peanuts. Food Sci. 2006, 71, 513–520. [Google Scholar] [CrossRef]
- Basha, S.M.; Young, C.T. Protein fraction producing off-flavor headspace volatiles inpeanut seed. J. Agric. Food Chem. 1996, 44, 3070–3074. [Google Scholar] [CrossRef]
- Young, C.T.; Hovis, A.R. A method for the rapid analysis of headspace volatiles of raw and roasted peanuts. Food Sci. 1990, 55, 279–280. [Google Scholar] [CrossRef]
- Coelho, G.; Mendes, M.F.; Pessoa, F. Handbook of Fruit and Vegetable Flavors; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Shu, Y.; Liu, Y.; Jiang, Y. Effect of roasting temperature of fresh peanut kernels on flavor and overall quality of peanut butter. Food Sci. 2020, 41, 8. [Google Scholar]
- Lou, F.; Liu, Y.; Sun, X. Pan, Y.; Zhao, J.; Zhao, Y. Identification of volatile flavor components in peanut butter. J. Food Sci. 2009, 30, 393–396. [Google Scholar]
- Hathorn, C.S.; Sanders, T.H. Flavor and Antioxidant Capacity of Peanut Paste and Peanut Butter Supplemented with Peanut Skins. Food Sci. 2012, 77, S407–S411. [Google Scholar] [CrossRef] [Green Version]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 1–19. [Google Scholar] [CrossRef]
- Schieberle, P. The carbon Module labeling (CAMOLA) technique: A useful tool for identifying transient intermediates in the formation of Maillard-type target molecules. Ann. N. Y. Acad. Sci. 2005, 1043, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, Q.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Kinetic study on the generation of furosine and pyrraline in a Maillard reaction model system of dglucose and l-lysine. Food Chem. 2020, 317, 126458. [Google Scholar] [CrossRef]
- Yu, H.; Seow, Y.-X.; Ong, P.K.C.; Zhou, W. Effects of high-intensity ultrasound on Maillard reaction in a model system of d-xylose and l-lysine. Ultrason. Sonochem. 2017, 34, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Jongen, W.; Boekel, M. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Newell, J.A.; Mason, M.E.; Matlock, R.S. Precursors of typical atypical roasted peanut flavor. Agric. Food Chem. 1967, 15, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Suri, K.; Singh, B.; Kaur, A.; Singh, N. Impact of roasting and extraction methods on chemical properties, oxidative stability and Maillard reaction products of peanut oils. J. Food Sci. Technol. 2019, 56, 2436–2445. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, C.; Zhang, K.; Tao, S.; Xue, W. Glycosylation between recombinant peanut protein Ara h 1 and glucosamine could decrease the allergenicity due to the protein aggregation. LWT 2020, 127, 109374. [Google Scholar] [CrossRef]
- Mason, M.E.; Newell, J.A.; Johnson, B.R.; Koehler, P.E.; Waller, G.R. Nonvolatile flavor components of peanut. Agric. Food Chem. 1969, 17, 728–732. [Google Scholar] [CrossRef]
- Buckholz, L.L. Microwave Browning and Baking to Give Surface Crust. US4943697-A, 24 July 1990. [Google Scholar]
- Momin, A.H.; Acharya, S.S.; Gajjar, A.V. Corriandrum Sativum review of advances in phytopharmacology. Int. J. Pharm. Sci. Res. 2012, 13, 1233–1239. [Google Scholar]
- Andreou, A.; Feussner, I. Lipoxygenases—Structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Alzagtat, A.A.; Alli, I. Protein-lipid interactions in food systems: A review. Int. Food Nutr. 2002, 53, 249–260. [Google Scholar] [CrossRef]
- Funes, J.A.; Weiss, U.; Karel, M. Effects of reaction conditions and reactant concentrations on polymerization of lysozyme reacted with peroxidizing lipids. Agric. Food Chem. 1982, 30, 1204–1208. [Google Scholar] [CrossRef]
- Vercellotti, J.R.; Mills, O.E.; Bett, K.L.; Sullen, D.L. Gas chromatographic analyses of lipid oxidation volatiles in foods. In Lipid Oxidation in Food; St. Angelo, A., Ed.; American Chemical Society: Washington, DC, USA, 1992; pp. 232–263. [Google Scholar]
- Williams, J.P.; Duncan, S.E.; Williams, R.C.; Mallikarjunan, K.; Eigel, W.N.; O’Keefe, S.F. Flavor fade in peanuts during short-term storage. Food Sci. 2006, 71, S265–S269. [Google Scholar] [CrossRef]
- Rey, F.; Melo, T.; Lopes, D.; Couto, D.; Marques, F.; Domingues, M.R. Applications of lipidomics in marine organisms: Progress, challenges and future perspectives. Mol. Omics 2022. [Google Scholar] [CrossRef]
- Girotti, A.W.; Korytowski, W. Intermembrane Translocation of Photodynamically Generated Lipid Hydroperoxides: Broadcasting of Redox Damage. Photochem. Photobiol. 2022. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, X.; Liu, S.Q. Aroma modulation of vegetable oils—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1740–1751. [Google Scholar] [CrossRef]
- Min, D.B.; Boff, J.M. Lipid Oxidation of Edible Oil; Department of Food Science and Technology, The Ohio State University: Columbus, OH, USA, 2002. [Google Scholar]
- Shahidi, F. Bailey’s Industrial Oil and Fat Products, Volume 3, Edible Oil and Fat Products: Specialty Oils and Oil Products, Part 2, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Pattee, H.E.; Isleib, T.G.; Giesbrecht, F.G.; Cui, Z. Prediction of parental genetic compatibility to enhance flavor attributes of peanuts. In Crop Biotechnology; Rajasekaran, K., Jacks, T.J., Finley, J.W., Eds.; American Chemical Society: Washington, DC, USA, 2002; pp. 217–230. [Google Scholar]
- Reed, K.A.; Sims, C.A.; Gorbet, D.W.; O’Keefe, S.F. Storage water activity affects flavor fade in high and normal oleic peanuts. Food Res. Int. 2002, 35, 769–774. [Google Scholar] [CrossRef]
- Mate, J.I.; Salveit, M.E.; Krochta, J.M. Peanut and walnut rancidity: Effects of oxygen, concentration and relative humidity. Food Sci. 1996, 61, 465–472. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Lipid Oxidation Pathways; AOCS: Champaign, IL, USA, 2003. [Google Scholar]
- Zhang, W.; Rui, W.; Yuan, Y.; Yang, T.; Liu, S. Changes in volatiles of palm kernel oil before and after kernel roasting. LWT Food Sci. Technol. 2016, 73, 432–441. [Google Scholar] [CrossRef]
- Kroh, L.W. Caramelisation in Food and Beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Hwang, H.I.; Hartman, T.G.; Rosen, R.T.; Lech, J.; Ho, C.T. Formation of pyrazines from the Maillard reaction of glucose and lysine-alpha-amine-N-15. Agric. Food Chem. 1994, 42, 1000–1004. [Google Scholar] [CrossRef]
- Friedman, M. Food browning and its prevention: An overview. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Beksan, E.; Schieberle, P.; Robert, F.; Blank, I.; Fay, L.B.; Schlichtherle-Cerny, H.; Hofmann, T. Synthesis and sensory characterization of novel umami-tasting glutamate glycoconjugates. Agric. Food Chem. 2003, 51, 5428–5436. [Google Scholar] [CrossRef] [PubMed]
- Chumngoen, W.; Chen, C.F.; Tan, F. Effects of moist- and dry-heat cooking on the meat quality, microstructure and sensory characteristics of native chicken meat. Anim. Sci. J. 2018, 89, 193–201. [Google Scholar] [CrossRef]
- Hou, C.; Li, X.; Wang, Z.; Huang, C.; Zhang, Q.; Luo, Z.; Zhang, D. Analysis of amino acid and fatty acid contents and nutritional value evaluation of different parts of yak meat. Meat Res. 2019, 33, 52–57. [Google Scholar]
- Kinsella, J.E. Functional properties of food proteins: A review. Crit. Rev. Food Sci. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Koumanov, A.; Ladenstein, R.; Karshikoff, A. Electrostatic interactions in proteins: Contribution to structure–function relationships and stability. Recent Res. Dev. Protein Eng. 2001, 1, 123–148. [Google Scholar]
- Schwenke, K.D. Reflections about the functional potential of legume proteins. Nahrung 2001, 45, 377–381. [Google Scholar] [CrossRef]
- Shichijo, K.; Yamada, T.; Shimizu, T. Independence of the goiter development to the concentration of circulating protein bound iodine. Endocrinol. Jpn. 1957, 4, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damodaran, S. Food proteins: An overview. In Food Proteins and Their Applications; Damodaran, S., Paraf, A., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 1–21. [Google Scholar]
- Morr, C.V. Current status of soy protein functionality in food systems. J. Am. Oil Chem. Soc. 1990, 67, 265–271. [Google Scholar] [CrossRef]
- Jiao, B. Preparation and Stabilization Mechanism of Peanut Protein-Polysaccharide Pickering Emulsion; Chinese Academy of Agricultural Sciences: Beijing, China, 2018. [Google Scholar]
- Monteiro, P.V.; Prakash, V. Alteration of functional properties of peanut (Arachis hypogaea L.) protein fractions by chemical and enzymatic modifications. J. Food Sci. Technol. 1996, 33, 19–26. [Google Scholar]
- Liu, L.; Shi, A.-M.; Liu, H.-Z.; Hu, H.; Wang, Q. Research progress on the structure and properties of peanut protein subunits. Chin. J. Cereals Oils 2016, 31, 151–156. [Google Scholar]
- Birch, G.G.; Kemp, S.E. Apparent specific volumes and tastes of amino acids. Chem. Sens. 1989, 14, 249–258. [Google Scholar] [CrossRef]
- Kilara, A.; Sharkasi, T.Y. Effects of temperature on food proteins and its implications on functional properties. Crit. Rev. Food Sci. Nutr. 1986, 23, 323–395. [Google Scholar] [CrossRef]
- Han, Z.; Guo, F.; Chen, J. Effects of different heat treatments on the main functional properties of peanut protein. Food Ind. Sci. Technol. 2000, 21, 37–38. [Google Scholar]
- Li, H.; Li, X.; Zhang, C.-H.; Wang, J.-Z.; Tang, C.-H.; Chen, L.-L. Flavor compounds and sensory profiles of a novel Chinese marinated chicken. J. Sci. Food Agric. 2016, 96, 1618–1626. [Google Scholar] [CrossRef]
- Wang, Q. Peanut Processing Characteristics and Quality Evaluation; Springer: Singapore, 2018. [Google Scholar]
- Bußler, S.; Steins, V.; Ehlbeck, J.; Schlüter, O. Impact of thermal treatment versus cold atmospheric plasma processing on the technofunctional protein properties from Pisum sativum ‘Salamanca’. J. Food Eng. 2015, 167, 166–174. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, M.; Liu, Y.; Cui, C. Effects of roasting on the structure and functional properties of peanut protein isolate. Food Ferment. Ind. 2009, 35, 20–23. [Google Scholar]
- Zhang, X.; Liu, Y.; Wang, X. Study on microwave modification of peanut protein concentrate. Grain Oil Process. 2010, 6, 19–22. [Google Scholar]
- Tu, Z.; Zhang, X.; Liu, C. Effects of ultra-high pressure microfluidics on the structure of peanut protein. Chin. J. Agric. Eng. 2008, 24, 306–308. [Google Scholar]
- Li, P.; Sheng, L.; Jin, Y. Using microwave-assisted phosphorylation to improve foaming and solubility of egg white by response surface methodology. Poult. Sci. 2019, 98, 5. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R. Functional and electrophoretic characteristics of succinylated peanut flour protein. J. Agric. Food Chem. 1977, 25, 258–261. [Google Scholar] [CrossRef]
- Schnabelrauch, M.; Schiller, J.; Möller, S.; Scharnweber, D.; Hintze, V. Chemically modified glycosaminoglycan derivatives as building blocks for biomaterial coatings and hydrogels. Biol. Chem. 2021, 402, 1385–1395. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Bo, Y. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2012, 131, 901–906. [Google Scholar] [CrossRef]
- Qi, J.; Weng, J.; Kang, Y. Preparation and characterization of soybean acid-soluble protein/soybean polysaccharide nanoemulsion. J. Mod. Food Technol. 2015, 6, 136–141. [Google Scholar]
- Zang, Y.; Zhao, Y.; Luo, S. Effects of ultrasonic and glycosylation compound modification on the properties and structure of wheat gluten. J. Food Sci. 2017, 38, 122–128. [Google Scholar]
- Liu, C.; Pei, R.; Heinonen, M. Faba bean protein: A promising plant-based emulsifier for improving physical and oxidative stabilities of oil-in-water emulsions. Food Chem. 2022, 369, 130879. [Google Scholar] [CrossRef]
- Aminigo, E.R.; Ogundipe, H.O. Effect of heat treatment on functional characteristics of peanut (Arachis hypogeae) meal. J. Food Sci. Technol. 2003, 40, 205–208. [Google Scholar]
- Beuchat, L.R.; Cherry, J.P.; Quinn, M.R. Physicochemical properties of peanut flour as affected by proteolysis. J. Agric. Food Chem. 2015, 23, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Martín, A.; Martínez, L.M.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Induced Changes in Aroma Compounds of Foods Treated with High Hydrostatic Pressure: A Review. Foods 2021, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Gao, A.; Zhao, Y.; Li, Y.; Chen, Y. Characterization of physicochemical and structural properties of atmospheric cold plasma (ACP) modified zein. Food Bioprod. Process. 2017, 106, 65–74. [Google Scholar] [CrossRef]
- Wang, Z. Crosslinked Recombinant-Arah1 Catalyzed by Microbial Transglutaminase: Preparation, Structural Characterization and Allergic Assessment. Foods 2020, 9, 1508. [Google Scholar]
- Lu, Y.X.H.; Zheng, Z. Study on functional improvement of peanut protein processing. Food Sci. 1995, 16, 6. [Google Scholar]
- Matheis, G. Phosphorylation of food proteins with phosphorus oxychloride-Improvement of functional and nutritional properties: A review. J. Food Chem. 1991, 39, 13–26. [Google Scholar] [CrossRef]
- Nam, S.C.; Sung, H.R.; Kang, S.H.; Joo, J.Y.; Lee, S.J.; Chung, Y.B.; Lee, C.K.; Song, S.G. Phosphorylation-dependent septin interaction of Bni5 is important for cytokinesis. J. Microbiol. 2007, 45, 227–233. [Google Scholar]
- Xiong, L.; Sun, G.; Wang, J. Study on phosphorylation modification of peanut protein isolate. J. Food Sci. 2010, 10, 35–41. [Google Scholar]
- Pan, Q.; Shen, B.; Cheng, S. Phosphorylation modification of peanut protein. J. China Oils Fats 2017, 22, 25–27. [Google Scholar]
- Mouécoucou, J.; Fremont, S.; Sanchez, C.; Villaume, C.; Méjean, L. In vitro allergenicity of peanut after hydrolysis in the presence of polysaccharides. Clin. Exp. Allergy 2004, 34, 1429–1437. [Google Scholar] [CrossRef]
- Liu, C.C.; Tellez Garay, A.M.; Castell Perez, M.E. Physical and mechanical properties of peanut protein films. LWT-Food Sci. Technol. 2004, 37, 731–738. [Google Scholar] [CrossRef]
- Marcela, J.P.; Zeng, T.; Temelli, F.; Chen, L. Understanding the stability mechanisms of lentil legumin like protein and polysaccharide foams. Food Hydrocolloid 2016, 61, 903–913. [Google Scholar]
- Yuan, Y. Study on the Interaction between Food Protein and Chitosan and Its Application in Food System; South China University of Technology: Guangzhou, China, 2014. [Google Scholar]
- Guo, R. Preparation and Application of Functional Soybean Protein; South China University of Technology: Guangzhou, China, 2011. [Google Scholar]
- Zhao, G.; Yan, L.; Zhao, M.; Ren, J.; Bo, Y. Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate. Food Chem. 2011, 127, 1438–1443. [Google Scholar] [CrossRef]
- Ambrosi, V.; Polenta, G.; Gonzalez, C.; Ferrari, G.; Maresca, P. High hydrostatic pressure assisted enzymatic hydrolysis of whey proteins. Innov. Food Sci. Emerg. Technol. 2016, 38, 294–301. [Google Scholar] [CrossRef]
- Sekul, A.A.; Vinnett, C.H.; Ory, R.L. Some functional properties of peanut proteins partially hydrolyzed with papain. J. Agric. Food Chem. 1978, 26, 855–858. [Google Scholar] [CrossRef]
- Li, N.; Liu, H.; Liu, L.; Wang, Q. Study on the preparation of peanut peptides by step-by-step enzymatic hydrolysis of peanut protein isolate by neutral protease. J. China Agric. Sci. 2013, 46, 5237–5247. [Google Scholar]
- Zhao, G.; Liu, Y.; Ren, J.; Zhao, M.; Yang, B. Effect of protease pretreatment on the functional properties of protein concentrate from defatted peanut flour. J. Food Process Eng. 2013, 36, 9–17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hu, H.; Liu, H.; Wang, Q. Recent Advances for the Developing of Instant Flavor Peanut Powder: Generation and Challenges. Foods 2022, 11, 1544. https://doi.org/10.3390/foods11111544
Liu Y, Hu H, Liu H, Wang Q. Recent Advances for the Developing of Instant Flavor Peanut Powder: Generation and Challenges. Foods. 2022; 11(11):1544. https://doi.org/10.3390/foods11111544
Chicago/Turabian StyleLiu, Yue, Hui Hu, Hongzhi Liu, and Qiang Wang. 2022. "Recent Advances for the Developing of Instant Flavor Peanut Powder: Generation and Challenges" Foods 11, no. 11: 1544. https://doi.org/10.3390/foods11111544
APA StyleLiu, Y., Hu, H., Liu, H., & Wang, Q. (2022). Recent Advances for the Developing of Instant Flavor Peanut Powder: Generation and Challenges. Foods, 11(11), 1544. https://doi.org/10.3390/foods11111544